Abstract
Introduction
Calcium phosphate cements (biocements) are alternative materials for use in vertebral augmentation procedures, and are a potential solution to problems associated with polymethylmethacrylate (PMMA) cements. The aim of this study is to demonstrate the utility of percutaneously injected biocements compared with PMMA in a validated animal model of osteoporosis.
Materials and methods
Fortyseven augmentation procedures were performed on 11 osteoporotic sheep. 9 vertebrae were augmented with PMMA and 38 with a biocement. The animals were killed in four groups: at 7 days, 3 months, 6 months, and 1 year after intervention. Radiological study and TC of the pieces were obtained to evaluate for leakage, cement diffusion, and integration. In total, 26 biomechanic studies and 27 histomorphometry analyses were performed, included control vertebrae.
Results
In 20.9% of the vertebrae, the hole was empty at sacrifice. The pattern of fracture was heterogeneous, and cement augmentation did not increase vertebral strength or decrease vertebral stiffness compared to control vertebrae, with neither PMMA or biocement. The rate of remodeling of the biocement was not predictable. In the single majority, there is peripheral remodeling, staying the volume of injected biocement stable.
Conclusions
Even though this animal model may not be useful to analyze the biomechanical pattern of treated vertebrae, it demonstrates that the percutaneous use of biocements in vertebral augmentation techniques is not predictable. This is one reason not to recommend its use presently as a substitute for PMMA in vertebral reinforcement procedures.
Keywords: Biocements, CaP cement, Vertebroplasty, Osteoporosis, Kyphoplasty
Introduction
Vertebral compression fractures are a common cause of pain and disability. There are various potential causes, including osteoporosis, trauma, and neoplasm. Osteoporosis is the most frequent cause among postmenopausal women and patients on long-term steroid therapy. Percutaneous polymethylmethacrylate (PMMA) vertebroplasty (PV) and kyphoplasty are two augmentation techniques that have become the standard of care for treatment of medically refractory vertebral compression fractures [1, 12, 22, 25].
PMMA has several disadvantages, including leakage [12, 22], exothermic reaction during polymerization of methyl methacrylate [14, 32], and incidence of adjacent fractures due to its excessive stiffness [23]. Due to these factors, other types of injectable materials have been proposed for use in stabilizing painful vertebral compression fractures. Hydroxyapatite-forming materials are an attractive alternative to PMMA cements due to their osteoconductivity and absence of exothermal properties.
Several ex vivo studies demonstrated that hydroxyapatite cements can restore the strength of fractured vertebrae [3, 15]. When tested in animal models, calcium phosphate (CaP) cement also appears to have excellent biological properties, although the capacity for bone resorption/replacement of the cement remains controversial [2], and most of these studies have been performed in craniofacial bones in small animals, such as rats or rabbits, or with open surgery [29].
The purpose of this study was to assess the biomechanical, histological, and histomorphometric characteristics of a calcium phosphate cement and compared these properties to PMMA for vertebral augmentation in a validated large animal model of vertebral osteoporosis treated with percutaneous injection, mimicking the routine clinical procedure [21, 33].
Materials and methods
The Institutional Animal Ethics Committee approved the study. 11 Merino female sheep were used. The animals were 4–6 years old (mean weight 61.3 ± 5 kg). They were ovariectomized, and then fed a diet restricted in calcium and vitamin D and were injected with methylprednisolone (O + D + S) for 7 months to develop an osteoporotic spine. A significant reduction in bone mineral density of cancellous lumbar vertebrae has been demonstrated with this model [21, 33].
To test our hypothesis, we designed an augmentation technique in the lumbar spine. The procedure plan was to perform kyphoplasty on L1, L2, L4, and L5 kyphoplasty and use L3 and L6 as control vertebrae. We used a 20 mm length kyphoplasty system (Kyphon™) in the first two sheep, but as we had several complications with the technique (fracture of the vertebral body, massive leakage into the spinal canal, and difficulties injecting the cement if a hole was not created), we altered the procedure to perform a hole manually with a 5 mm drill.
