Abstract
Increased tone of orexigens mediating reward occurs upon repeated consumption of sweet foods. Interestingly, some of these reward orexigens, such as opioids, diminish activity of neurons synthesizing oxytocin, a nonapeptide that promotes satiety and feeding termination. It is not known, however, whether consumption-related activity of the central oxytocin system is modified under chronic sugar feeding reward itself. Therefore, we examined how chronic consumption of a rewarding high-sucrose (HS) vs. bland cornstarch (CS) diet affected the activity of oxytocin cells in the hypothalamus at the time of meal termination. Schedule-fed (2 hrs/day) rats received either a HS or CS powdered diet for 20 days. On the 21st day, they were given the same or the opposite diet, and food was removed after the main consummatory activity was completed. Animals were perfused 60 minutes after feeding termination and brains were immunostained for oxytocin and the marker of neuronal activity, c-Fos. The percentage of c-Fos-positive oxytocin cells in the hypothalamic paraventricular nucleus was significantly lower in rats chronically exposed to the HS than to the CS diet, regardless of which diet they received on the final day. A similar pattern was observed in the supraoptic nucleus. We conclude that the chronic rather than acute sucrose intake reduces activity of the anorexigenic oxytocin system. These findings indicate that chronic consumption of sugar blunts activity of pathways that mediate satiety. We speculate that a reduction in central satiety signaling precipitated by regular intake of foods high in sugar may lead to generalized overeating.
Keywords: Sucrose, Starch, Obesity, Palatability, Reward, Satiation, Oxytocin
1. Introduction
Palatability serves as one of the most potent reinforcers of consummatory behavior. Consequently, intake of palatable tastants often exceeds levels necessary to satisfy energy needs of the organism and addition of such ingestants to a daily diet (e.g., in a cafeteria diet paradigm) predisposes to generalized overconsumption. Foods and solutions rich in sugar belong to the most readily consumed ingestants. It has been proposed that intake of high-sugar diets is driven by the reward system. Regular and frequent exposure to sugar-enriched tastants appears to be particularly effective in propelling activity of the reward circuitry and it is reflected by a changed expression of multiple genes and/or release of neuroactive agents. For example, it has been shown that deltaFosB, a transcription factor implicated in the development of substance abuse, also mediates enhanced appetite for sucrose and food-reinforced instrumental behavior [25, 40]. Dopamine overflow in the nucleus accumbens is induced by both actual consumption of sugar as well as oral stimulation with sucrose [16]. Finally, mRNA levels of genes coding for opioid peptides and receptors are altered in animals chronically exposed to sugar [33, 38], whereas injections of opioid receptor ligands have a particularly potent effect on consumption of sweet diets [30, 42].
It should be noted that consumption of high-sucrose foods does not stem solely from the increased motivation to seek and eat such ingestants. Sugar overconsumption seems also possible due to an elevated food load “threshold” to induce satiation. Importantly, some reward mediators, e.g., opioid receptor agonists, have been shown to reduce activity of neurons involved in inhibition of consummatory behavior, whereas antagonists produce an opposite effect [4, 28]. One population of affected neurons synthesizes a nonapeptide oxytocin strongly linked with feeding termination mechanisms. Intracerebroventricular administration of oxytocin and oxytocin receptor agonists decreases food intake [2, 26]. Peripheral oxytocin injections have also been shown to inhibit consumption [2, 3], while administration of oxytocin receptor antagonists prevents this inhibition [2, 3, 9, 27]. Recently gathered evidence allows us to hypothesize that the satiety-related responsiveness of the oxytocin system may be particularly vulnerable to the consumption of sugar and the associated activity of the reward circuitry. Injections of butorphanol tartrate, an opioid receptor agonist, given at a dose known to cause hyperphagia, decreases sucrose intake-driven activity of oxytocin neurons [31]. Oxytocin knock-out mice consume more sucrose than wild type mice do [23]. Olszewski et al.[30] demonstrated that oxytocin gene expression is increased in the hypothalamus following intake of palatable sucrose pellets. Furthermore, subcutaneous oxytocin administration attenuates the hyperphagic response to acute morphine injections over a 24-hour period [14].
