Abstract
Introduction
Studies have reported higher cancer risk in individuals with psoriasis, a chronic inflammatory autoimmune disease; however, adjustment for potential confounders was lacking.
Methods
We examined the association of psoriasis with cancer incidence in 32,910 women after age 65 in the IWHS cohort linked to Medicare. Psoriasis was defined as: 2+ psoriasis claims from any Medicare file during 1991–2004 or 1+ psoriasis claim from a dermatologist (n = 719). Severe psoriasis was defined as 4+ psoriasis claims from a dermatologist in any year (n = 121). Cox proportional hazards regression, with psoriasis as a time-dependent variable was conducted to calculate hazard ratios (HR) and 95% confidence intervals (CI) of total (n = 6,488), breast (n = 2,066), lung (n = 742), and colon cancers (n = 947).
Results
With age-adjustment, psoriasis (yes vs. no) was associated with increased risk of lung 1.9 (95% CI: 1.2–3.0), colon 1.6 (95% CI: 1.1–2.5), and total cancer 1.2 (95% CI, 1.0–1.4). After further adjustment for smoking, body mass index, education, physical activity, and hormone therapy use, only the association for colon cancer remained statistically significant (HR = 1.6, 95% CI: 1.0–2.4) and was stronger for severe psoriasis.
Conclusion
The observed association between psoriasis and colon cancer may reflect inflammatory or unidentified processes.
Keywords: Colon cancer, Inflammation, Psoriasis, Immune disease, Medicare
Introduction
Psoriasis is among the most common inflammatory autoimmune diseases of the skin; prevalence is 1–3% in the United States, and approximately 7.5 million people are affected [1]. Onset peaks in young adulthood and again at age 60, but psoriasis can begin at any age and last a lifetime [2, 3]. Psoriasis with an early age at onset is thought to be hereditary and is often associated with human leukocyte antigen (HLA-Cw6), whereas psoriasis that starts in older age is sporadic [4, 5]. Most psoriasis is managed with topical therapies. However, approximately 25% of patients have moderate to severe psoriasis which requires treatment with phototherapy, systemic agents, and/or immunomodulators. There is mounting evidence that people with more severe psoriasis have increased incidence of cardiovascular disease, hypertension, diabetes, and cancer [6]. Many studies showed that psoriasis is positively associated with increased risk of lymphoma and non-melanoma skin cancer [3, 7–13]. Far fewer studies focused on solid organ cancers; reported observations include positive associations with lung, colorectal, breast, pancreas and some other cancers [7, 8]. The main limitation of those studies was potential bias due to the lack of adjustment for potential confounding factors. To our knowledge, only one prior study on psoriasis and cancer controlled for potential confounders [9].
Furthermore, most studies of psoriasis and cancer have involved specific groups of patients either hospitalized with psoriasis [7, 8, 11–13] or those treated with phototherapy or systemic medications [14–16]. We sought to address limitations of previous studies in the current study.
We hypothesized that psoriasis is associated with the risk of total cancers as well as solid organ cancers including breast, lung, and colon in a population-based cohort of elderly Iowa women, after adjusting for potential confounders. We hypothesized that the strongest association would be with colon cancer since psoriasis is an inflammatory disease and colon cancer has been consistently shown to be associated with inflammation. To test these hypotheses, we identified all women with psoriasis at or after age 65 years using Medicare data linked to the Iowa Women’s Health Study (IWHS) during 1991–2004.
Materials and methods
This study combined three data sources: the IWHS, Medicare data and the Iowa SEER cancer registry.
IWHS study design
Detailed descriptions of IWHS have been published previously [17–19]. Briefly, the IWHS cohort included 41,836 women aged 55–69 years recruited via a baseline questionnaire mailed in 1986. Follow-up questionnaires were mailed in 1987, 1989, 1992, and 1997 to update information about participants’ residence, vital status, and other characteristics. Deaths were ascertained by annual linkage to Iowa death certificates, supplemented by linkage to the National Death Index for non-respondents and emigrants from Iowa. Emigration rate from Iowa was less than 1% [19]. The University of Minnesota Institutional Review Board approved this study, and all participants gave informed consent.
