Abstract
Data concerning nine forms of human stone disease, along with observations on normal people give new insights into formation of interstitial apatite plaque and intra-tubular crystal deposits. In general, across multiple disease states, one can reproduce the same relationships between plaque abundance as is seen among patients within individual disease states, so that the link between plaque and high urine calcium excretion, and low urine volume and pH seems increasingly secure. From this, one can propose a specific model of plaque formation, susceptible to experimental test. In many diseases, formation of inner medullary collecting duct and Bellini duct deposits is compatible with simple crystallization driven by urine supersaturations; this is expected in that these segments contain tubule fluid quite close in composition to final urine. But in ileostomy, small bowel disease and obesity bypass patients, crystals found in deposits are not those expected: apatite and urates in deposits, despite formation of highly acidic urine. Also, this discrepancy suggests the possibility of divergence between bulk urine pH and pH of focal collecting ducts, a new kind of possibility that is susceptible to experimental test.
Nature of Plaque and Deposits
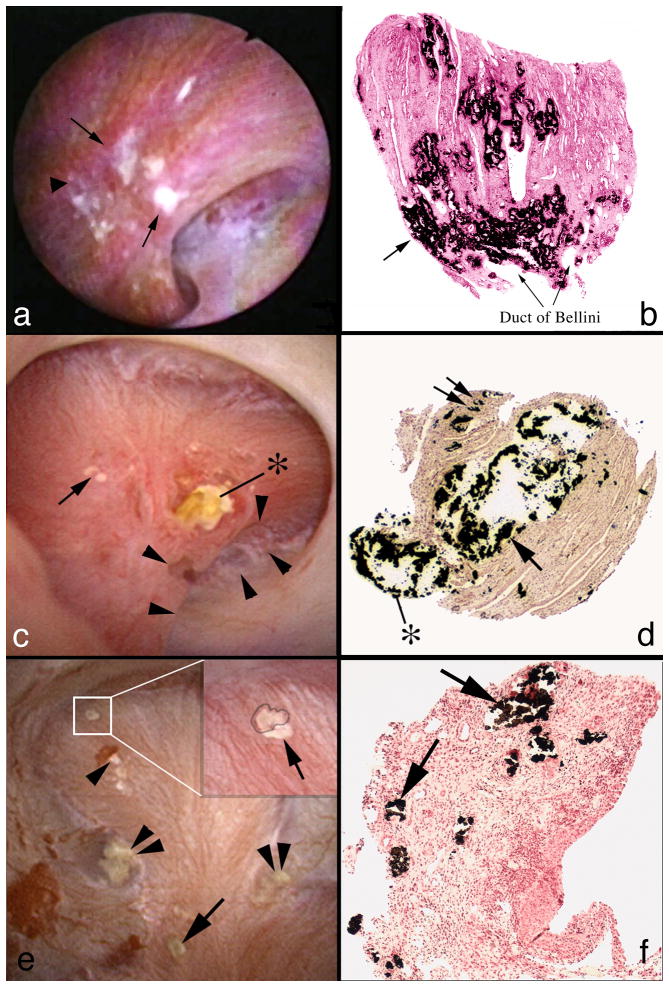
Work to date permits comparison of plaque and intra-tubular mineral deposits in 9 disease states and normal people – 10 groups in all (Table 1). Plaque, as referred to in this paper is interstitial apatite deposit within the renal papilla (Figures 1a & b). It is known to form in the basement membranes of thin loops of Henle [1], and migrate from there to locations beneath the urothelium where it appears as white streaks (Figure 1a). Its importance lies in being an anchored initiating site for overgrowth of the common calcium oxalate (CaOx) stone [2]. Plaque is found in five of the diseases (Table 2) at abundances above those seen in normal people [3;4]. Tubule deposits of apatite, CaOx, cystine, and urate species (Table 2) occur in all stone diseases studied thus far except for ICSF and normal people [4-7]. They are purely apatite only in primary hyperparathyroidism, pure CaOx only in primary hyperoxaluria and otherwise mixtures (Table 2). They appear as elongate yellow tinted sub-urothelial risings and often protrude (Figure 1c) from the mouths of massively dilated ducts of Bellini (BD). Unlike interstitial plaque that evokes no inflammatory response or injury, deposits destroy epithelial cells (Figure 1d) and induce interstitial fibrosis.