The goal of the current set of experiments was to elucidate whether consumption-related activity of the central oxytocin system is modified under chronic feeding reward precipitated by sucrose intake. Therefore, we examined how chronic and acute consumption of a rewarding high-sucrose vs. bland cornstarch diet affected the activity of oxytocin cells in the paraventricular and supraoptic hypothalamic nuclei at the time of meal termination. Schedule-fed rats received either a sucrose- or cornstarch-rich powdered diet for 20 days. On the 21st day, they were given the same or the opposite diet. Brains were immunostained for oxytocin and the marker of neuronal activity, c-Fos. In addition, several feeding-related sites were analyzed for the presence of Fos-positive nuclear profiles in biochemically undefined neurons following the aforementioned treatments. It should be noted that under a scheduled meal regimen, ingestive behavior is extremely robust and, as all caloric needs must be met within a short timeframe, it often reaches a “ceiling effect” regardless of a rewarding value provided by a given diet. Hence, accessibility of food and its texture (affecting, e.g., ease of mastication) play a significant role. Since we intended to study neuronal activity at the end of a sucrose vs. cornstarch meal after chronic diet exposure, we maximized food intake (thus, made it equal) in animals receiving either type of food, by providing tastants in the form of a very fine powder. It allowed us to avoid introducing pair-feeding in sucrose-fed rats that would otherwise be necessary to achieve similar energy consumption and body weight.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (n=28; Charles River Laboratories, Wilmington, MA) weighing 280–300 g were individually housed in conventional hanging cages with a 12:12 LD photoperiod (lights on at 0700) in a temperature-controlled room (21–22 °C). They had unlimited access to tap water throughout the entire experiment, whereas standard chow (Teklad Global Diet 2018) was available ad libitum as basic food until the beginning of diet manipulations. The study was approved by the University of Minnesota Institutional Animal Care and Use Committee.
2.2. Feeding Regimen
The rats were schedule-fed. To allow the animals to adjust to the scheduled feeding regimen, during the first three days, they were allowed a 4 h/day access to standard chow; then the food availability period was shortened to 2 h/day for 7 days. Daily feeding periods occurred in the first half of the light cycle. On the subsequent four days of the feeding regimen, standard chow was offered in a powdered form. After the adaptation to the feeding schedule, half the rats were placed on a high-sucrose powder diet and the other half were placed on an isocaloric cornstarch powder diet for the next 20 days. Details of the composition of these diets are provided in Table 1. On Day 21, half the rats in the sucrose-fed group and half the rats in the cornstarch-fed group were given the opposite diet. Diets were presented for only 45 minutes on this day as the main consummatory activity was completed within this timeframe.
Table 1.
Composition and Caloric Density of Sucrose and Cornstarch Diets.
| Sucrose Diet (g) (3.89 kcal/g) |
Cornstarch Diet (g) (3.89 kcal/g) |
|
|---|---|---|
| Casein | 20.0 | 20.0 |
| DL-methionine | 0.3 | 0.3 |
| Corn Oil | 5.0 | 5.0 |
| Corn Starch | 15.0 | 65.0 |
| Sucrose | 50.0 | 0.0 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1.0 |
| Choline Chloride | 0.2 | 0.2 |
| Cellulose | 5.0 | 5.0 |
| 100.0 g | 100.0 g |
2.3. Perfusion protocol
On Day 21, 60 minutes after food was removed from cages, the rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused via the aorta with 50 ml of ice-cold saline followed by 500 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed and postfixed overnight in the same fixative at 4°C.
2.4. Immunohistochemical Staining
Coronal brain sections (50-µm thick) were cut on a vibratome and processed as free-floating tissue for single (c-Fos) or double (c-Fos and oxytocin) immunostaining. Every fourth brain section encompassing the PVN, SON, nucleus of the solitary tract (NTS) or central nucleus of the amygdala (AMYG) was used in the single c-Fos staining, and every fourth one containing the PVN and SON was stained for c-Fos and oxytocin. Boundaries of the anatomical regions of interest have been defined according to the standard rat brain atlas by Paxinos and Watson [32]. There were equal distances between sections used in each of the IHC staining protocols.
Sections were pretreated for 10 minutes in 3% H2O2 and 10% methanol (diluted in TBS; pH 7.4) and incubated for 48 h at 4°C in the primary goat anti-Fos antibody (1:1000; Santa Cruz, Burlingame, CA). Subsequently, tissue was incubated for 1 h in the rabbit anti-goat antibody (1:400; Vector Laboratories, Burlingame, CA) at room temperature. After a 1-h incubation in the avidin-biotin complex (1:800; Vector Laboratories, Burlingame, CA), peroxidase was visualized with 0.05% diaminobenzidine (DAB; Sigma Diagnostics, St. Louis, MO), 0.01% H2O2, and 0.3% nickel sulfate. All incubations in antibodies were performed in the mixture of 0.5% Triton-X (Sigma Diagnostics, St. Louis, MO) and 0.25% gelatin (Sigma Diagnostics, St. Louis, MO) in TBS. Intermediate rinsing steps were done in TBS.