The operation was performed with the animal under general anesthesia after overnight fasting. Induction of anesthesia was achieved via intravenous 2 mg/kg xylazine and 13 mg/kg ketamine as a bolus injection. Thiopental sodium was used for maintenance of anesthesia as a continuous intravenous infusion at 10–15 mg/kg/h. The subjects were taken to the operating theater and placed in the lateral decubitus position so that right side would be superior. After sterile preparation with povidine–iodine solution, the surgical field was covered with sterile drapes. The vertebrae were identified with radiographic control. A 5 mm drill with a long guide was placed on the medial column of the vertebral body, aiming for placement approximately 1 cm from the superior endplate. The guide was fixed to the cortex. A hole was created manually, attempting to penetrate the opposite side of the vertebrae without breaking the cortical bone. The guide was used as a cannula, and the cement was injected under radiological control, aiming to fill the holes and imbricate the cement into the adjacent trabeculae. PMMA (Osteopal®V, Biomet, Varsaw, Indiana, USA) and CaP cement (Calcibon®, Biomet, Varsaw, Indiana, USA) were used.
Buprenorphine (0.01 mg/kg twice a day) was administered for 5 days as postoperative analgesia. Anteroposterior (AP) and lateral (L) radiographs of the spine were obtained after surgery in every animal, in order to assess for complete filling or the absence of cement in the hole. The subjects were then followed and killed while under xylazine and ketamine anesthesia, with the application of 3 mL 2% lysthenone forte injection at baseline (2 animals), and at 3, 6, and 12 months postoperative (three animals in each group). The spines were excised and the implants were removed to eliminate possible artifacts on CT scans. AP and L radiographs were again obtained, followed by a dual-energy X-ray absorptiometry (DXA) (QDR 2000, Hologic Inc, Waltham, MA, USA) with standard software (Version 7.10b) to measure bone mineral density (BMD) of the un-augmented vertebrae. The vertebrae were compared with a control group of six sheep without osteoporosis. A CT scan was obtained in all explanted vertebrae to detect the presence of leakage and cement in the holes.
Vertebrae were divided in two groups: one for histological analysis and the second for biomechanical analysis. For biomechanical analysis, vertebrae with better filling of the holes on CT were selected.
Mechanical testing
A total of 26 vertebrae were used for ex vivo mechanical testing, grouped according to duration of follow-up and type of cements. The vertebrae were embedded in PMMA between two parallel plates and a pure compressive load was applied at 1 mm/min using a Shenck-Trebel 10 Tm with a 1Tm sense (Schenck Corporation, Deer Park, NY, USA). Force and displacement data were recorded in real time. Force displacement curves were used to determine the compressive fracture strength of each vertebral body as the maximum force on the curve. Ultimate failure load (kN) and elastic stiffness (kN/mm) were determined.
Histomorphometry
Twenty-seven extracted vertebrae were fixed in 70% ethyl alcohol and embedded in methyl metacrylate (MMA). Sections of 10 μm thickness were obtained with a microtome (Leica 22550, Leyca Microsystems GmbH, Germany), and specimens′ sections were deplasticized with methyl-acetate and stained with Von Kossa′s (VK) and Goldner′s trichrome (TRI). The sections were taken in all specimens from the middle of the implant. A total of four sections per block were VK and TRI stained for conventional light microscopy. Qualitative histology was performed from stained sections using a microscope (Olympus BX51, Germany) with attached digital camera (DP70, Olympus, Germany). The parameters were determined and utilized according to the Parfitt standardization of 1987 [24].
Statistical analysis
The vertebral strength and stiffness with the different cements and in control vertebrae were compared using non-parametric Mann–Whitney test with a confidence level of 0.95. Statistical analyses were performed using Stata 11 software.