IWHS-Medicare linkage
IWHS identifiers were linked to Centers for Medicare Services (CMS) claims data from 1986 until 2004 via social security number, first and last names, and date of birth [20]. Linkage was conducted for participants aged 65+, since Medicare provides payment for health benefits for US residents 65 years and older. The linkage was successful for 99% of the cohort that survived to age 65 (n = 40,668).
Information about Medicare participants was collected from four files (Denominator, MedPar, Carrier, and Outpatient files). The annual Denominator file was used to identify periods of enrollment into Medicare and managed care. Information about inpatient services, including discharge codes, has been available since 1986, whereas data about outpatient services, including diagnosis codes, have been available since 1991 (Outpatient and Carrier files). In addition, information about the specialty of providers was obtained from Carrier files. The data about most prescription drugs in the Medicare database was not available until 2006 [20].
IWHS-Iowa SEER cancer registry linkage
Incident cancer cases from 1991 through 2006 (except for non-melanoma skin cancer) were ascertained via linkage of the IWHS cohort to the State Health Registry of Iowa, a participant in the Surveillance, Epidemiology, and End Results Program (SEER). Sites were defined using ICD-O codes (International Classification of Diseases for Oncology, 3rd ed) [21].
Analytical cohort
Our goal was to ascertain psoriasis, which is usually treated in a clinic setting and most psoriasis claims are found in the Medicare Carrier file available in 1991–2004. Thus, our analytical sample included only participants who, since 1991, were enrolled in at least 1 month of fee-for-service Part A and Part B Medicare coverage after reaching 65 years (n = 39,503). Those participants who did not reside in Iowa at the start of their follow-up were excluded because their cancers would not be identified by SEER (n = 1,354). Participants were followed from the start of their follow-up until disenrollment from full Medicare fee-for-service benefits, emigration from Iowa, cancer diagnosis in Iowa, death, or end of follow-up on 31 December 2006, whichever occurred earlier. Further, women who had cancer at baseline or were diagnosed with cancer before the start of their follow-up (except non-melanoma skin cancer) were excluded (n = 4,883). After these exclusions, 33,266 women were left in the cohort.
Psoriasis ascertainment
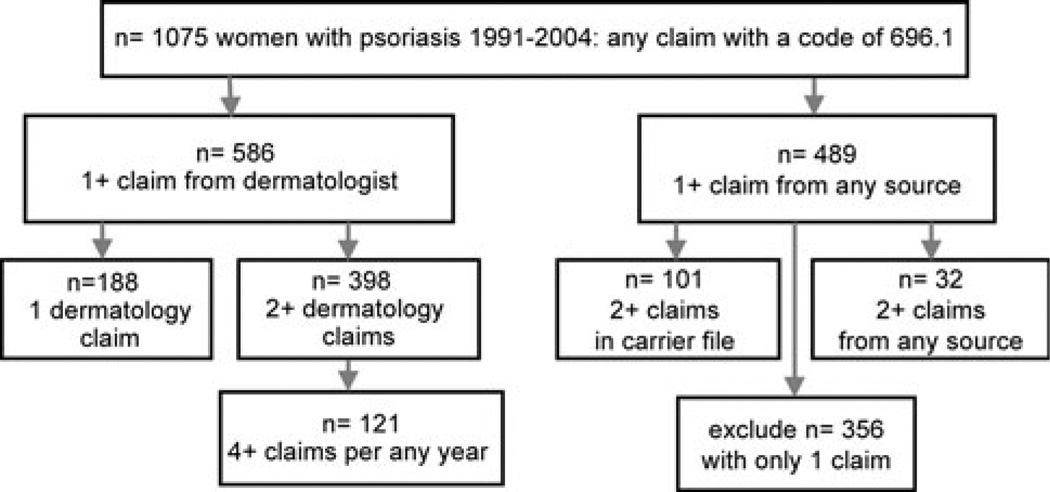
Psoriasis diagnoses were identified using the International Classification of Diseases (ICD-9) diagnosis code 696.1 in Medicare claims data. This is the only ICD-9 code for psoriasis. In our analytical cohort, there were 1,075 women who had 696.1 code. Only those women with 1+ dermatology claim with a psoriasis diagnosis code or those with 2+ claims on different days at any time during follow-up were included (Fig. 1). Thus, 719 patients among 32,910 women free of cancer (about 2.2%) had a diagnosis of psoriasis at 65+ in 1991–2004 in IWHS/CMS. The prevalence of psoriasis in the cohort prior to excluding cancer was also 2.2%. This agrees with the reported psoriasis prevalence of 1–3% in the United States [1]. Consistent with clinical patterns, 96% of psoriasis cases were identified from Carrier files and 4% from MedPAR or Outpatient files only. Within the Carrier file, 85% of all psoriasis claims were submitted by dermatologists, which is consistent with National Ambulatory Medical Survey data—82% of visits for psoriasis were to dermatologists in 1990–2001 in the United States [22].