Table 1. Diseases Studied.
| Idiopathic CaOx stone formers (ICSF) |
| Obesity bypass (BP) |
| Ileostomy (ILEO) |
| Small bowel resection (SBR) |
| Hyperparathyroidism (HPT) – high stone phosphate |
| Distal renal tubular acidosis (RTA) |
| Brushite stones (BR) |
| Cystinuria (Cys) |
| Primary hyperoxaluria with ESRD (HOX) |
| Normal people (N) – Controls |
Figure 1.
Surgical anatomy and histopathology of plaque and deposits in three stone diseases. Plaque is seen as a white sub-urothelial reposition during endoscopic surgery (panel a, arrows) in an ICSF. On biopsy, plaque is stained black with Yasue stain; tubule lumens show through the interstitial deposits (panel b). In a brushite SF (panel c) a large deposit extends out of the mouth of a greatly dilated Bellini ducts (BD) (*). Arrowheads outline a deformity of the papillum representing fibrosis. Yellow plaque, the gross reflection of inner medullary collecting ducts (IMCD) deposits is at the arrow. On biopsy (panel d) the large IMCD and BD deposit(arrow) has destroyed epithelial cells and protrudes (*); interstitial fibrosis surrounds the dilated tubule. Double arrow points to interstitial plaque. In a hyperparathyroid stone former (panels e & f) a small CaOx stone is attached over white plaque (insert box and blowup, panel e). Double arrowheads point to yellow IMCD lumen deposits. Single arrow points to white plaque. The arrowhead points to white plaque where an attached stone has been removed. Biopsy shows numerous plugged IMCD (panel f, arrows) with extensive interstitial fibrosis.
Table 2. Crystals in Plaque and Deposits.
| Location | Crystal | Found in | |
|---|---|---|---|
| Plaque | Interstitial | Apatite | ICSF SBR BR HPT ILEO |
| Deposits | In tubules | Apatite | HPT |
| A + CAOX | BP SBR RTA BR | ||
| A + CYS | CYS | ||
| A + UA* | ILEO | ||
| CAOX | HOX WITH ESRD |
Sodium and ammonium urates
A apatite
CAOX, calcium oxalate
ICSF, idiopathic CaOx stone formers
SBR, small bowel resection
BR, brushite stone formers
HPT, primary hyperparathyroid stone formers
ILEO, ileostomy stone formers
RTA, renal tubular acidosis
CYS, cystinuria
HOX, primary hyperoxaluria with end stage renal disease.
Whereas plaque is a proven anchoring nucleus for CaOx stone overgrowth [2] tubule deposits are not generally proven anchors for stones although in some cases stones have been found extending from a protruding deposit. In some diseases (Table 2) plaque and deposits coexist (Figures 1e & f). In this example of primary hyperparathyroidism, a small stone (inset box at arrow) is growing over plaque; multiple deposits are seen as well at arrowheads. Large deposits dilate BD (Figure 1 lower right panel) and destroy their epithelial cell linings; interstitial fibrosis is present. Overall, plaque and deposits are different forms of renal crystallization. The former is a benign initiating site for CaOx stones that occur in patients with no systemic diseases. The latter are destructive and create a crystal mediated form of tubulointerstitial papillary renal disease.