Following the completion of c-Fos staining, PVN and SON sections were further processed for visualization of oxytocin. The procedure was similar to that used to stain for c-Fos. However, rabbit anti-oxytocin was used as the primary antibody (1:15000; Millipore, Temecula, CA), and nickel sulfate was omitted from the DAB solution so as to obtain a brown stain.
Sections were mounted on gelatin-coated slides, dried, dehydrated in ascending concentrations of alcohol, soaked in xylene and embedded in DPX (Fluka, Germany).
2.5. Staining Analysis
In the single staining analysis, the number of Fos-IR nuclei in the regions of interest was counted bilaterally in at least 5 sections per region per animal and the density of Fos-positive nuclear profiles (per mm2 of each neuroanatomical area) was calculated. Images provided by the camera attached to the Nikon microscope were analyzed using the NIH 1.51 Image software (NIH, Bethesda, MD). Densities of Fos-positive nuclei per mm2 were averaged per animal and then per experimental group.
In the double staining analysis, the following estimates were assessed per section and then per region: the total number of oxytocin neurons and the number of oxytocin neurons positive for c-Fos. Cells were counted bilaterally, and the percentage of neurons containing c-Fos-positive nuclei was tabulated.
2.6. Statistical Analysis
Results are presented as means ± SEM. Body weight data were analyzed using independent samples t-tests and feeding data were analyzed using t-tests and two-way ANOVAs. Immunohistochemical data were analyzed using two-way ANOVAs. Values were considered significantly different when p<0.05. All analyses were conducted with SPSS software.
3. Results
3.1. Body Weight and Food Intake
A repeated measures ANOVA was run (with time as the repeated measure) for body weight. There was a significant effect of day, but the interaction of diet and day was not significant. Subsequent t-tests did not find any significant differences in bodyweight as a function of diet during the course of the experiment. (t-test; Figure 1).
Figure 1.
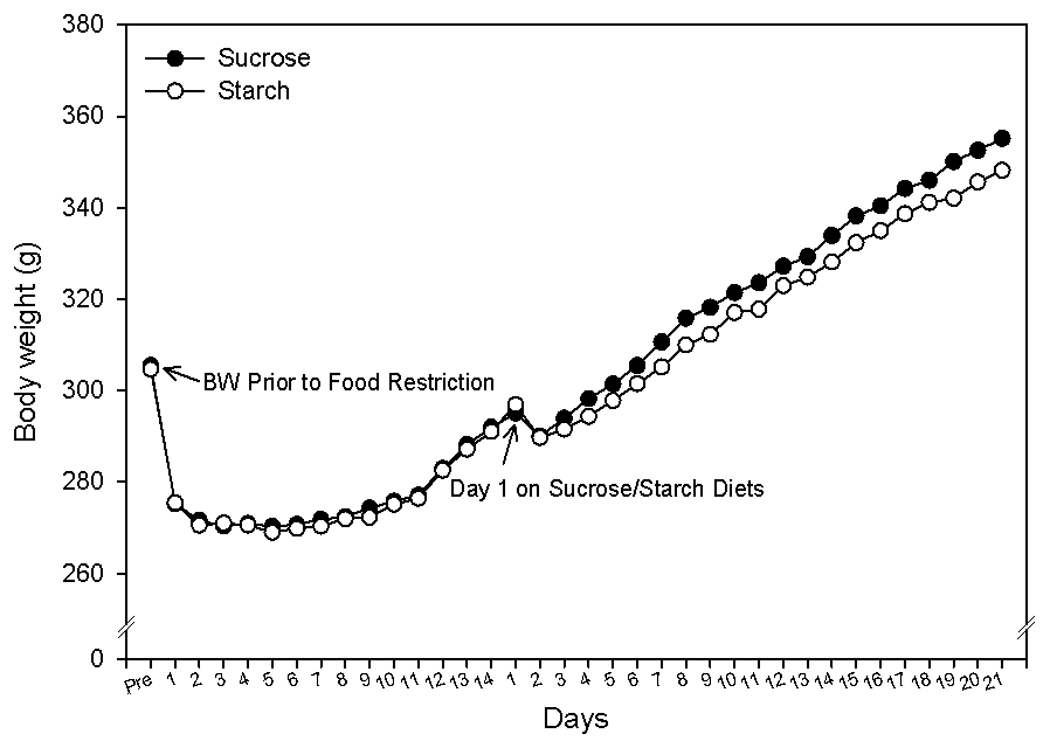
Body weights of rats fed sucrose or cornstarch diets. All data points represent means ± SEM with n = 14 per group.