Fig. 1.
Number of cancer-free women with psoriasis in IWHS/Medicare, 1991–2004 (After excluding women with only one non-dermatology claim, 719 women with psoriasis remained in the cohort (n = 188 + 398 + 101 + 32 = 719)
We used the number of visits to a dermatologist with a psoriasis diagnostic claim as a surrogate measure of severity. Severe psoriasis was defined as having 4+ visits to a dermatologist in any year (n = 121). Four visits were selected as a cut-off because patients on oral medications or UV light treatment are usually seen every 3 months by dermatologists.1 All other cases of psoriasis were considered mild (n = 598). We did not have data about psoriasis diagnoses before enrollment in Medicare; thus we examined women who had “active” psoriasis at age 65+ years.
Other exposure information
The baseline questionnaire (1986) collected information about age, education level, smoking status and pack-years of smoking, anthropometric characteristics, usual alcohol intake within the last year, physical activity level, reproductive history, use of oral contraceptives and hormone therapy (HT) [17, 23]. Information about HT use, diabetes mellitus, heart disease, and hypertension was collected at baseline and each follow-up.
Cancer ascertainment
In our analytical cohort, there were 6,488 total cancers (excluding non-melanoma skin cancer), 2,066 breast, 742 lung, and 947 colon cancers. Due to insufficient number of cases (less than 10 among psoriatic patients), we were unable to investigate the association of psoriasis with less common neoplasms, such as cancers of the pancreas, bladder, hematopoietic or several others, for which associations were observed in some studies. We also did not have information about non-melanoma skin cancers.
Statistical analysis
Characteristics of women with and without psoriasis were compared using the chi-square tests and t tests for categorical and continuous variables, respectively. Prevalent odds ratio for psoriasis (age- and multivariate-adjusted) for selected characteristics were calculated using logistic regression.
Age-adjusted cancer incidence rates for psoriatic and non-psoriatic groups were compared using Poisson regression. To further investigate the association of “active” psoriasis at 65+ years with each cancer, we used a Cox proportional hazards model with psoriasis as a time-dependent covariate [24].
The following variables, associated with cancer and psoriasis, were included into a multivariate-adjusted model: age at the start of follow-up, BMI, education, smoking status, pack-years, HT use, physical activity, and, for breast cancer, number of live births. Further adjustment for alcohol intake, WHR, history of diabetes, oral contraceptives use, and the start of follow-up did not materially change associations, and these variables were not included in the final model.
We also examined associations between psoriasis “severity” and risk of total and specific cancers by calculating hazard ratios (HR) for each cancer in groups with severe and mild psoriasis versus “no psoriasis,” although there was limited power to conduct these analyses. We tested effect modification of the psoriasis—cancer association by smoking and BMI, since these are risk factors for cancer and are related to psoriasis: no interactions were detected (p = 0.3–0.8). All analyses were performed using SAS 9.2, all p tests were two-sided.
Results
The analytical cohort included 32,910 initially cancer-free women with a total of 389,180 person-years of follow-up. Mean age at start of follow-up was 68.1 years and over 99% women were White. Consistent with findings from other studies, psoriasis was positively associated with higher education, smoking, lack of regular physical activity, diabetes, hypertension, and history of HT use (Table 1) [1, 25–29]. We conducted a sensitivity analysis by including into psoriasis group only women with 2+ claims for psoriasis, i.e., women with a more stringent definition for diagnosis, and all the associations were practically unchanged.
Table 1.