Quantification of Plaque and Deposits
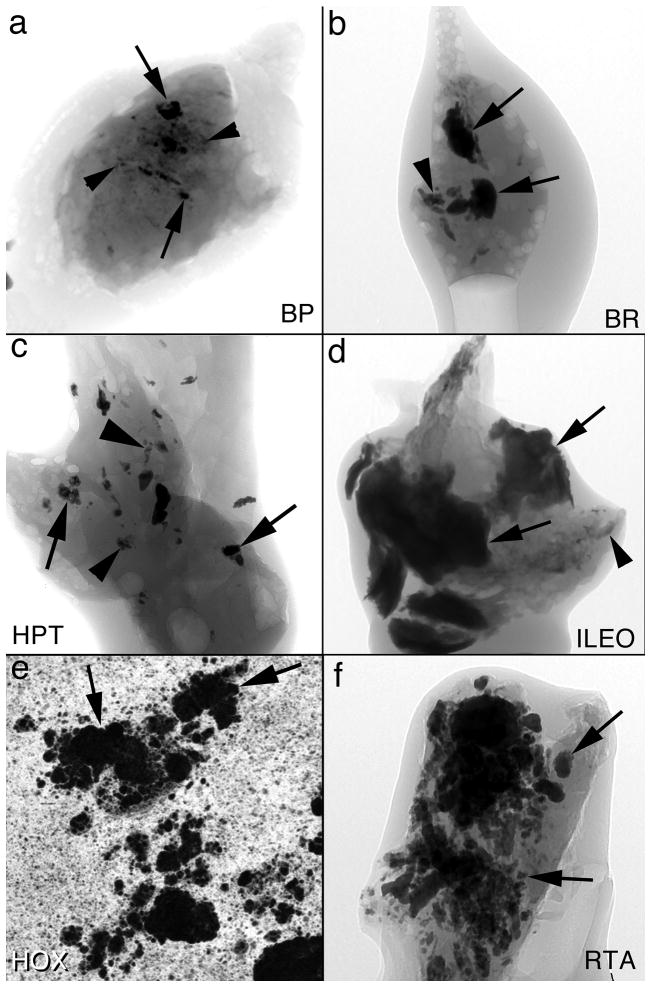
Plaque can be quantified by percent of papillary surfaces covered. To do this, plaque areas on intra-operative images (Figure 1a) are outlined and the fraction of surface measured using digital processing. Deposits can be quantified in terms of number per unit of papillary tissue volume, and in terms of their own average volume. To do this, papillary biopsies are visualized using high resolution CT scanning. In obesity bypass and brushite stone formers (Figures 2a & b) deposits occupy a minority of tissue volume; they are small in bypass patients but individually large in brushite patients. Deposits are more numerous and space filling in hyperparathyroidism and ileostomy patients (Figures 2c & d); the former form deposits of modest individual size, the latter very large individual deposits. In renal tubular acidosis and primary hyperoxaluria deposits are individually large and fill a large fraction of tissue volume (Figures 2e & f).
Figure 2.
Micro-CT analysis of deposits in six stone diseases. In all 6 panels, arrows point to intratubular deposits and arrowheads to interstitial plaque. From the micro-CT reconstructions we calculate density of deposits in cubic mm of tissue as well as the size of the deposit in mm. Panels A, B, C, D and F are micro-CT shadow images while panel E is a micro-CT cross sectional image. Abbreviations as in Table 1.
Relationship Between Plaque and Deposits
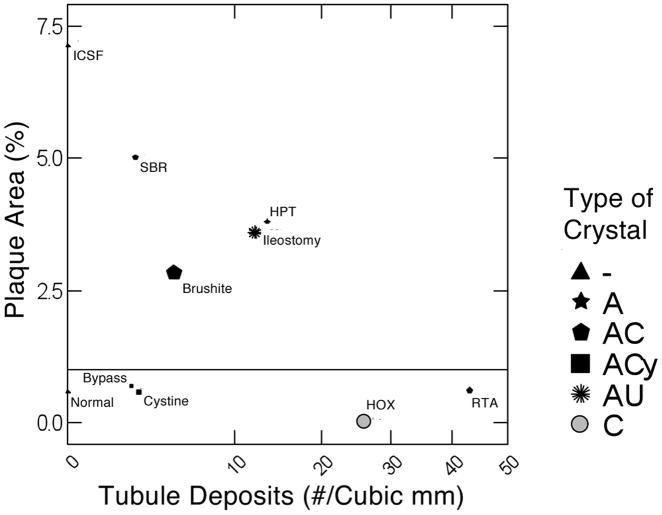
When plaque area is plotted against number of tubule deposits per unit of tissue volume (Figure 3) and the size of the symbol scaled to mean individual deposit size, the 9 diseases and normal people distribute themselves into discrete groups. Obesity bypass, cystinuria, primary hyperoxaluria and renal tubular acidosis patients form no more plaque than normal people and therefore cluster with normals below the horizontal line that denotes the upper fringe of normal plaque abundance. Normals, of course, have no deposits, so their symbol size denotes an individual deposit size of 0 for reference. ICSF have abundant plaque but, like normals, have no deposits, and therefore reside at the extreme upper left of the graph. Small bowel resection, ileostomy, brushite and hyperparathyroid stone formers are alike in having abundant plaque and deposits. If one for a moment lays normals and bypass and cystinuria aside, plaque abundance and tubule deposit density seem roughly inverse to each other, forming a crude but recognizable diagonal distribution from upper left to lower right of the graph. This is as if abundant deposits somehow do not well coexist with abundant plaque. Mean size of deposits and their number per cubic mm of tissue volume do not well correspond; for example renal tubular acidosis has the highest number per unit volume but the deposits are rather small, whereas brushite patients form among the largest individual deposits but fill only a modest fraction of tissue volume with them.
