Abstract
A wide spectrum of quality exists among deceased donor organs available for liver transplantation. It is unknown whether some transplant centers systematically use more low quality organs, and what factors might influence these decisions. We used hierarchical regression to measure variation in Donor Risk Index (DRI) in the U.S. by region, Organ Procurement Organization (OPO), and transplant center. The sample included all adults who underwent deceased donor liver transplantation between January 12, 2005 and February 1, 2009 (n=23,810). Despite adjusting for geographic region and OPO, transplant centers’ mean DRI ranged from 1.27–1.74, and could not be explained by differences in patient populations such as disease severity. Larger volume centers and those having competing centers within their OPO were more likely to use higher risk organs, particularly among recipients with lower Model for End-stage Liver Disease (MELD) scores. Centers using higher risk organs had equivalent waiting list mortality rates, but tended to have higher post-transplant mortality (hazard ratio 1.10 per 0.1 increase in mean DRI). In conclusion, the quality of deceased donor organ patients receive is variable and depends in part on characteristics of the transplant center they visit.
Introduction
It is well recognized that significant geographic variation exists in the availability of liver transplantation within the United States(1, 2). Less commonly appreciated, however, is that long-term survival for a patient with end-stage liver disease is determined not only by the receipt of a liver transplant but also by the quality of the organ received. This quality can be measured by the Donor Risk Index (DRI), which combines factors such as donor age and mechanism of death to predict the risk of graft failure associated with a particular organ. Among organs currently transplanted, the 3-year risk of graft survival ranges from 81% with organs having DRI <1 to <65% with organs having DRI≥2 (3). Thus, donor factors play a larger role in determining outcome than many recipient factors such as the pre-transplant Model for End-stage Liver Disease (MELD) score(4).
Each time an organ becomes available, the physician and patient must decide whether to accept that organ or wait in hopes that a better one will come along. Because this is an area of significant medical uncertainty, it is likely that variation in clinical practice exists. We previously demonstrated decision-making about the use of high risk organs to be susceptible to external pressures such as changes in allocation policy(5). It is unknown whether other non-clinical factors such as transplant volume, or competition for organs, might be associated with some centers using higher risk organs than others. Variations in clinical practice represent an important target for understanding and improving health care(6). Therefore, the aim of this study was to determine the magnitude and correlates of variation in organ quality between liver transplant centers.
Methods
Data source and preparation
This study used data from the Scientific Registry of Transplant Recipients (SRTR)(7). The data included all adults age ≥18 who underwent deceased donor liver transplantation between January 12, 2005 and February 1, 2009 (n=23,810). The beginning of this period was chosen to correspond with the start of the Share-15 policy, which mandates regional sharing of organs when no eligible candidate on the local list has a MELD score of at least 15. Approval was obtained from the University of Michigan Institutional Review Board to conduct this study.
Organ quality was calculated using the DRI,(3) a continuous measure of the risk of graft failure that is composed of factors such as donor age, size, race, cause of death, cold ischemia time, and location (local, regional, or national). In order to avoid bias associated with listwise deletion of large numbers of observations, we imputed the cold ischemia time using regression estimation with distance from the donor hospital to transplant center and other components of the DRI for 2,937 (12.3%) subjects(8). An imputation flag was created and included in subsequent analyses to ensure that no bias was introduced by the imputation(5). In order to exclude inactive transplant centers, we removed from the analysis centers performing fewer than ten transplants during the 4-year study period. Small amounts of missing data in other components of the DRI resulted in a final sample of 23,631 patients transplanted at 105 centers in 51 Organ Procurement Organizations (OPOs) and 11 Regions.
Statistical analysis
A map displaying mean DRI by Region was created using ArcGIS 9.3.1 software (ERIS corporation, Redlands, CA). All other analyses were performed using Stata 11.0 (StataCorp, College Station, TX).