A repeated measures ANOVA was run (with time as the repeated measure) for food intake. Food intake was measured every other day, and the repeated measures ANOVA was run on data collected on the odd days with the exception of Day 19, where we had incomplete data; we instead substituted data from Day 20 for Day 19. There was a significant interaction between day and diet, however subsequent t-tests failed to show any significant differences in sucrose versus starch intake from Days 1–20 (Figure 2). A two-way ANOVA comparing intake on the final day indicated an effect of both the chronic diet [F(1,24)= 6.95, p=0.014] and an effect of the acute diet [F(1,24)= 10.22, p=0.004], and an interaction that approached significance (p=0.053) (Figure 3).
Figure 2.
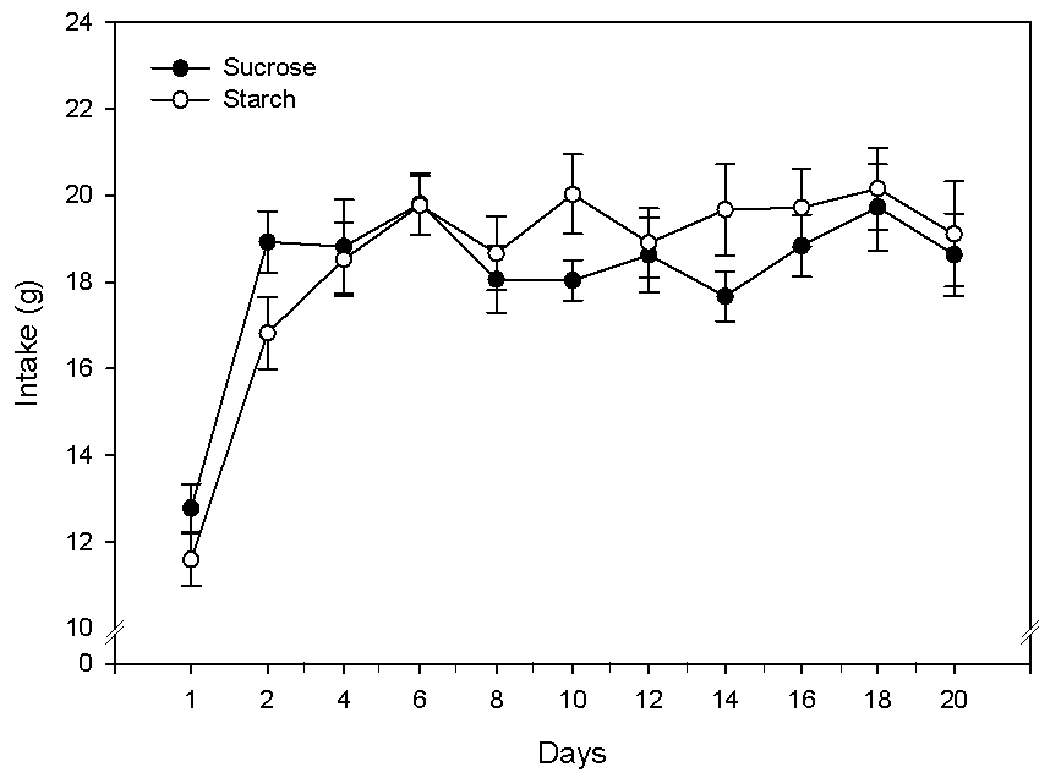
Food intake of rats fed cornstarch or sucrose diets. All data points represent means ± SEM with n = 14/group.
Figure 3.

Food intake on the last (21st) day of scheduled feeding. Rats were fed either a sucrose or a cornstarch diet for 20 days (chronic condition) (n=14/group); half of these rats then received the opposite diet on the last (21st) day (acute condition). All data points represent means ± SEM. (* indicates a significant difference in food intake on Day 21 for the chronic sucrose-acute cornstarch group, p< 0.01).
When the intakes on the final day were compared using independent group t-tests, the sucrose-starch group (which received sucrose from Day 1–20, and received starch acutely on Day 21) had a significantly lower intake on the final day as compared to the sucrose-sucrose group [t(12)=5.134, p=0.01], the starch-starch group [t(12)=3.175, p=0.08] and the starch-sucrose group [t(12)=4.404, p=0.001] (Figure 3).
3.2. Immunohistochemistry
A two-way ANOVA revealed a significant effect of the chronic diet in the PVN [F(1,24)=9.76, p =0.005] and the SON [F(1,24)=6.92, p=0.015]: the rats maintained on a chronic starch diet had a significantly greater percentage of oxytocin cells that were c-Fos-positive as compared with the rats maintained on a chronic sucrose diet. No effect of the acute diet was observed in either the PVN or the SON (See Figure 4).