Figure 3.
Plaque surface coverage (y-axis) vs. tubule deposit density (x-axis) in 9 conditions. Disease abbreviations as in Table 1. Size of symbol gauges mean size of individual deposits. Deposit density and size are from micro-CT analysis. The horizontal line represents the upper boundary of plaque abundance in normal renal tissue. A, apatite; C, CaOx; Cy, cystine; AU, sodium and ammonium urate crystals; - denotes no deposits, shown as triangles for normal and ICSF.
Relationship Between Plaque Abundance and Renal Physiology
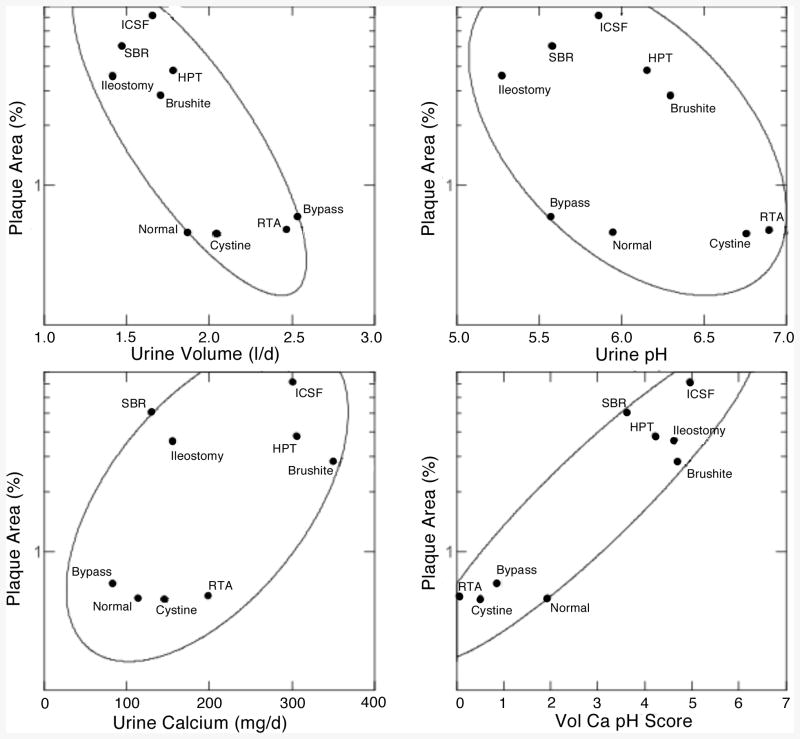
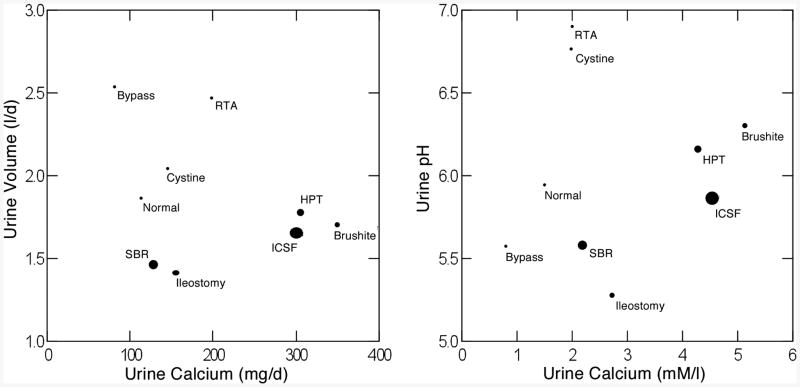
We have observed that among ICSF, bypass stone formers, and normals, plaque abundance was inverse to urine volume and pH and proportional to urine calcium excretion [3]. Among the 10 conditions we have such measurements for all but primary hyperoxaluria. When mean values for plaque abundance for normals and for individual diseases are plotted against corresponding mean values for urine volume (Figure 4 upper left panel) we recover the same inverse relationship as observed among the individual patients within our initial three conditions – normals, ICSF and bypass stone formers. Bypass, cystinuria, and renal tubular acidosis have high volumes and low plaque area; normals, however, have volumes overlapping with the 5 high plaque diseases at the upper left of the panel. Likewise, renal tubular acidosis patients and cystinuria patients have the highest urine pH and low plaque abundance (Figure 4, upper right panel, lower right of panel) but normals and bypass patients have urine pH values that overlap with the high plaque patients, and brushite patients, with abundant plaque have a urine pH considerably above that of normals. However, urine calcium excretion is quite low in bypass and normals, compared to most of the high plaque group (Figure 4, lower left panel). As we found among individual patients with ICSF or bypass stones, and among normals, one needs to concatenate all three variables to achieve an ideal separation. To do this we performed multivariate linear regression to create a volume, calcium pH score (Figure 4, lower right panel) that separates the high and low plaque groups completely. Altogether, one can find across the diseases and normals the same relationships between plaque and urine volume, pH and calcium excretion as is found among the individuals within a single disease category, meaning that this particular example of correspondence between renal physiology and renal crystallization is very robust and probably reflective of important pathogenetic mechanisms.