To examine variation in DRI (i.e., organ quality) at the region, OPO, and transplant center levels, we used hierarchical linear regression(9). The benefits of this method are that it can estimate the degree of clustering in the dependent variable (in this case DRI), as well as control for this clustering when testing hypotheses. For example, Center A might use higher risk organs than Center B simply because of differences in organ availability by region or OPO, not because the centers are inherently different in their decision making. Although most nationwide database analyses ignore clustering, it is an inherent in a country where organ procurement and transplantation are divided geographically. This model provided estimates of the proportion of total variance in organ quality that was due to between-transplant center differences in DRI, as opposed to between-OPO or between-region differences(10), as measured by the intra-class correlation coefficient (ICC). In other words, this model controlled for: a) variation at the regional level (possibly attributable to geographic variability in age, medical comorbidities, and causes of death among the general population)(11) and for b) differences between OPO’s in their average donor organ quality(12). The significance of the variance estimates was determined using the likelihood ratio (LR) test(13). Standard diagnostics were performed to ensure model fit and calibration(9).
To control for possible differences due to systematic variation in patient factors between sites, these models were repeated controlling for patient-level variables. These patient factors were chosen a priori based upon our prior work, and included the MELD score immediately prior to transplant, age, gender, history of a prior liver transplant, encephalopathy, ascites, hepatitis C, and receipt of a MELD exception(5). Exceptions are typically awarded for life-threatening diagnoses not captured by the MELD score, such as hepatocellular carcinoma. However, regional variations exist in the frequency with which these exceptions are awarded(14). Furthermore, some of these patients do have varying degrees of underlying liver dysfunction. For these reasons, we used laboratory MELD score plus exception status rather than assigned MELD.
Next, center-level variables hypothesized to affect variation in organ quality were added to the model as fixed effects, including: 1) mean MELD at transplant as a measure of local organ shortage, 2) center volume expressed as the number of liver transplants performed per year, 3) the presence or absence of another transplant center within the same OPO, as a measure of competition, and 4) proportion of transplant recipients at each center who received MELD exceptions.
A random coefficient model was then created allowing the slope of individual MELD scores to vary by center, in order to investigate the hypothesis that centers using higher risk organs are more likely than other centers to use these organs in lower MELD patients. This model was compared to the random intercept model by LR test in order to determine statistical significance; both models contained all the previously described patient and center covariates. This concept was then analyzed visually by ranking centers according to their mean DRI, dichotomizing at the median into “high DRI” and “low DRI” centers, and examining the patient-level correlation between DRI and MELD at transplant among these two subgroups.
Finally, in order to determine the clinical significance of variation in organ quality, Cox proportional hazards regression was used to determine whether the mean DRI at each transplant center was associated with waiting list or post-transplant survival at that center. This analysis was performed among 39,341 waiting list candidates during the study period, as well as 18,014 recipients transplanted at least one year prior to the end of the study period. Time spent on hold was included in time at risk for death on the waiting list. The date of death used was from the Social Security Master Death file, and could have occurred on the list or after removal. We adjusted for all patient-level variables described above, and stratified by transplant center in order to generate robust standard errors.
Results
Among the 23,631 liver transplants performed during the study period, the distribution of DRI is shown in Figure 1. At the patient level, the mean DRI was 1.46, median 1.40, and inter-quartile range 1.14–1.71. Mean DRI by region ranged from 1.30–1.63 as shown in Figure 2, with the highest DRI (highest risk) organs being transplanted in Region 9 (New York and Western Vermont). There was slightly more variability in mean DRI by OPO (ranging from 1.25–1.65), and by transplant center (ranging from 1.24–1.74). The magnitude of variation by transplant center approached the inter-quartile range of the patient-level DRI, and exceeded the variation by OPO and region.
Figure 1.
Distribution of Donor Risk Index (DRI) among livers transplanted into adult recipients, 2005–2009.
Figure 2.