Results
All surgical interventions were performed without complications. The animals recovered well from surgery with normal locomotion, except one case that developed paraplegia and was sacrificed the third day after the procedure. We did not find any major problems with the injection of the cement into the vertebral body, and in all cases postprocedural radiologic imaging demonstrated cement filling of the created holes.
Radiographic evaluation
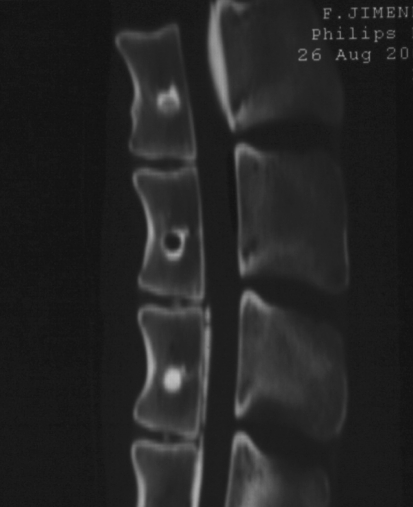
The CaP and PMMA cements were clearly distinguished from the surrounding trabecular bone on the postoperative radiographs because of their higher radio density. The holes were a mean of 8.55 mm (7–12 mm) from the superior endplate. In the radiographs and CT obtained after euthanasia, 9 of 43 vertebrae studied after 3 months (20.9%) had holes that were completely or partially empty (Fig. 1).
Fig. 1.
TC obtained following euthanasia 6 months after surgery. Three different patterns are observed in the same animal: a hole with partial replacement of the CaP cement by bone, an empty hole, and a hole completely filled with CaP cement
Leakage were observed in 48.9% of the vertebrae. In three cases, cement was into the canal (2 cases with PMMA and 1 with CaP. In 21 cases, the cement was surrounding the puncture entry or exit of the vertebral body.
Bone mineral density
Twenty-two un-augmented vertebrae of the osteoporotic sheep had a mean BMD of 0.77 g/cm2 (SD 0.10), which was 20.78% lower than that of the 12 control group vertebrae (mean 0.98 g/cm2, SD 0.05).
Biomechanical study
The pattern of fracture was heterogeneous, and in most cases, the fracture line was observed in the middle of the vertebral body, below the created cavity, instead of in the superior endplate. Results are summarized in Table 1. The cement augmentation did not increase vertebral strength with either PMMA or the biocement compared with control vertebra (p = 0.3320 for PMMA and p = 0.7231 for CaP cement). Augmentation did not decrease vertebral stiffness (p = 0.4562 for PMMA and p = 0.6548 for CaP cement). No changes were observed over time with the biocement.
Table 1.
Vertebral strength and stiffness of augmented and un-augmented vertebrae
| N | Strengh (kN) | Stiffness (kN/mm) | |
|---|---|---|---|
| mean (SD) | mean (SD) | ||
| Control | 10 | 13,465 (3,240) | 8,757 (2,368) |
| PMMA | 4 | 12,604 (2,009) | 8,024 (1,616) |
| CaP cement | 12 | 13,529 (3,020) | 7,404 (2,41) |
| CaP cement <3 months | 6 | 13,430 (2,537) | 7,459 (2,59) |
| CaP cement 6–12 months | 6 | 13,949 (1029) | 7,327 (2,70) |
Histological study
Vertebrae appeared heterogenous on histologic analysis. Vertebral sites containing PMMA were characterized by new bone on the cement and surrounding trabecular surfaces. A thin fibrous membrane incompletely surrounded the residual PMMA bolus centrally. PMMA was frequently noted to be present in the vessels on the canal floor.
After 3 months, in the CaP cement group the presence of newly formed islets of osteoids in direct contact with the remaining cement was occasionally seen. In addition, many multinucleated cells, most likely osteoclasts, were found mainly in the area of the original defect. In 3 of 10 vertebrae studied (30%) the hole was empty.