Selected characteristics in relation to psoriasis, IWHS/Medicare, 1991–2004
| Characteristics | No psoriasis n = 32,191 (97.8%) |
Psoriasis n = 719 (2.2%) |
Age-adjusted odds ratiosb of psoriasis (95% CI) |
Multivariate-adjustedb,c odds ratios of psoriasis (95% CI) |
|---|---|---|---|---|
| Mean age at the start of follow-up ± SD (years) | 68.1 ± 3.2a | 67.8 ± 3.0a | 1.0 (0.9–1.0) | |
| BMI categories in 1986 (kg/m2) (%)d | ||||
| <24.9 | 39.5 | 41.3 | 1 | 1 |
| 25–30 | 37.3 | 33.4 | 0.9 (0.7–1.0) | 0.9 (0.7–1.1) |
| ≥30 | 23.2 | 25.3 | 1.0 (0.9–1.3) | 1.1 (0.9–1.4) |
| p = 0.7 | p = 0.3 | |||
| Education in 1986 (%)d | ||||
| Less than high school | 19.6 | 17.9 | 1 | 1 |
| High school | 42.2 | 38.2 | 1.0 (0.8–1.2) | 1.0 (0.8–1.3) |
| More than high school | 38.1 | 43.9 | 1.3 (1.0–1.6) | 1.3 (1.0–1.6) |
| p = 0.03 | p = 0.02 | |||
| Smoking in 1986 (%)d | ||||
| Never | 67.6 | 53.1 | 1 | 1 |
| Former | 18.6 | 25.0 | 1.4 (1.1–1.8) | 1.4 (1.1–1.7) |
| Current | 13.8 | 22.0 | 1.5 (1.1–2.0) | 1.5 (1.1–2.0) |
| p = 0.004 | p = 0.006 | |||
| Alcohol intake in 1986 (%)d | ||||
| Never | 56.9 | 52.8 | 1 | 1 |
| <4 g/day | 23.6 | 22.4 | 1.0 (0.9–1.2) | 1.0 (0.8–1.2) |
| ≥4 g/day | 19.5 | 24.8 | 1.4 (1.1–1.6) | 1.1 (0.9–1.4) |
| p = 0.0007 | p = 0.3 | |||
| Regular physical activity in 1986 (%) | ||||
| No | 57.9 | 63.7 | 1 | 1 |
| Yes | 42.1 | 36.3 | 0.8 (0.7–0.9) | 0.8 (0.7–1.0) |
| History of HT use up to 2004 (%) | ||||
| Never | 56.1 | 51.7 | 1 | 1 |
| Ever | 43.9 | 48.3 | 1.2 (1.0–1.4) | 1.19 (1.0–1.4) |
| Diabetes up to 2004 (%) | ||||
| Never | 84.2 | 80.7 | 1 | 1 |
| Ever | 15.8 | 19.3 | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) |
| Heart disease up to 2004 (%) | ||||
| Never | 86.6 | 85.4 | 1 | 1 |
| Ever | 13.4 | 14.6 | 1.2 (1.0–1.4) | 1.1 (1.0–1.3) |
| Hypertension up to 2004 (%) | ||||
| Never | 57.8 | 54.1 | 1 | 1 |
| Ever | 42.2 | 45.9 | 1.3 (1.07–1.5) | 1.2 (1.1–1.5) |
Mean ± standard deviation
Prevalent odds ratio
Adjusted for age, education, smoking status, pack-years of smoking, alcohol, and BMI
These variables were adjusted for each other in multivariate-adjusted analyses
Age-adjusted incidence rates of lung, colon, and total cancers were higher for those with psoriasis than for those without (Table 2). Age-adjusted hazard ratios were increased for lung, 1.9 (95% CI: 1.2–3.0); colon, 1.6 (95% CI: 1.0–2.5) and total cancer, 1.2 (95% CI: 1.0–1.4). There was no association between psoriasis and breast cancer (Table 2). After multivariate adjustment, all observed associations were attenuated: hazard ratios of lung, colon, and total cancer were 1.3 (95% CI: 0.8–2.0), 1.6 (95% CI: 1.0–2.4) and 1.1 (95% CI: 0.9–1.4), respectively. This attenuation was largely due to smoking: after adjusting for all the covariates except smoking, hazard ratios for all cancers were virtually the same as in the age-adjusted analyses. Compared to those without psoriasis, hazard ratios of colon cancer were 1.5 for mild and 1.9 for severe psoriasis (p trend = 0.03) (Table 3).
Table 2.