Figure 4.
Relationship between plaque surface area and urine chemistry measurements. Ellipses are 68% non-parametric containment estimates. Vol Ca pH score, multivariate combined effects of urine volume, pH and calcium excretion.
When abundance is presented as a function of two of the three variables at a time, their effects on plaque, and the differences between high and low plaque states becomes visually dramatic. The high plaque diseases (Figure 5, left panel, large dots) run along a low diagonal from lower left to mid-upper right when urine volume is plotted against urine calcium excretion and plaque area is symbolized by size of the dots. Above the 5 high plaque states, the 4 low plaque states float in the region of high volume and lower calcium. Between them is a large space denoting the very different physiologies. If we combine urine volume and calcium excretion into calcium molarity (Figure 5, right panel) the high plaque states all lie to the right, high molarity, compared to the low plaque states, and in the region of overlap, renal tubular acidosis and cystinuria – low plaque – vs. small bowel resection and ileostomy – high plaque states, they differ most markedly in urine pH. So the pH effect is most important when calcium molarities are close, least when they differ markedly.
Figure 5.
Relationship between urine volume and calcium excretion (left panel) and urine pH and urine calcium molarity (right panel) in 9 conditions. Size of symbol denoted plaque surface area. Note clustering of high (large symbols) plaque states to the lower right of both panels and marked separation from low plaque states.
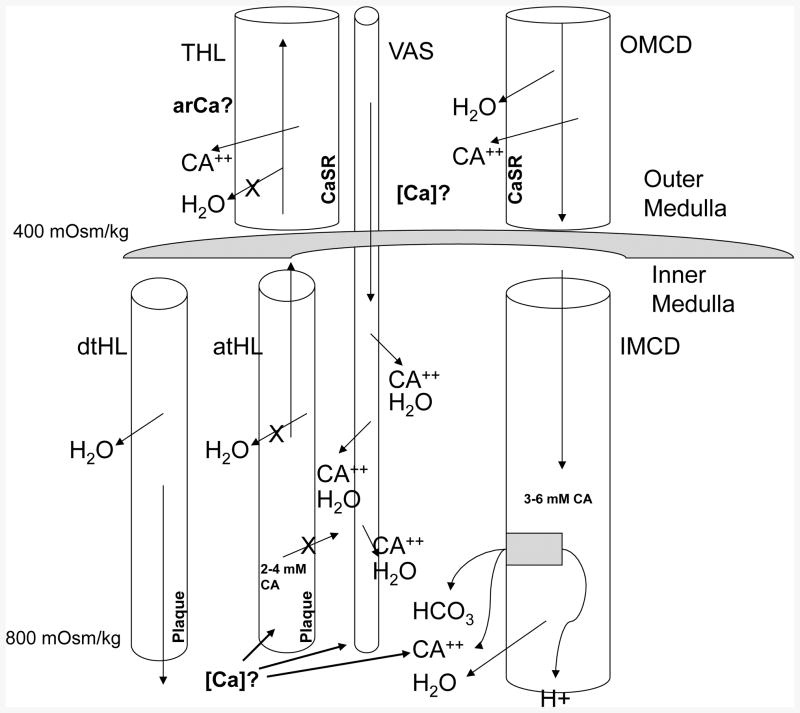
We do not know how urine calcium molarity and pH influence plaque so strongly, but have a viable theory (Figure 6) that can be tested and would be consistent with what we observe. The thick ascending limb of Henle's loop (THL) reabsorbs calcium without water into the outer medullary interstitium. Given that the vasa recta counter-current trap solutes there, calcium molarity must be above blood levels, although no one has as yet measured it. Blood descending the vas along the long loops of juxta-medullary nephrons must be enriched with calcium, so the outer surface of the thin loop basement membranes would be exposed to a calcium rich interstitium. This same loop has a fluid whose calcium molarity is higher – at least in rodents [8] – than blood because of water extraction in the descending thin segments so that the basement membrane is essentially sandwiched in between two calcium rich environments. High urine calcium reflects idiopathic hypercalciuria, a condition in which delivery of calcium out of the proximal tubule is increased above normal [9]; therefore delivery of calcium to THL is increased and THL could, in principle reabsorb the extra calcium raising outer medullary calcium molarity above normal, fostering plaque via wash down by vas recta. Higher delivery into the thin segment could also increase calcium molarity of loop fluid if water extraction were efficient. Outer medullary collecting ducts (OMCD) also reabsorb calcium and deliver it into outer medulla, but with water, so the effect is less important. Finally, collecting duct calcium reabsorption may be increased in hypercalciuria. When urine pH is lowered, protons are secreted into collecting duct fluid; bicarbonate must exit on the basolateral side, making the interstitium more alkaline. Since plaque is apatite, which forms best in alkaline media, a more acid urine could foster plaque. Critical tests of this hypothesis are clearly interstitial and loop calcium molarities in patients and normal subjects.