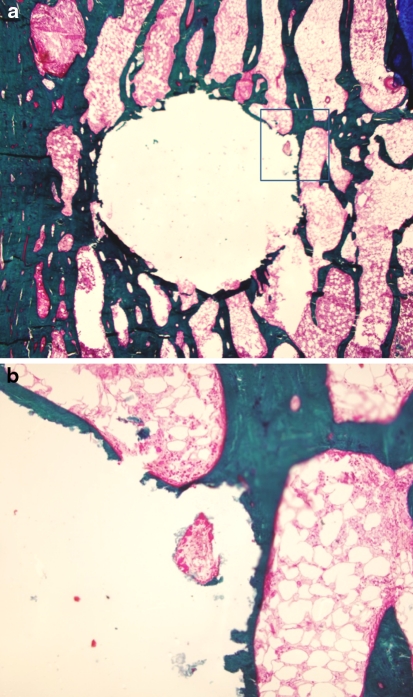
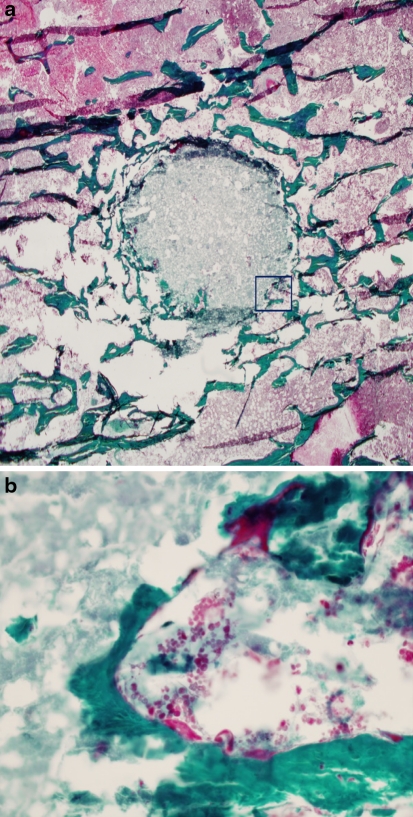
For the 6 and 12 months groups, the results were similar. 6 of 14 (42.8%) vertebrae had an empty hole (Fig. 2). The majority of the remaining specimens demonstrated marginal resorption. The cell population was composed of a few macrophages-like cells visible around the original bone defect, and osteoclasts were not found on the outside edge. Osteoblasts were slightly active, producing new osteoid and bone (Fig. 3). In three cases, a large area of the original cement was replaced with osteoid and new bone. Both osteoblast and osteoclast-like cells were visible within the remodeling lacunae formed in the cement crevices, where they penetrated and grew from the edge of the bone cement (Fig. 4).
Fig. 2.
Histologic image of von Kossa stain showing complete resorption of the CaP cement, leaving an empty hole with some peripheral bone formation. a ×20, b ×100
Fig. 3.
Histologic pictures of specimens at 6 months after implantation. Images show osteoid and new bone formation, bone remodeling, and peripheral cement resorption. a. Goldner trichrome ×20, b ×400
Fig. 4.
Histologic section of a specimen 12 months after treatment, where complete replacement of the CaP cement can be observed. a Stain von Kossa, ×20. b Osteoid and osteoblasts cells are observed in new trabeculae. Goldner trichrome, ×400
Discussion
Vertebroplasty has become an important treatment option in the management of osteoporotic vertebral fractures. PMMA is the most commonly used cement; however, there are some possible complications with PMMA. Several studies reported concerns about subsequent vertebral compression fractures after vertebroplasty [9, 18, 30]. Augmentation using PMMA can alter the normal spinal biomechanics and may result in subsequent vertebral compression fractures. In contrast, osteoconductive filler materials such as CaP may prevent fractures. CaP cement has additional advantages including the absence of exothermic effects and osteoconductive activity [16, 19, 20, 28]. However, biodegradation is an open question: when treating osteoporotic vertebral fractures by cement augmentation, rapid cement resorption without new bone formation will have a negative effect, as the induced resorption may weaken the vertebral body and promote further collapse. The aim of this study was to assess the biological behavior and dynamics of biodegradation in a standardized animal model.