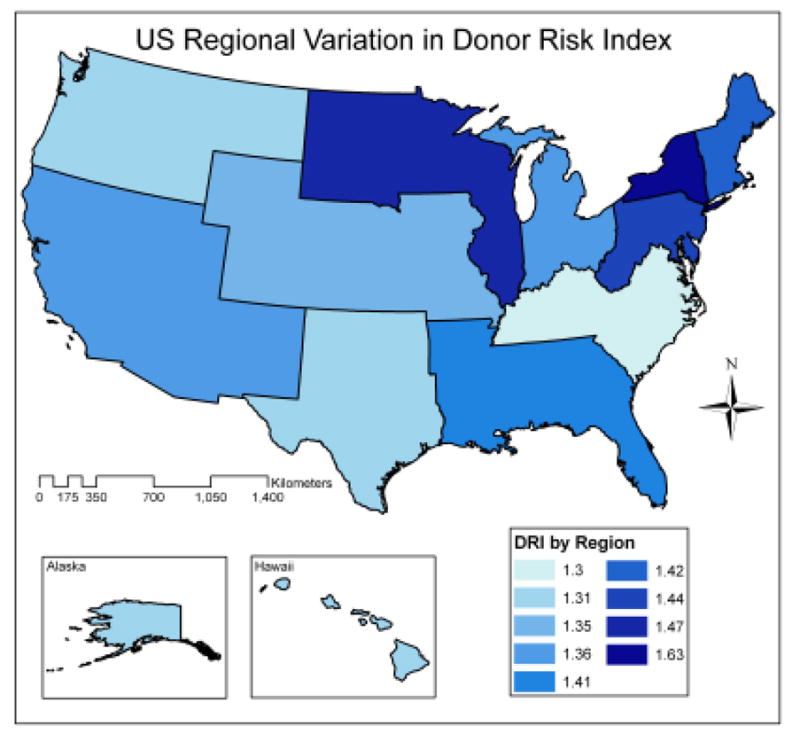
Mean DRI by transplant Region of the United States. The highest risk livers were used in Region 9 (New York and western Vermont), while the lowest risk livers were used in Region 11 (Kentucky, North Carolina, South Carolina, Tennessee, and Virginia).
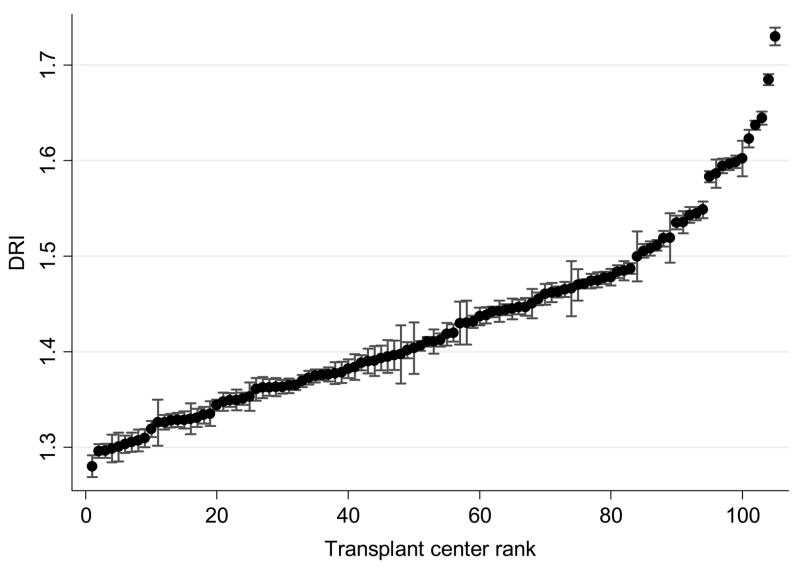
Overall, 3.4% of the variation in organ quality (i.e., ICC=.034) was attributable to variation between transplant centers, compared to 1.2% at the OPO level and 2.9% at the region level (all p values <0.001, by LR test). The remainder of the variation existed within centers (at the patient level). In other words, although the majority of variation existed at the patient level, transplant centers differed significantly in the quality of organs they used, even after controlling for variation in organ availability by region and OPO. Adjusting for patient-level factors such as severity of liver disease did not substantially affect these center differences (adjusted center ICC=0.029), suggesting that these findings were not due to differing patient populations across centers. As shown in Table 1, older patients and those with more encephalopathy tended to receive higher DRI organs, whereas male patients, those with higher MELD scores, those who received a MELD exception, had a prior liver transplant, or had hepatitis C all tended to receive lower DRI organs (p<0.001). Figure 3 shows the mean DRI by transplant center, which varied from 1.27–1.74 after adjustment for patient-level covariates and geographic variation by region and OPO. Confidence intervals for most of the centers do not overlap, indicating that these differences were statistically significant.