Figure 4.
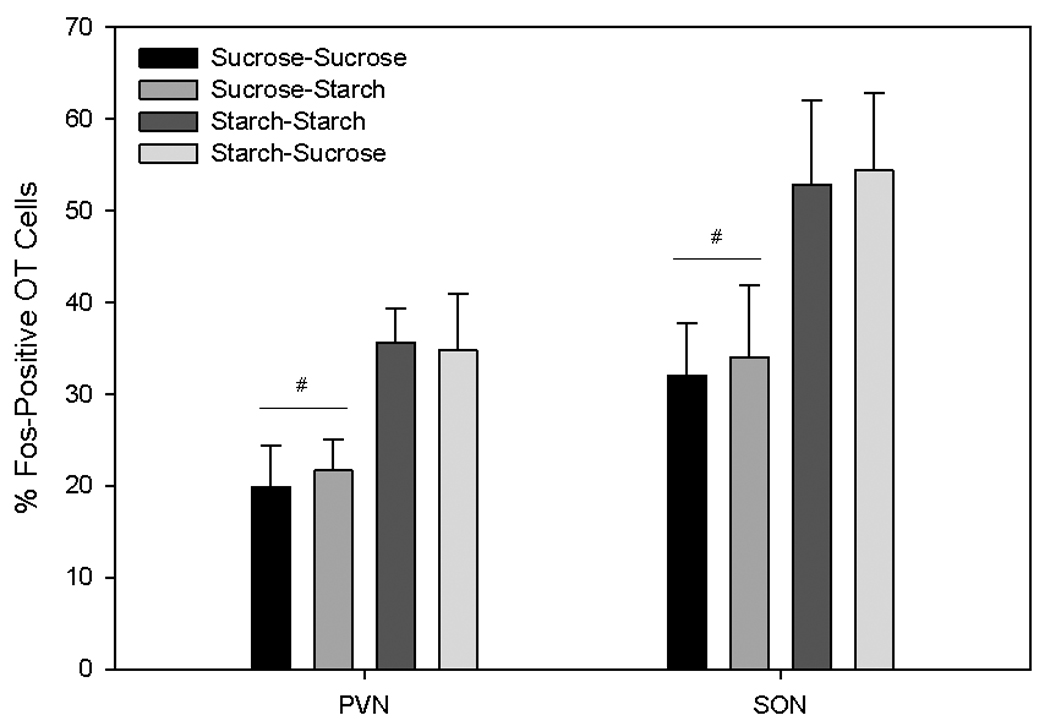
The percentage of Fos-positive oxytocin cells in the paraventricular (PVN) and supraoptic (SON) hypothalamic nuclei. # indicates a significant effect of chronic diet (Days 1 – 20 on sucrose vs. cornstarch), p< 0.05).
A two-way ANOVA indicated a significant effect of receiving the chronic versus acute diet, such that rats receiving an acute diet on the final day had significantly increased density of c-Fos positive nuclei in the AMYG [F(1,23)=6.189, p=0.021] and NTS [F(1,23)=7.551, p=0.011]. A distinct trend towards an increased density of c-Fos-positive nuclei was detected in the PVN and SON in the rats that had received the acute diet on the final day (PVN, p = 0.062; SON, p=0.090). This effect was seen regardless of whether the acute diet presented was sucrose or starch (See Figure 6).
Figure 6.

Density of Fos-positive nuclei in the paraventricular (PVN) and supraoptic (SON) hypothalamic nuclei, central nucleus of the amygdala (AMYG) and nucleus of the solitary tract (NTS). # indicates a significant difference in AMYG and NTS, p< 0.05).