When validated, the significant impact of osteoporosis on the mechanical loading characteristics of the lumbar spine in the osteoporotic sheep model confirmed the suitability of this model for developing and testing new surgical strategies for the treatment of vertebral osteoporosis, including augmentation techniques [21, 33]. However, in the present study we found that the high trabecular density of the vertebral body of these sheep does not allow direct injection of a cement, unless it is overly liquified. The model is, however, useful for the study of percutaneous augmentation technique such as kyphoplasty, reproducing the technical difficulties and the bioactivity of the CaP cements.
The augmentation materials are a mix of liquid and powder and have a progressive setting time that affects the ultimate strength. Increasing pressure on the mix, with the liquid-to-powder ratio (L/P) maintained constant, causes the liquid to be absorbed onto the particles; the cement paste thus becomes dryer, making it impossible to inject [8, 10]. Using these materials in liquid form increases the injectability properties, but increases the risk of venous leakage and pulmonary embolism [17]. In this study TC analysis confirmed the unpredictable pattern of the biocement when injected percutaneously, mimicking clinical practice, with 20.9% of the vertebrae having an empty hole at the time of the sacrifice. Most animal studies on biocements were performed using open surgery to fill the created defect [5, 6, 29, 31]. In our study, we were not able to complete filling of the cavity with the cement, so the cement could be washed by venous drainage.
We confirmed that this animal model is not useful for in vivo biomechanical analysis of biocements. The shape of the vertebral body is different than in humans, the augmentation was performed in a single small hole on the upper third of the vertebral body, and the fracture pattern is dissimilar from humans. An open model, creating a larger hole similar to kyphoplasty, would be recommended [29].
Histologic analysis confirms that the biocement used here is stable, with cement resorption occurring slowly over a prolonged period. In order to accelerate bone tissue colonization and resorption of the cement implant, several authors have improved macroporosity, although this can be link to a decrease in strength [7, 26, 27].
The clinical usefulness of biocement remains controversial. In several clinical trials, biocements were used with kyphoplasty with disappointing results, including fracture progression in many cases [4, 11, 14].
While this study has several limitations, including the fact that the histologic analysis was performed on vertebrae where the cement filling of the holes observed by TC was lower, we can assess that this animal model is limited in its utility for biomechanical testing of this biocement, especially if used percutaneously. However mimicks the bioactivity of the biocements when injected percutaneously. The findings are too unpredictable, suggesting that performance of vertebral augmentation using CaP cement should be reconsidered, and the development of new filler materials is warranted.
Conflict of interest
None.