Associations between psoriasis and risk of total cancer and cancers of breast, lung, and colon, IWHS/Medicare, 1991–2006
| Incident cancer | Psoriasis | Number of cancers | Person-years | Incidence rate per 1,000, age-adjusted |
HR, age-adjusted (95% CI) |
HR, multivariate- adjusteda (95% CI) |
|---|---|---|---|---|---|---|
| Total | No | 6,381 | 384,037 | 16.5 | 1 | 1 |
| Yes | 107 | 5,141 | 20.8 | 1.2 (1.0–1.4) | 1.1 (0.9–1.4) | |
| Breastb | No | 2,037 | 402,199 | 5.1 | 1 | 1 |
| Yes | 29 | 5,477 | 5.3 | 1.0 (0.7–1.5) | 1.0 (0.7–1.5) | |
| Lung | No | 722 | 414,074 | 1.7 | 1 | 1 |
| Yes | 20 | 5,703 | 3.5 | 1.9 (1.2–3.0) | 1.3 (0.8–2.0) | |
| Colon | No | 925 | 411,031 | 2.2 | 1 | 1 |
| Yes | 22 | 5,637 | 3.9 | 1.6 (1.1–2.5) | 1.6 (1.0–2.4) |
Adjusted for age at the start of follow-up, BMI, education, smoking status, pack-years, physical activity, and history of HT use
Additionally adjusted for number of live births
Table 3.
Multivariate-adjusted hazard ratios (HR) of total cancer and cancers of breast, lung, and colon, according to psoriasis severity, 1991–2006
| Cancer | No psoriasis | Mild psoriasis | Severe psoriasis | p trendb | |||
|---|---|---|---|---|---|---|---|
| Number of cancers | HR | Number of cancers | HR (95% CI) | Number of cancers | HR (95% CI) | ||
| Total | 6,381 | 1 | 85 | 1.1 (0.9–1.4) | 22 | 1.2 (0.8–1.8) | 0.3 |
| Breasta | 2,037 | 1 | 24 | 1.4 (0.8–2.2) | 5 | 1.0 (0.4–2.7) | 0.4 |
| Lung | 722 | 1 | 16 | 1.1 (0.7–1.6) | 4 | 1.0 (0.4–2.3) | 0.9 |
| Colon | 925 | 1 | 17 | 1.5 (0.9–2.4) | 5 | 1.9 (0.8–4.7) | 0.03 |
Adjusted for age at the start of follow-up, BMI, education, smoking status, pack-years, physical activity, and history of HT use
Additionally adjusted for number of live births
Test for trend across psoriasis groups was calculated by putting a psoriasis severity variable (0—no psoriasis, 1—mild, 2—severe psoriasis) as a continuous variable into the model
In addition, sensitivity analyses were conducted. To reduce ambiguity for a temporal relation between psoriasis and cancer, we included only women who had at least 2 or 5 years of cancer-free follow-up time. The results were similar to those in the main analysis. For instance, hazard ratios of colon cancer, for psoriatic versus non-psoriatic patients, were 1.6 (95%CI: 1.0–2.4) and 1.84 (95%CI: 1.8–2.9) for those with at least 2 and 5 years of follow-up, respectively.
Further, most of the covariates were ascertained in 1986, while the follow-up started in 1991. Because some of the covariates could have changed during this time gap, we conducted analyses stratified by the median time between 1986 and the start of follow-up (~5 years); similar associations between psoriasis and colon cancer were observed in both subgroups: HR = 1.6 (95% CI: 0.9–2.9) for those with a time gap <5 years and HR = 1.8 (95% CI: 0.9–3.4) for those with a time gap ≥5 years.
Finally, we stratified colon cancer into proximal and distal. The association of psoriasis with proximal colon cancer (HR = 2.0, 95% CI: 1.2–3.1) was stronger than with overall colon cancer. We were unable to examine psoriasis in relation to distal colon cancer due to small case numbers.
Discussion
In a prospective analysis, having psoriasis at age 65+ was associated with higher subsequent colon cancer risk after adjustment for age, BMI, education, smoking, physical activity, and HT use. The hazard ratio for colon cancer was higher among those with severe compared to mild psoriasis. We also observed increased age-adjusted hazard ratios for lung and total cancer for psoriatic versus non-psoriatic patients but this was primarily due to confounding by cigarette smoking.