Table 1.
Patient and transplant center characteristics
| Patient characteristics at transplant (n=23,631) | Age, mean (range) | 53 (18–83) |
| Gender | 68% male | |
| MELD, mean (range) | 21 (6–40) | |
| Received priority exception | 30% | |
| Prior liver transplant | 8% | |
| Hepatitis C | 38% | |
| Ascites | None: 21% Mild: 52% Severe: 27% |
|
| Encephalopathy | None: 32% Mild: 56% Severe: 12% |
|
| Transplant center characteristics (n=105) | Liver transplants performed per year, mean (range) | 88 (3–199) |
| Mean MELD at transplant | Range: 12–31 IQR: 20–23 |
|
| Sole center within OPO | 21/105 (20%) | |
| Proportion of transplants with MELD exceptions | Range: 12%–51% IQR: 22%–37% |
MELD = Model for End-stage Liver Disease (based upon laboratory score, not including assigned priority exception scores), IQR = inter-quartile range, OPO = organ procurement organization
Figure 3.
Mean DRI by transplant center, adjusted for patient-level covariates and random effects of transplant region and OPO. The error bars represent 95% confidence intervals surrounding each center’s mean DRI, such that lack of overlap indicates statistical difference between centers.
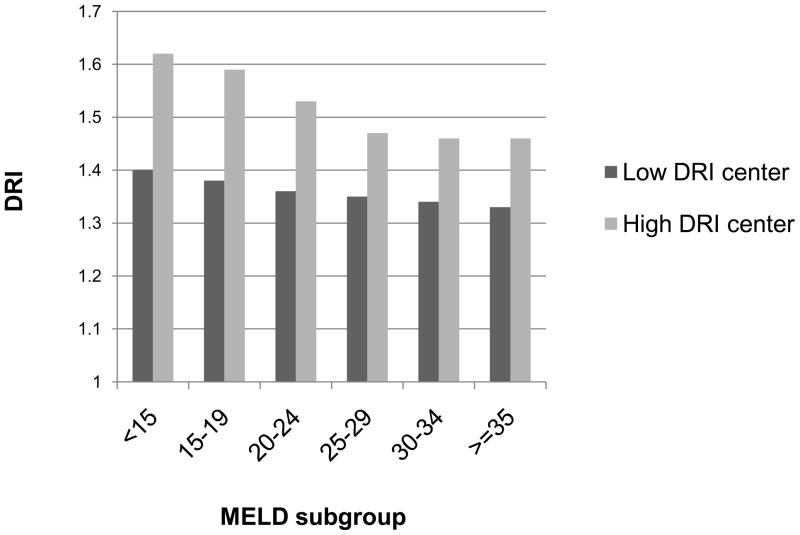
An examination of center attributes revealed that higher volume centers were significantly more likely to use higher DRI organs, as were those having competing centers within their OPO. These attributes explained some but not all of the variation between centers (adjusted center ICC decreased from 0.029 to 0.023). In the unadjusted analysis, centers at the bottom decile of transplant volume had mean DRI of 1.40, compared to 1.57 among centers at the top decile of transplant volume. In the multivariable analysis this effect was smaller but still statistically significant: as shown in Table 2, for every 10 transplant/year increase in a center’s volume there was on average a 0.006 increase in mean DRI. Thus, the mean DRI among the centers at the top decile of volume was 1.64, compared to 1.40 among the centers at the bottom decile of volume. Transplant centers having competing centers within their OPO had on average 0.05 higher mean DRI in the multivariable analysis than centers without local competition (p=0.013). In contrast, there was no association between the proportion of patients with MELD exceptions and DRI. There was also no association between the centers’ mean MELD at transplant and DRI. Further, centers that systematically used higher risk organs tended to use the higher risk organs in patients with low MELD scores more frequently than the low DRI centers (see Figure 4).