References
- 1.Alvarez L, Alcaraz M, Pérez-Higueras A, Granizo JJ, Miguel I, Rossi RE, Quiñones D. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine. 2006;31:1113–1118. doi: 10.1097/01.brs.0000216487.97965.38. [DOI] [PubMed] [Google Scholar]
- 2.Ambard AJ, Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont. 2006;15:321–328. doi: 10.1111/j.1532-849X.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 3.Belkoff SM, Mathis JM, Jasper LE, Deramond H. An ex vivo biomechanical evaluation of a hydroxyapatite cement for use with vertebroplasty. Spine. 2001;26:1542–1546. doi: 10.1097/00007632-200107150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Blattert TR, Jestaedt L, Weckbach A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation: a controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine. 2009;34:108–114. doi: 10.1097/BRS.0b013e31818f8bc1. [DOI] [PubMed] [Google Scholar]
- 5.Bodde EW, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. The kinetic and biological activity of different loaded rhBMP-2 calcium phosphate cement implants in rats. J Biomed Mater Res A. 2008;87:780–791. doi: 10.1002/jbm.a.31830. [DOI] [PubMed] [Google Scholar]
- 6.Bodde EW, Cammaert CT, Wolke JG, Spauwen PH, Jansen JA. Investigation as to the osteoinductivity of macroporous calcium phosphate cement in goats. J Biomed Mater Res B Appl Biomater. 2007;83:161–168. doi: 10.1002/jbm.b.30780. [DOI] [PubMed] [Google Scholar]
- 7.del Real RP, Wolke JG, Vallet-Regí M, Jansen JA. A new method to produce macropores in calcium phosphate cements. Biomaterials. 2002;23:3673–3680. doi: 10.1016/S0142-9612(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 8.Fernández E, Gil FJ, Ginebra MP, Driessens FC, Planell JA, Best SM. Production and characterization of new calcium phosphate bone cements in the CaHPO4-alpha-Ca3(PO4)2 system: pH, workability and setting times. J Mater Sci Mater Med. 1999;10:223–230. doi: 10.1023/A:1008958112257. [DOI] [PubMed] [Google Scholar]
- 9.Fribourg D, Tang C, Sra P, Delamarter R, Bae H. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29:2270–2276. doi: 10.1097/01.brs.0000142469.41565.2a. [DOI] [PubMed] [Google Scholar]
- 10.Ginebra MP, Driessens FC, Planell JA. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials. 2004;25:3453–3462. doi: 10.1016/j.biomaterials.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Grafe IA, Baier M, Nöldge G, Weiss C, Da Fonseca K, Hillmeier J, Libicher M, Rudofsky G, Metzner C, Nawroth P, Meeder PJ, Kasperk C. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine. 2008;33:1284–1290. doi: 10.1097/BRS.0b013e3181714a84. [DOI] [PubMed] [Google Scholar]
- 12.Hadjipavlou AG, Tzermiadianos MN, Katonis PG, Szpalski M. Percutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumours. J Bone Joint Surg Br. 2005;87:1595–1604. doi: 10.1302/0301-620X.87B12.16074. [DOI] [PubMed] [Google Scholar]
- 13.Haung KY, Yan JJ. Lin RM (2005) Histologic findings of retrieved speciments of vertebroplasty with polymethylmethacrylate cement. Spine. 2005;30:E585–E588. doi: 10.1097/01.brs.0000182226.56498.55. [DOI] [PubMed] [Google Scholar]
- 14.Heo HD, Cho YJ, Sheen SH, Kuh SU, Cho SM, Oh SM. Morphological changes of injected calcium phosphate cement in osteoporotic compressed vertebral bodies. Osteoporosis Int. 2009;20:2063–2070. doi: 10.1007/s00198-009-0911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitchon PW, Goel V, Drake J, Taggard D, Brenton M, Rogge T, Torner JC. Comparison of the biomechanics of hydroxyapatite and polymethylmethacrylate vertebroplasty in a cadaveric spinal compression fracture model. J Neurosurg. 2001;95:215–220. doi: 10.3171/spi.2001.95.2.0215. [DOI] [PubMed] [Google Scholar]
- 16.Hong SJ, Park YK, Kim JH, Lee SH, Ryu KN, Park CM, Kim YS. The biomechanical evaluation of calcium phosphate cements for use in vertebroplasty. J Neurosurg. 2006;4:154–159. doi: 10.3171/spi.2006.4.2.154. [DOI] [PubMed] [Google Scholar]