Figure 6.
Schematic diagram of outer and inner medulla showing elements of proposed vas washdown hypothesis – details presented in text. THL, thick ascending limb of Henle's loop; Ca, calcium ion; [Ca}, interstitial calcium concentration; CaSR calcium sensing receptor; OMCD, outer medullary collecting duct; dtHL and atHL, descending and ascending limbs of Henle's loop; Vas, vas recta; X, blockade of transepithelial movement; estimates of osmolality and tubule fluid concentrations are for illustrative purposes.
How will this model work in conditions other than idiopathic hypercalciuria? In brushite and primary hyperparathyroid stone formers, vas wash down will be driven by hypercalciuria (Table 3). In small bowel resection and ileostomy high water extraction in the thin loops is expected as is higher proton pumping into tubule fluid with raised inner medullary interstitial pH. In normals delivery to THL is not high, by definition, urine neither scanty nor acidic; the other low plaque conditions are like the normal in lacking the combination of high calcium delivery, low volume and pH.
Table 3. Mechanisms for Plaque.
|
Variation of Deposit Invasion Among the Diseases
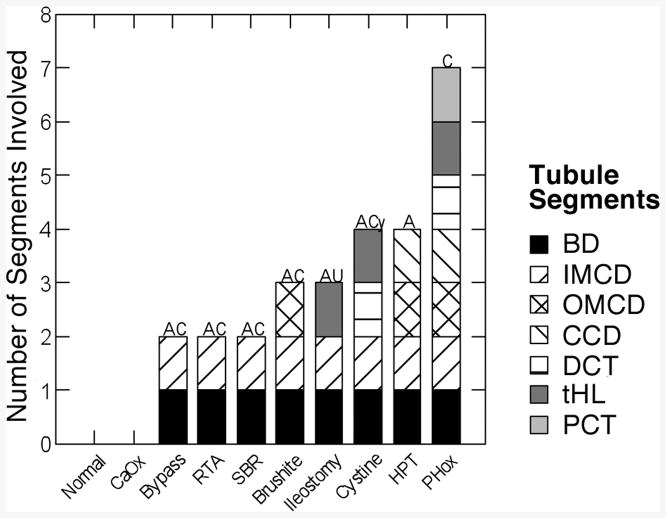
As mentioned, normals and ICSF have no deposits (Figure 7) and therefore no invasion of their tubule epithelial compartments. All other patients have deposits in their BD (Figure 7, solid black bars) and inner medullary collecting ducts (right diagonal bars). Brushite, primary hyperparathyroid and primary hyperoxaluria patients have deposits that extend further, into their outer medullary collecting ducts (Crosshatch bars). Ileostomy, cystine and primary hyperoxaluria patients have deposits in their thin limbs; this is not contiguous with collecting duct deposits in that thick ascending limbs are never involved. Primary hyperparathyroid patients are special in that contiguous involvement ranges from BD up to cortical collecting ducts (left diagonal bars). By contiguous we do not mean continuous deposits have been documented in particular nephrons but only that involved segments are linearly contiguous in nephrons. Primary hyperoxaluria is unique in that deposits involve all segments, but our cases are all end stage renal disease; we do not have samples from earlier stages of the disease. One is tempted to attempt an explanation for the variations of involvement but that would be mostly speculation at this time. However one might note that ileostomy is a condition of extreme and chronic restriction of urine volume [4], so thin limb fluid concentrations might be expected to be very far above normal, and cystinuria is a condition in which clinicians usually prescribe very high doses of alkali for solubilization of cystine over many years. This latter would raise thin limb fluid pH and might promote crystallizations. As mentioned, deposits contain apatite (Figure 7, ‘A’) often combined with traces of calcium oxalate (Figure 7, ‘C’), cystine (‘Cy’) or the sodium or ammonium salts of uric acid (‘AU’). Primary hyperoxaluria tissue in our end stage patients contains only calcium oxalate deposits.