Table 2.
Patient and transplant center characteristics associated with DRI of liver transplants
| Coefficient | P value | ||
|---|---|---|---|
| Patient characteristics | Age, per decade | .02 | <0.001 |
| Encephalopathy (mild, moderate, severe) | .02 | <0.001 | |
| Male gender | −.06 | <0.001 | |
| MELD, per ten points | −.05 | <0.001 | |
| Priority exception (yes/no) | −.04 | <0.001 | |
| Prior liver transplant (yes/no) | −.09 | <0.001 | |
| Hepatitis C (yes/no) | −.03 | <0.001 | |
| Ascites (mild, moderate, severe) | −.01 | 0.07 | |
| Transplant center characteristics | Liver transplants performed per year, per ten transplants | .006 | <0.001 |
| Mean MELD at transplant/10 points | .01 | 0.5 | |
| Multiple centers within OPO (competition) | .05 | 0.013 | |
| Proportion of transplants with MELD exceptions | −0.04 | 0.57 |
DRI = Donor Risk Index. MELD = Model for End-stage Liver Disease, OPO = Organ Procurement Organization. Based upon multivariable mixed-effects hierarchical regression which adjusts for clustering at the center, OPO, and Region levels. Statistically significant results are in bold. For the purpose of reference, a difference of 0.1 in DRI is associated with an approximately 2% difference in risk of graft failure at 3 years after transplant(3).
Figure 4.
Mean DRI by Model for End-stage Liver Disease (MELD) subgroup. The greatest discrepancy between low DRI centers and high DRI centers can be seen among the patients with low MELD scores, suggesting that centers that are more aggressive about using high risk organs do so primarily among the low MELD patients. P<0.001 by LR test in hierarchical modeling, adjusted for patient and center covariates (see Methods).
Centers that used higher risk organs did not have lower waiting list mortality than those using lower risk organs (hazard ratio adjusted for patient characteristics = 1.0002 per 0.1 increase in mean DRI, p=0.9). However, they did have higher post-transplant mortality (hazard ratio 1.10 per 0.1 increase in mean DRI), despite adjustment for patient characteristics. These data indicate that centers at the 80th percentile for DRI have more than a 30% higher risk of post-transplant mortality than those at the 20th percentile.
Discussion
This study demonstrated that liver transplant patients systematically receive different quality organs at different transplant centers within the United States. These differences appear to be clinically significant, and cannot be explained by differences in the clinical characteristics of the patients being served or by geographic variation in the underlying donor pool. Large volume transplant centers and those that have competing centers within their OPO are more likely to use high risk organs, and these centers tend to use the lower quality organs in patients with lower MELD scores. A compelling reason to use higher risk organs would be to decrease patients’ overall mortality through timely transplantation. However, we found that centers that use higher risk organs do not have lower waiting list mortality; rather, they tended to have higher post-transplant mortality compared to centers using lower risk organs.
Based on this observational study we cannot establish causality or the full impact of these practices on individual patients. In other words, this study is not intended to lay blame or judge the clinical appropriateness of centers’ decisions. Nonetheless, it is noteworthy that centers which systematically use higher risk organs are even more likely than other centers to use these high risk organs in patients with low MELD scores. There are certainly some patients in whom the MELD score does not accurately reflect their mortality risk, who would benefit from early transplantation with a high risk organ. However, for the majority of patients the available evidence indicates that those with low MELD scores are least likely to benefit from receipt of a high risk organ because these are the patients who can afford to wait for a better organ to come along(4). It is possible, therefore, that some centers are concentrating more on the waiting list as a whole than on the risks and benefits for individual patients. Transplant centers in the current era experience a vast number of regulatory and oversight pressures. Utilization of high risk organs certainly expands the donor pool, reducing the waiting time by making more organs available for all patients. In current practice, the burden of this expansion appears to be falling primarily on the patients with low MELD scores.