The majority of previous studies of psoriasis and cancer examined total, skin, and lymphoproliferative cancers, whereas only a few studies investigated associations with solid organ cancers. Several cohorts from Scandinavian countries, which included patients of wide age ranges, observed increased standardized incidence ratios (SIR) among psoriatic patients versus the general population for cancers as follows: total (by 27–40%), lung (by 40–110%), and colon (by 30–40%) [7, 8, 11–13]. This increase was largely explained by an excess risk of skin cancer and lymphoproliferative cancers and/or cancers related to smoking. The main limitation of these Scandinavian studies was a potential bias due to lack of data about confounding variables. Another limitation is that those studies included only hospitalized patients, the majority of whom were hospitalized for psoriasis [7, 8, 11]. Such patients likely have more severe psoriasis and, possibly, different cancer risk than other psoriatic patients [30].
Only a few population-based studies have investigated psoriasis in relation to cancer, particularly solid organ cancers. A US study using the Medicaid administrative database reported an increase in total cancer risk by 78% for patients with severe psoriasis (those treated with systemic medication) and by 13% for those with mild disease compared to hypertensive patients [3]. Recently, a nested case–control study by Brauchli et al. [9] was the first to examine a link between psoriasis and cancer after adjusting for confounding variables, such as age, sex, calendar time, BMI, smoking, and benign tumors. In that study, psoriatic patients, 55% of whom were younger than 50 years at the time of psoriasis diagnosis, had slightly increased odds ratio of total cancer (OR = 1.13, 95% CI: 1.02–1.26), which was higher for psoriasis with duration ≥4 years: (OR = 1.50, 95% CI: 1.30–1.74) [9]. The odds ratio for colorectal cancers was also increased with longer-duration psoriasis: (OR ~ 2.5, statistically significant, but exact data were not reported). The results for total and colorectal cancer are in agreement with the findings from the multivariate analysis in our study.
The biological mechanism explaining the relation between psoriasis and cancer is under investigation. Psoriasis is considered a systemic inflammatory disease not limited to the epidermis. It is characterized by immune deregulation: Th1 cells infiltrate the skin of a psoriatic patient and release inflammatory cytokines, such as interleukins (IL-1, IL-6), interferon-γ, and tumor necrosis factor-α, and stimulate dendritic cells, macrophages, and neutrophils, which induce epidermal hyperproliferation and hyperplasia [25, 31–33]. A deregulated immune system together with chronic inflammation may lead to mutations in dividing cells and errors in elimination of malignant cells, resulting in increased risk of cancer, especially, of lymphoproliferative cancers [34, 35].
Several lines of evidence link inflammation to colorectal cancer risk. Colorectal cancer is the only cancer for which the protective effect of an anti-inflammatory drug—aspirin has been clinically established [36]. Several studies reported positive associations between C-reactive protein, a systemic inflammatory marker, and risk of colorectal cancer [37–40]. In addition, positive associations have been shown between inflammatory bowel disease and colorectal cancer [36, 41] and between inflammatory bowel disease and psoriasis [42, 43]. Thus, chronic inflammation associated with psoriasis could result in subsequent colon cancer.
The major strength of our study is use of data from a large prospective population-based cohort linked to the already existing longitudinal Medicare dataset, which allowed for a cost-efficient analysis. To our knowledge, this is the first attempt to comprehensively establish a diagnosis of psoriasis through Medicare. Ascertainment of psoriasis via Medicare claims is free from usual bias such as non-response or recall. Furthermore, to our knowledge, this is only the second study that examined an association between psoriasis and incident cancers after adjustment for confounding variables.
Our study has limitations. Because we used the Medicare database for psoriasis ascertainment, we cannot exclude the possibility of incomplete coding and coding errors [44, 45]. The precise date of psoriasis onset was also unknown. We did not validate psoriasis diagnosis. However, validation studies conducted for other autoimmune diseases in Medicare, which used medical records as the gold standard, reported high sensitivities of the physician claims (0.90 for rheumatoid arthritis and 0.85 for systemic lupus erythematosus), and high positive predictive values (0.90 for both of these diseases) [44]. Further, we conducted several sensitivity analyses and their results were robust. Finally, the most common diseases that can be misdiagnosed as psoriasis are seborrheic dermatitis and eczematous skin conditions, which are not known to be related to cancer [7].