Figure 7.
Segments containing deposits in 10 conditions. BD, Bellini ducts; IMCD, OMCD and CCD, inner and outer medullary and cortical collecting ducts; DCT, distal convoluted tubule; tHL, thin limbs of Henle's loop; PCT, proximal tubule.
Relationship of Deposits to Urine Composition
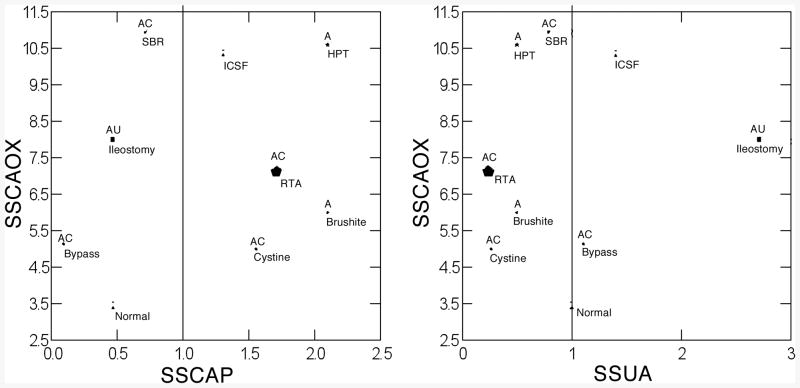
Being in BD and inner medullary collecting ducts for the most part, deposit crystals form in tubule fluid close to the composition of urine. So one expects a general correspondence between urine supersaturations (SS) and crystal composition. Normals, as noted before, form no deposits, and produce urine with low CaOx SS and no CaP SS, on average (Figure 8 left panel); likewise they have no uric acid SS (Figure 8, right panel). So their lack of deposits is compatible with their generally low or absent SS values. Bypass, renal tubular acidosis, and primary hyperoxaluria stone formers have substantial CaOx SS and form deposits containing that crystal (Figure 8, left panel, ‘C’ above symbol); SS values are not available for our cases but are assumed present given the disease and studies by others. Brushite patients also have substantial CaOx SS and in one deposit from one patient we have observed traces of CaOx, so although the symbol is not marked as such – our published work does not describe this deposit – we have evidence of the crystal in at least tiny amounts. Brushite, cystine, primary hyperparathyroid and renal tubular acidosis stone formers have very substantial CaP SS and form deposits containing apatite (Figure 8 left panel, ‘A’ above symbol). So one can find a wide range of concordances supporting the notion that high SS promotes deposits perhaps in a rather direct manner: free solution nucleation.
Figure 8.
Relationship between urine CaOx and CaP (left panel) and CaOx and uric acid SS (right panel) in 9 conditions. Size of symbol reflects density of deposits in number per cubic mm of tissue assessed by micro-CT analysis. Vertical lines denote SS values of 1, the point of solubility. Type of crystal deposit is above each symbol: A, apatite; C, CaOx; Cy, cystine; U, sodium and ammonium urate; -, no deposits occur. Details presented in text.
But small bowel resection, ileostomy and bypass patients deny the general truth of the hypothesis; in all three, urine is acidic, and CaP SS not present, yet apatite is abundant, in fact the main component of BD deposits (Figure 8 left panel). As well, ileostomy patients form deposits that contain the sodium and ammonium salts of uric acid, which form at pH values of 6 or more, but no detectable traces of uric acid itself, that crystallizes at the pH values – 5 – 5.5 of the urine these patients produce. So in these three types of patient's deposits and urine SS are discordant, and some additional mechanisms must be assumed to operate.