4. Discussion
Most mammals, including humans, favor foods and liquids rich in sugar [20, 34, 35]. Preference for this macronutrient stems from both orosensory and post-ingestive properties [12, 24] and it is regulated at the brain level [13, 15, 41]. Several central mechanisms underlie the drive to consume sucrose. For example, multiple studies assessing operant behavior have shown increased motivation to obtain sweet foods. Furthermore, sucrose intake – especially that of a chronic nature - activates components of the central reward circuitry by, for example, modifying expression levels of genes encoding opioid peptides and their receptors as well as by affecting the release of neuropeptides and neurotransmitters such as dopamine and opioids [30]. One mechanism, however, that has not been extensively studied thus far is the relationship between sugar consumption and activity of neural systems that mediate satiety. The present set of experiments provides evidence that chronic sucrose intake downregulates activity of the anorexigenic oxytocin system at meal termination. In our study, rats chronically exposed only to sucrose (including the final experimental day) had fewer Fos-positive oxytocin neurons than animals exposed exclusively to cornstarch, following the consumption of the same amount of food. Therefore, regular intake of a palatable sugar diet decreases feeding-related activation of the anorexigenic oxytocin system compared to the effect evoked by the bland food. This lower activity of the oxytocin neuronal population obviously does not have to translate immediately to an increase in feeding as other environmental factors and neuroendocrine processes affect the animal’s transient feeding status. It may contribute, however, to somewhat “incomplete” or “impaired” satiation, which predisposes to undertaking consummatory behavior sooner despite the sufficient energy stores. This is a particularly exciting concept taking into account numerous reports showing elevated caloric intake in “cafeteria” diet rodent models that employed high-sugar foods (for review, see [29]). In many of these paradigms, increased energy consumption was not directed just toward high-sugar foods, but it was more generalized and included also tastants regarded as less attractive than sweet ingestants. This is in line with the current data that rats chronically eating sucrose displayed a lower number of Fos-positive oxytocin neurons also when they were given a cornstarch meal. This particular aspects of our of results needs to be corroborated by future studies as the sucrose-cornstarch group was the only one that ate less food on the final day. The level of consummatory activity was lower due to a decreased rewarding value of a cornstarch meal in comparison to the customary sucrose diet. Nonetheless, this outcome provides evidence that the palatability of the sucrose diet was greater than that of the cornstarch one.
It is interesting that a single sucrose exposure in rats that had previously been fed cornstarch did not result in a diminished activity of oxytocin cells at meal termination; then the percentage of Fos-positive oxytocin neurons was the same as in rats offered the sucrose-free diet throughout the entire experiment. Our finding appears to be of importance as not only does it show the link between sucrose intake and oxytocin neuronal activity, but it also emphasizes the influence of long-term repeated ingestion of palatable sucrose as the factor modifying activation of cells involved in satiety responsiveness.
An important question due to the apparent food intake ceiling effect observed herein (equal amounts of palatable and non-palatable powder diets were consumed) emerges as to whether the relationship between sucrose ingestion and activation of the oxytocin system parallels different levels of sugar consumption. Our previous study utilizing scheduled intake of sweet versus standard pellets (instead of powder) produced significant differences in consumption of each diet according to their rewarding values [30]. We then also measured relative expression of the oxytocin gene in the hypothalamus and found that it reached higher levels, but only upon excessive intake of the sugar chow. Altogether, it suggests that oxytocin neurons do respond to sucrose ingestion, however, their response is diminished compared to that observed when a bland diet is offered. Perhaps the increased activity of the oxytocin system evoked by extreme sucrose overeating reflects more the homeostatic role of oxytocin (thus, preventing extreme stomach distension or osmotic load) than its direct involvement in satiation. This hypothesis is in line with the main consumption-related roles of two distinct subpopulations of oxytocin neurons: parvo- and magnocellular. Magnocellular cells located in the PVN and SON project to the pituitary and supply oxytocin to the general circulation. Parvocellular neurons are located only in the PVN and approximately 10% of them project to the brainstem sites crucial in the exchange of the brain-periphery feeding-related information, including GI tract motility and chemical plasma profile [11, 17, 21, 39]. While the parvocellular neurons are thought to affect satiety-related responses, the magnocellular cells in conjunction with the parvocellular ones are thought to participate in the development and maintenance of food avoidance due to aversion or osmolality [29, 31].
One should note that experiments involving oxytocin knock-out mice support our notion of a strong link between sucrose ingestion and the oxytocin system (e.g. [1, 8, 36]). Mice lacking the oxytocin gene exhibit increased preference toward and higher intake of solutions rich in sugar [1]. Exogenous oxytocin delivered via injections eliminates this phenotypic manifestation of oxytocin gene deletion. On the other hand, injection studies on effects of exogenous oxytocin on intake of sweet carbohydrates are very limited. For example, Lokrantz et al. administered oxytocin centrally and measured intraoral intake of glucose in deprived and non-deprived rats. They found that oxytocin administration reduced intake in deprived rats, but did not have a comparable effect on non-deprived animals. Pretreatment with an oxytocin receptor antagonist prevented the suppression of the intake previously seen in the deprived rats [18].