A limitation in our study is that power was limited for examining colon cancer risk in different subgroups. However, we observed a statistically significant increase in hazard ratio of proximal colon cancer by ~100% for psoriatic versus non-psoriatic patients. An additional limitation is that we lacked data about treatment. Although it is unlikely that systemic drugs or phototherapy affect the risk of solid organ cancers (other than skin cancer), this possibility cannot be fully excluded. Another potential concern is surveillance bias: patients with psoriasis could be more likely screened for cancer. Finally, our study was conducted among white elderly women in Iowa; results may not be generalizable to other age, race, gender, or other geographic settings.
In summary, our analysis showed that there was a positive association between psoriasis and colon cancer incidence. This supports the view that inflammation may play a role in colon carcinogenesis.
Acknowledgments
The authors are thankful to the IWHS staff for consultation and assistance in data preparation. The authors are especially thankful to Bill Baker for help with the analysis of Medicare data. This study was supported by National Cancer Institute grant R01 CA39742.
Footnotes
This study was presented at the Annual Society for Epidemiological Research Meeting in 2010 and was the First Prize Winner.
E. Warshaw, Department of Dermatology, University of Minnesota, October, 2009, personal communication.
Conflict of interest The authors state no conflict of interest.
Contributor Information
Anna E. Prizment, Email: prizm001@umn.edu, Division of Epidemiology and Community Health, University of Minnesota, 1300 2nd Street South, Suite 300, Minneapolis, MN 55455, USA.
Alvaro Alonso, Division of Epidemiology and Community Health, University of Minnesota, 1300 2nd Street South, Suite 300, Minneapolis, MN 55455, USA.
Aaron R. Folsom, Division of Epidemiology and Community Health, University of Minnesota, 1300 2nd Street South, Suite 300, Minneapolis, MN 55455, USA Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
Rehana L. Ahmed, Department of Dermatology, University of Minnesota, Minneapolis, MN, USA
Beth A. Virnig, Division of Health Policy and Management, University of Minnesota, Minneapolis, MN, USA
Erin M. Warshaw, Department of Dermatology, University of Minnesota, Minneapolis, MN, USA Department of Dermatology, VA Medical Center, Minneapolis, MN, USA.
Kristin E. Anderson, Division of Epidemiology and Community Health, University of Minnesota, 1300 2nd Street South, Suite 300, Minneapolis, MN 55455, USA Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
References
- 1.Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 3.Margolis D, Bilker W, Hennessy S, et al. The risk of malignancy associated with psoriasis. Arch Dermatol. 2001;137:778–783. [PubMed] [Google Scholar]
- 4.Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767–768. doi: 10.1136/jmg.39.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13:450–456. doi: 10.1016/s0190-9622(85)70188-0. [DOI] [PubMed] [Google Scholar]
- 6.Kimball AB, Gladman D, Gelfand JM, et al. National psoriasis foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008;58:1031–1042. doi: 10.1016/j.jaad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boffetta P, Gridley G, Lindelof B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117:1531–1537. doi: 10.1046/j.0022-202x.2001.01520.x. [DOI] [PubMed] [Google Scholar]
- 8.Ji J, Shu X, Sundquist K, Sundquist J, et al. Cancer risk in hospitalised psoriasis patients: a follow-up study in Sweden. Br J Cancer. 2009;100:1499–1502. doi: 10.1038/sj.bjc.6605027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brauchli YB, Jick SS, Miret M, et al. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009;129:2604–2612. doi: 10.1038/jid.2009.113. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand JM, Shin DB, Neimann AL, et al. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126:2194–2201. doi: 10.1038/sj.jid.5700410. [DOI] [PubMed] [Google Scholar]
- 11.Hannuksela-Svahn A, Pukkala E, et al. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J Invest Dermatol. 2000;114:587–590. doi: 10.1046/j.1523-1747.2000.00898.x. [DOI] [PubMed] [Google Scholar]
- 12.Olsen JH, Frentz G, Moller H. Psoriasis and cancer. Ugeskr Laeger. 1993;155:2687–2691. [PubMed] [Google Scholar]
- 13.Frentz G, Olsen JH. Malignant tumours and psoriasis: a follow-up study. Br J Dermatol. 1999;140:237–242. doi: 10.1046/j.1365-2133.1999.02655.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindelof B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108–112. doi: 10.1046/j.1365-2133.1999.02928.x. [DOI] [PubMed] [Google Scholar]