There are several possible policy implications of our findings. First, we contend that there should be a comprehensive education process for all patients regarding the quality of organ they might receive. Since organs often become available in the middle of the night and decision-making is often time-pressured, this process may best begin early in the process of listing for transplantation(15). Whether accepting a high risk organ or waiting for a better one to come along, these decisions are associated with high risk and low certainty, and are consequently difficult for patients to grasp. We are currently in the process of developing a patient education tool and decision aid for this purpose, building upon prior work which has demonstrated that complex risk communication is best done 1) using graphs, 2) using absolute rather than relative risks, and 3) providing contextual information to account for innumeracy(16–18). Second, we propose that center-level organ quality measures should be provided as feedback to centers(19). This feedback would allow centers to compare their practices to national standards, and in the absence of published guidelines such comparison may provide some standardization or at least guide centers when making these complicated decisions. Third, current allocation policy does not consider organ quality, and these findings suggest that perhaps it should. One option would be adopting the proposed survival benefit model of liver allocation, which incorporates not only pre-transplant mortality predictors, but also post-transplant mortality predictors including donor characteristics(20). Changing to this allocation model might better optimize liver allocation practices because the priority list would be donor-specific. Finally, it should be noted that while the available data supports use of the higher DRI organs in patients at higher risk of dying on the waiting list, this practice is associated with increased costs to transplant centers(21). Consideration should be given to revising the payment structure in an effort to shift use of high risk organs to the patients most likely to benefit from them.
Although we would have liked to conduct an analysis looking at variation in acceptance rates for high risk organs, this was not possible because each center sets criteria for organs it will consider. We therefore cannot be certain that some of the observed center variation was not caused by variation in the available donor pool rather than acceptance practices. However, adjustment for region and OPO level variation should have accounted for most of this potential confounding. We were also unable to analyze or understand variations in individual physician decision making within transplant centers; the use of hierarchical regression does not change the observational nature of this study. An additional limitation relates to our measurement of organ quality. DRI lacks important information - particularly the degree of steatosis, which is available in only a small percent of donors, and biopsies are generally not performed on donor livers that do not appear to be steatotic. It is possible that centers using high DRI organs are doing so because these organs have less steatosis and could therefore be associated with the same overall outcome as lower DRI organs with more steatosis. Finally, it should be noted that the transplant center contributes a relatively small proportion of the total variation between patients in the quality of organs they receive – not surprisingly, most of this variation appears to be due to chance. Nonetheless, it is striking that there is more variation at the center level, within OPOs and regions rather than between them. This suggests that physician factors, not just coincidence or donor/recipient characteristics, play a key role in determining which patients receive high risk organs. Most importantly, the observed differences between centers were sufficiently large to translate into a measurable impact on patient survival.
In summary, the quality of the liver transplant organ patients receive is determined not only by their clinical characteristics, but also by the transplant center they visit. Notably, liver transplant centers with higher volumes and more local competition are significantly more likely to use lower quality donor livers, and these lower quality livers are preferentially used in patients with low MELD scores. These findings provide further evidence that decision making about organ quality is influenced by external forces, and emphasizes the importance of transparency in organ acceptance practices.
Acknowledgments
This study was supported by K23-DK085204 from the National Institute of Diabetes and Digestive and Kidney Diseases and a Research Scholar Award from the American Gastroenterological Association (MLV). The sponsors had no role in design, conduct, or interpretation of the study, or approval of the manuscript.