An interesting issue arises as to what other neural factors that mediate sugar reward diminish the activity of the oxytocin system either directly (via synaptic connectivity) or indirectly (as part of a larger network). Opioids are likely candidates for this interaction. Opioid receptor agonists are particularly effective at inducing intake of palatable foods, including those high in sugar; conversely, antagonists block reward-driven consumption. Regular exposure to high sucrose foods evokes changes in expression of genes coding opioid peptides and receptors. Interestingly, opioids do not seem just to stimulate feeding, but they have been suggested to maintain consummatory behavior by inhibiting activity of neural systems associated with termination of feeding [4, 28]. Preliminary studies have suggested that activation of oxytocin neurons at the end of a meal may be particularly vulnerable to modification by opioids. Administration of a wide-spectrum opioid receptor agonist, butorphanol tartrate, inhibits activity of PVN oxytocin cells upon feeding termination; this effect is particularly pronounced when a high-sugar diet is given [29]. A connection between opioids and oxytocin in other aspects of ingestive behavior has also been shown. For example, opiates reduce taste aversion and accompanying oxytocin neuronal activation [31], whereas antagonism of opioid receptors potentiates taste aversions [22] and oxytocin cellular activity induced by other agents and, by itself, can lead to aversion as well. Finally, other functional and neuroanatomical data point to the opioid-oxytocin system relationship. To name a few: opioid receptors are expressed by oxytocin neurons in the PVN and SON [37], opioids and oxytocin are co-expressed and – under some circumstances – co-released from the same terminals [7], activity of oxytocin cells has been modified by a treatment with several opioid ligands [19], and opioid addiction withdrawal also affects activation of oxytocin neurons [10].
Aside from studying the effect of sucrose intake on oxytocin neuronal activation, we also assessed c-Fos IR in four areas known to mediate feeding behavior and contain the components of both reward- and energy-related feeding circuitry. It is striking that changes in the density of Fos-positive nuclear profiles in two of these areas, the AMYG and NTS, seemed to be associated with the novelty of a presented diet (and likely with learning mechanism-related categorization of each of these diets in terms of their nutritional and homeostatic value [5]) rather than with composition, chronic exposure effects, palatability and/or satiety. Authors investigating a specific subtype of learned consummatory responses, namely, a conditioned taste aversion, have already suggested the involvement of the AMYG and NTS in this process [6]. One should note that in the NTS and AMYG, we investigated only plain c-Fos immunoreactivity. It is therefore possible that if phenotypes of specific subpopulations of neurons within these sites had been defined, changes in activity of certain groups of cells could have been detected. In addition, future studies should assess whether the neuronal activity pattern differs under similar conditions in the meal anticipatory phase, i.e. at the time of expected food presentation without the actual access to food on the perfusion day.
In summary, the present study shows that long-term consumption of foods high in sugar downregulates activity of the anorexigenic oxytocin system associated with meal termination; it supports the initial findings obtained in KO rodent models suggesting a strong link between the oxytocin tone and sugar intake. It can be speculated that under less restrictive food availability schedules than the one tested herein, this decrease in anorexigenic signaling precipitated by regular intake of foods rich in sucrose may serve as one of the mechanisms promoting more generalized overconsumption, ultimately propelling excessive weight gain.
Figure 5.

Photomicrographs showing immunohistochemistry for c-Fos and oxytocin. A: Details of double staining shown at a high magnification. Distribution of c-Fos-positive nuclear profiles and oxytocin cells visualized in coronal sections of the PVN (B) or SON (C). Rats were given either sucrose (B and C top) or cornstarch (B and C bottom) diets for 21 days preceding the perfusion. Open arrows, oxytocin neurons devoid of c-Fos; thin arrows, c-Fos-IR oxytocin neurons; otr, optic tract.