- 17.Krebs J, Aebli N, Goss BG, Sugiyama S, Bardyn T, Boecken I, Leamy PJ, Ferguson SJ. Cardiovascular changes after pulmonary embolism from injecting calcium phosphate cement. J Biomed Mater Res B Appl Biomater. 2007;82:526–532. doi: 10.1002/jbm.b.30758. [DOI] [PubMed] [Google Scholar]
- 18.Lee WS, Sung KH, Jeong HT, Sung YS, Hyun YI, Choi JY, Lee KS, Ok CS, Choi YW. Risk factors of developing new symptomatic vertebral compression fractures after percutaneous vertebroplasty in osteoporotic patients. Eur Spine J. 2006;15:1777–1783. doi: 10.1007/s00586-006-0151-7. [DOI] [PubMed] [Google Scholar]
- 19.Libicher M, Hillmeier J, Liegibel U, Sommer U, Pyerin W, Vetter M, Meinzer HP, Grafe I, Meeder P, Nöldge G, Nawroth P, Kasperk C. Osseous integration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: initial results from a clinical and experimental pilot study. Osteoporos Int. 2006;17:1208–1215. doi: 10.1007/s00198-006-0128-8. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman IH, Togawa D, Kayanja MM. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5:305S–316S. doi: 10.1016/j.spinee.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Lill CA, Gerlach UV, Eckhardt C, Goldhahn J, Schneider E. Bone changes due to glucocorticoid application in an ovariectomized animal model for fracture treatment in osteoporosis. Osteoporos Int. 2002;13:407–414. doi: 10.1007/s001980200047. [DOI] [PubMed] [Google Scholar]
- 22.McGirt MJ, Parker SL, Wolinsky JP, Witham TF, Bydon A, Gokaslan ZL. Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: an evidenced-based review of the literature. Spine J. 2009;9:501–508. doi: 10.1016/j.spinee.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Nouda S, Tomita S, Kin A, Kawahara K, Kinoshita M. Adjacent vertebral body fracture following vertebroplasty with polymethylmethacrylate or calcium phosphate cement: biomechanical evaluation of the cadaveric spine. Spine. 2009;34:2613–2618. doi: 10.1097/BRS.0b013e3181abc150. [DOI] [PubMed] [Google Scholar]
- 24.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 25.Peh WC, Munk PL, Rashid F, Gilula LA. Percutaneous vertebral augmentation: vertebroplasty, kyphoplasty and skyphoplasty. Radiol Clin North Am. 2008;46:611–635. doi: 10.1016/j.rcl.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Sarda S, Nilsson M, Balcells M, Fernández E. Influence of surfactant molecules as air-entraining agent for bone cement macroporosity. J Biomed Mater Res A. 2003;65:215–221. doi: 10.1002/jbm.a.10458. [DOI] [PubMed] [Google Scholar]
- 27.Takagi S, Chow LC. Formation of macropores in calcium phosphate cement implants. J Mater Sci Mater Med. 2001;12:135–139. doi: 10.1023/A:1008917910468. [DOI] [PubMed] [Google Scholar]
- 28.Tomita S, Kin A, Yazu M, Abe M. Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J Orthop Sci. 2003;8:192–197. doi: 10.1007/s007760300032. [DOI] [PubMed] [Google Scholar]
- 29.Turner TM, Urban RM, Singh K, Hall DJ, Renner SM, Lim TH, Tomlinson MJ, An HS. Vertebroplasty comparing injectable calcium phosphate cement compared with polymethylmethacrylate in a unique canine vertebral body large defect model. Spine J. 2008;8:482–487. doi: 10.1016/j.spinee.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 31.Vlad MD, Sindilar EV, Mariñoso ML, Poeată I, Torres R, López J, Barracó M, Fernández E. Osteogenic biphasic calcium sulphate dihydrate/iron-modified alpha-tricalcium phosphate bone cement for spinal applications: in vivo study. Acta Biomater. 2010;6:607–616. doi: 10.1016/j.actbio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Willert HG. Tissue reactions around joint implants and bone cement. In: Chapchl G, editor. Arthroplasty of the Hip. Stuttgraft: Thieme; 1973. pp. 11–21. [Google Scholar]
- 33.Zarrinkalam MR, Beard H, Schultz CG, Moore RJ. Validation of the sheep as a large animal model for the study of vertebral osteoporosis. Eur Spine J. 2009;18:244–253. doi: 10.1007/s00586-008-0813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]