Footnotes
This study utilized the Measurement Core of the Michigan Diabetes Research & Training Center (NIDDK of The National Institutes of Health [P60 DK-20572]). The data reported here have been supplied by the Arbor Research Collaborative for Health (Arbor Research) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Volk ML, Choi H, Warren GJ, Sonnenday CJ, Marrero JA, Heisler M. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9(9):2113–2118. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 2.Ellison MD, Edwards LB, Edwards EB, Barker CF. Geographic differences in access to transplantation in the United States. Transplantation. 2003;76(9):1389–1394. doi: 10.1097/01.TP.0000090332.30050.BA. [DOI] [PubMed] [Google Scholar]
- 3.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 4.Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8(2):419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 5.Volk ML, Lok AS, Pelletier SJ, Ubel PA, Hayward RA. Impact of the model for end-stage liver disease allocation policy on the use of high-risk organs for liver transplantation. Gastroenterology. 2008;135(5):1568–1574. doi: 10.1053/j.gastro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Wennberg J, Gittelsohn Small area variations in health care delivery. Science. 1973;182(117):1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson DM, Dykstra DM, Levine GN, Li S, Welch JC, Webb RL. Transplant data: sources, collection and research considerations, 2004. Am J Transplant. 2005;5(4 Pt 2):850–861. doi: 10.1111/j.1600-6135.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 8.Allison PD. Missing data. Thousand Oaks, Calif: Sage Publications; 2002. [Google Scholar]
- 9.West B, Welch KB, Galecki AT. Linear mixed models: a practical guide using statistical software. Boca Raton: Chapman & Hall/CRC; 2007. [Google Scholar]
- 10.Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA. 1999;281(22):2098–2105. doi: 10.1001/jama.281.22.2098. [DOI] [PubMed] [Google Scholar]
- 11.Sheehy E, Conrad SL, Brigham LE, Luskin R, Weber P, Eakin M, et al. Estimating the number of potential organ donors in the United States. N Engl J Med. 2003;349(7):667–674. doi: 10.1056/NEJMsa021271. [DOI] [PubMed] [Google Scholar]
- 12.Tuttle-Newhall JE, Krishnan SM, Levy MF, McBride V, Orlowski JP, Sung RS. Organ donation and utilization in the United States: 1998-2007. Am J Transplant. 2009;9(4 Pt 2):879–893. doi: 10.1111/j.1600-6143.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 13.Stram DO, Lee JW. Variance components testing in the longitudinal mixed effects model. Biometrics. 1994;50(4):1171–1177. [PubMed] [Google Scholar]
- 14.Rodriguez-Luna H, Vargas HE, Moss A, Reddy KS, Freeman RB, Mulligan D. Regional variations in peer reviewed liver allocation under the MELD system. Am J Transplant. 2005;5(9):2244–2247. doi: 10.1111/j.1600-6143.2005.01008.x. [DOI] [PubMed] [Google Scholar]
- 15.Halpern SD, Shaked A, Hasz RD, Caplan AL. Informing candidates for solid-organ transplantation about donor risk factors. N Engl J Med. 2008;358(26):2832–2837. doi: 10.1056/NEJMsb0800674. [DOI] [PubMed] [Google Scholar]
- 16.Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ. Making numbers matter: present and future research in risk communication. Am J Health Behav. 2007;31 (Suppl 1):S47–56. doi: 10.5555/ajhb.2007.31.supp.S47. [DOI] [PubMed] [Google Scholar]
- 17.Zikmund-Fisher BJ, Fagerlin A, Roberts TR, Derry HA, Ubel PA. Alternate methods of framing information about medication side effects: incremental risk versus total risk of occurrence. J Health Commun. 2008;13(2):107–124. doi: 10.1080/10810730701854011. [DOI] [PubMed] [Google Scholar]
- 18.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. “If I'm better than average, then I'm ok?”: Comparative information influences beliefs about risk and benefits. Patient Educ Couns. 2007;69(1–3):140–144. doi: 10.1016/j.pec.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axelrod DA, Kalbfleisch JD, Sun RJ, Guidinger MK, Biswas P, Levine GN, et al. Innovations in the assessment of transplant center performance: implications for quality improvement. Am J Transplant. 2009;9(4 Pt 2):959–969. doi: 10.1111/j.1600-6143.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- 20.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7(4):990–997. doi: 10.1111/j.1600-6143.2006.01724.x. [DOI] [PubMed] [Google Scholar]