Acknowledgements
Supported by National Institute on Drug Abuse Grants R01DA021280, National Institute of Diabetes and Digestive and Kidney Diseases P30DK50456, National Institute of Dental and Craniofacial Research Grant T32DE007288, Swedish Research Council (VR), Swedish Brain Research Foundation and the Novo Nordisk Foundation. We would like to thank Ross A. Avant for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R1806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- 2.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- 3.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48:825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 4.Beckman TR, Shi Q, Levine AS, Billington CJ. Amygdalar opioids modulate hypothalamic melanocortin-induced anorexia. Physiol Behav. 2009;96:568–573. doi: 10.1016/j.physbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein IL, Koh MT. Molecular signaling during taste aversion learning. Chem Senses. 2007;32:99–103. doi: 10.1093/chemse/bjj032. [DOI] [PubMed] [Google Scholar]
- 7.Bicknell RJ. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J Endocrinol. 1985;107:437–446. doi: 10.1677/joe.0.1070437. [DOI] [PubMed] [Google Scholar]
- 8.Billings LB, Spero JA, Vollmer RR, Amico JA. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res. 2006;171:134–141. doi: 10.1016/j.bbr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 10.Brown CH, Russell JA. Cellular mechanisms underlying neuronal excitability during morphine withdrawal in physical dependence: lessons from the magnocellular oxytocin system. Stress. 2004;7:97–107. doi: 10.1080/10253890410001727776. [DOI] [PubMed] [Google Scholar]
- 11.Buijs RM. Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron microscopy. J Histochem Cytochem. 1980;28:357–360. doi: 10.1177/28.4.6989899. [DOI] [PubMed] [Google Scholar]
- 12.De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- 13.Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes (Lond) 2009;33 Suppl 2:S54–S58. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- 14.Gulati K, Ray A, Sharma KK. Effects of acute and chronic morphine on food intake in rats: modulation by oxytocin and vasopressin. Pharmacol Biochem Behav. 1991;40:27–32. doi: 10.1016/0091-3057(91)90316-t. [DOI] [PubMed] [Google Scholar]
- 15.Hajnal A, Norgren R, Kovacs P. Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann N Y Acad Sci. 2009;1170:347–364. doi: 10.1111/j.1749-6632.2009.03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Sved AF, Stricker EM. Vasopressin and oxytocin release evoked by NaCl loads are selectively blunted by area postrema lesions. Am J Physiol Regul Integr Comp Physiol. 2000;278:R732–R740. doi: 10.1152/ajpregu.2000.278.3.R732. [DOI] [PubMed] [Google Scholar]
- 18.Lokrantz CM, Uvnas-Moberg K, Kaplan JM. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav. 1997;62:347–352. doi: 10.1016/s0031-9384(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig M, Brown CH, Russell JA, Leng G. Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J Physiol. 1997;505(Pt 1):145–152. doi: 10.1111/j.1469-7793.1997.145bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miceli D, Marfaing-Jallat P, Le Magnen J. Non-specific enhancement of ethanol-induced taste aversion by naloxone. Pharmacol Biochem Behav. 1979;11:391–394. doi: 10.1016/0091-3057(79)90113-8. [DOI] [PubMed] [Google Scholar]
- 23.Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1063–R1068. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- 24.Nissenbaum JW, Sclafani A. Sham-feeding response of rats to Polycose and sucrose. Neurosci Biobehav Rev. 1987;11:215–222. doi: 10.1016/s0149-7634(87)80029-5. [DOI] [PubMed] [Google Scholar]
- 25.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 27.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991;129:785–791. doi: 10.1210/endo-129-2-785. [DOI] [PubMed] [Google Scholar]
- 28.Olszewski PK, Levine AS. Minireview: Characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology. 2004;145:2627–2632. doi: 10.1210/en.2004-0016. [DOI] [PubMed] [Google Scholar]
- 29.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav. 2007;91:506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Olszewski PK, Shaw TJ, Grace MK, Hoglund CE, Fredriksson R, Schioth HB, et al. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides. 2009;30:226–233. doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olszewski PK, Shi Q, Billington CJ, Levine AS. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1504–R1511. doi: 10.1152/ajpregu.2000.279.4.R1504. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Sydney ; Orlando: Academic Press; 1986. [Google Scholar]
- 33.Rask-Andersen M, Olszewski PK, Levine AS, Schioth HB. Molecular mechanisms underlying anorexia nervosa: Focus on human gene association studies and systems controlling food intake. Brain Res Rev. 2009 doi: 10.1016/j.brainresrev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA. Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr. 2004;79:372–378. doi: 10.1093/ajcn/79.3.372. [DOI] [PubMed] [Google Scholar]
- 35.Sclafani A. Starch and sugar tastes in rodents: an update. Brain Res Bull. 1991;27:383–386. doi: 10.1016/0361-9230(91)90129-8. [DOI] [PubMed] [Google Scholar]
- 36.Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–R1833. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MJ, Wise PM. Localization of kappa opioid receptors in oxytocin magnocellular neurons in the paraventricular and supraoptic nuclei. Brain Res. 2001;898:162–165. doi: 10.1016/s0006-8993(01)02154-0. [DOI] [PubMed] [Google Scholar]
- 38.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Voorn P, Buijs RM. An immuno-electronmicroscopical study comparing vasopressin, oxytocin, substance P and enkephalin containing nerve terminals in the nucleus of the solitary tract of the rat. Brain Res. 1983;270:169–173. doi: 10.1016/0006-8993(83)90809-0. [DOI] [PubMed] [Google Scholar]
- 40.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]