- 15.Paul CF, Ho VC, McGeown C, et al. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol. 2003;120:211–216. doi: 10.1046/j.1523-1747.2003.12040.x. [DOI] [PubMed] [Google Scholar]
- 16.Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142:1132–1135. doi: 10.1001/archderm.142.9.1132. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Kaye SA, Sellers TA, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269:483–487. [PubMed] [Google Scholar]
- 18.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 19.Bisgard KM, Folsom AR, Hong CP, et al. Mortality and cancer rates in nonrespondents to a prospective study of older women: 5-year follow-up. Am J Epidemiol. 1994;139:990–1000. doi: 10.1093/oxfordjournals.aje.a116948. [DOI] [PubMed] [Google Scholar]
- 20.Virnig B, Durham SB, Folsom AR, et al. Linking the Iowa Women’s Health Study cohort to Medicare data: linkage results and application to hip fracture. Am J Epidemiol. 2004;172:327–333. doi: 10.1093/aje/kwq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritz A, Percy C, Jack A. International classification of disease for oncology (ICD-O) 3rd edn. Geneva: WHO; 2000. [Google Scholar]
- 22.Pearce DJ, Stealey KH, Balkrishnan R, et al. Psoriasis treatment in the United States at the end of the 20th century. Int J Dermatol. 2006;45:370–374. doi: 10.1111/j.1365-4632.2006.02532.x. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR, Anderson JP, Ross JA. Estrogen replacement therapy and ovarian cancer. Epidemiology. 2004;15:100–104. doi: 10.1097/01.ede.0000091606.31903.8e. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbaum D, Klein M. Survival analysis: a self-learning text. 2nd edn. New York: Springer; 2005. [Google Scholar]
- 25.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 26.Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:121–128. doi: 10.2174/1568010043343958. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setty AR, Curhan G, Choi HK. Smoking and the risk of psoriasis in women: Nurses’ Health Study II. Am J Med. 2007;120:953–969. doi: 10.1016/j.amjmed.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plunkett A, Marks R. A review of the epidemiology of psoriasis vulgaris in the community. Australas J Dermatol. 1998;39:225–232. doi: 10.1111/j.1440-0960.1998.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 30.Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 31.Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64:30–36. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 33.Tonel G, Conrad C. Interplay between keratinocytes and immune cells—recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41:963–968. doi: 10.1016/j.biocel.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Bernatsky S, Ramsey-Goldman R, Clarke A. Malignancy and autoimmunity. Curr Opin Rheumatol. 2006;18:129–134. doi: 10.1097/01.bor.0000209423.39033.94. [DOI] [PubMed] [Google Scholar]
- 35.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 36.Puntoni M, Marra D, Zanardi S, et al. Inflammation and cancer prevention. Ann Oncol. 2008;19:225–229. doi: 10.1093/annonc/mdn442. [DOI] [PubMed] [Google Scholar]
- 37.Erlinger TP, Platz EA, Rifai N, et al. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 38.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the Health Aging and Body Composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 39.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 40.Otani T, Iwasaki M, Sasazuki S, et al. Japan Public Health Center-Based Prospective Study Group. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-Based Prospective Study. Cancer Epidemiol Biomarkers Prev. 2006;15:690–695. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–16. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 42.Gulliver W. Long-term prognosis in patients with psoriasis. Br J Dermatol. 2008;159:2–9. doi: 10.1111/j.1365-2133.2008.08779.x. [DOI] [PubMed] [Google Scholar]
- 43.Yates VM, Watkinson G, Kelman A. Further evidence for an association between psoriasis, Crohn’s disease and ulcerative colitis. Br J Dermatol. 1982;106:323–330. doi: 10.1111/j.1365-2133.1982.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 44.Katz JN, Barrett J, Liang MH, et al. Sensitivity and positive predictive value of Medicare part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40:1594–1600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- 45.Icen M, Crowson CS, McEvoy MT, et al. Potential misclassification of patients with psoriasis in electronic databases. J Am Acad Dermatol. 2008;59:981–985. doi: 10.1016/j.jaad.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]