Additional mechanisms must be assumed for ICSF, hyperparathyroid and ileostomy stone formers in a different sense. All three have significant CaOx SS, quite high in the first two instances, and yet their deposits contain no CaOx at all. Likewise ICSF have considerable urine CaP SS and no deposits. ICSF and to a greater extent, ileostomy and bypass patients have significant urine uric acid SS but no deposits contain uric acid. So divergences are easy to demonstrate in a negative as well as a positive direction, leading one to ask what mechanisms might be in play beside the obvious one of mere over saturation and crystallization that is simply not sufficient.
A Catalog of Discordances and Proposed mechanisms (Table 4)
Table 4. Mechanisms of Deposit Formation.
| Mechanism | Disease | Consequence |
|---|---|---|
| Oxalate causes micro-crystal cell injury | SBR BP | Apatite forms |
| Uric acid injures cells and plugs BD | ILEO | Apatite and urates form |
| Apatite injures cells and plugs BD | RTA HPT BR | CaOx anchors and grows |
| Cystine plugs BD + alkali loading | Cystinuria | Apatite forms |
| Oxalate deluge injures cells | HOX+ESRD | Massive CaOx plugging |
| Lack of CaP and minimal UA SS | ICSF N | No cell injury, no crystal plugging |
The finding of apatite in deposits of small bowel resection, ileostomy and bypass patients who lack any evidence of urine CaP SS, the presence of sodium and ammonium urates in deposits of ileostomy patients who form a frankly acid urine, and the absence of uric acid in tubule of these same patients lead one to only one plausible hypothesis: tubule fluid pH must exceed that of bulk urine pH in at least some BD and inner medullary collecting ducts whereas in the main tubule fluid pH and bulk urine pH must match. In other words one is forced to presume that localized renal tubular acidification defects are rife and lead to alkaline crystal deposits.
The other discordances seem less compelling if perhaps instructive. High CaOx SS in primary hyperparathyroidism and ileostomy with no CaOx in tubule deposits may represent simply sampling issues in that our published reports to date include only a handful of cases for each. But ICSF are a different matter; we have reported on many and none ever have deposits. We suspect that an initial cell injury must precede CaOx attachment so that deposits of this crystal can form, and ICSF have no candidate initiating injury. Apatite and or urates could cause an initial injury in the other two diseases, but ICSF have very low CaP SS. This is also the probable reason they never form apatite deposits. The same for uric acid in ICSF and bypass: SS values for uric acid are too slight to produce crystals that can plug tubules and be retained.
If we place this entire ensemble of diseases in an historical context, our results support the notion of Finlayson [10] that CaOx is formed too slowly and in too small an amount to plug a renal tubule without assuming some anchoring site. So, except for the onslaught of oxalate in primary hyperoxaluria this material is not present in tubules except as an accompaniment to apatite. Apatite can form in abundance because large amounts of phosphate and calcium are excreted so given a high enough tubule fluid pH plugging occurs. If pH is low, one must assume some initial local form of ‘renal tubular acidosis’ involving individual BD or collecting ducts, and speculate that an initial CaOx or uric acid crystal injury caused it. Whether true or not, this is testable if one could measure tubule fluid pH in individual BD of humans, and such an experiment, especially in ileostomy patients, seems especially worthwhile.
Acknowledgments
Grant Support: PO1 DK56788
Reference List
- 1.Evan AP, Lingeman JE, Coe FL, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evan AP, Coe FL, Lingeman JE, et al. Anat Rec. Vol. 290. Hoboken: 2007. Mechanism of formation of human calcium oxalate renal stones on Randall's plaque; pp. 1315–1323. [DOI] [PubMed] [Google Scholar]
- 3.Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall's plaque. Kidney Int. 2003;64:2150–2154. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 4.Evan AP, Lingeman JE, Coe FL, et al. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int. 2009;76:1081–1088. doi: 10.1038/ki.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evan AP, Coe FL, Lingeman JE, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 6.Evan AE, Lingeman JE, Coe FL, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int. 2008;74:223–229. doi: 10.1038/ki.2008.161. [DOI] [PubMed] [Google Scholar]
- 7.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 8.Asplin JR, Mandel NS, Coe FL. Evidence of calcium phosphate supersaturation in the loop of Henle. Am J Physiol. 1996;270:F604–F613. doi: 10.1152/ajprenal.1996.270.4.F604. [DOI] [PubMed] [Google Scholar]
- 9.Worcester EM, Coe FL, Evan AP, et al. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol. 2008;295:F1286–F1294. doi: 10.1152/ajprenal.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlayson B, Reid F. The expectation of free and fixed particles in urinary stone disease. Invest Urol. 1978;15:442. [PubMed] [Google Scholar]