Abstract
Introduction
Lumbar fusion in elderly patients is increasingly common. This study prospectively investigated the clinical and radiological outcome of osteoporotic patients >70 years with degenerative lumbar instability treated with fusion using a new cannulated, cemented, pedicle screw instrumentation augmented with PMMA.
Materials and Methods
The surgical protocols, patient records, densitometry, imaging studies, and pre- and postoperative patient-reported outcome questionnaires of 23 patients (mean age, 77 years) with a follow-up of 20–49 months were reviewed. All patients underwent postoperative 3D CT scan control to assess cement leakage and instrumentation position. Serial radiological controls were analyzed for secondary complications, i.e., adjacent fractures, hardware mobilization and radiological evidence of fusion.
Results
Pain and function improved at 6 months and were maintained at the final follow-up. No clinical complications secondary to PMMA leakage developed. No clinical or radiological cases of non-union were observed with a mean of 1.8 levels fused. No fractures occurred in adjacent segments. There were four cases of adjacent disc disease. Three deep infections required surgical revision without removal of material and one superficial infection, all with complete remission.
Conclusion
This new instrumentation for degenerative lumbar disease in elderly patients is safe and effective.
Keywords: Pedicle screw augmentation, Polymethylmethacrylate, Osteoporosis, Degenerative lumbar surgery, Elderly patients
Introduction
Instrumented spinal fusion in older-aged patients is becoming an increasingly common and requested procedure [1]. The average life expectancy is increasing, and the global trend toward aging societies is well established, with increasing proportions of older people with disabilities [2].
Elderly patients have an increased risk of complications during lumbar surgery, especially when they also have a high degree of comorbidity [3–5]. Osteoporosis is the most frequent skeletal disease in this context, affecting up to 50% of women older than 65 years. The technical difficulties and high rate of complications associated with osteoporotic bone fixation are well documented in the literature [6]. The torque and pullout strength of pedicle screws have a linear correlation with bone mineral density [7, 8]. Many screw augmentation techniques have been proposed, and cemented polymethylmethacrylate (PMMA) augmentation appears to be the most effective augmentation method in biomechanical tests [9–13]. Most of the studies published on screw cement augmentation are experimental. There are few clinical reports on the application of these techniques in clinical practice [14].
The purpose of the current study was to analyze the results of lumbar fusions in elderly patients with poor bone quality performed with rigid instrumentation based on a new controlled PMMA pedicle screw augmentation technique through cannulated screws.
Methods
Study design
Twenty-three consecutive elderly patients (20 female and 3 male) aged over 70 (mean 77, range 70–81) years with lumbar degenerative spondylolisthesis with instability, or lumbar stenosis requiring aggressive decompression, underwent a spinal fusion with PMMA-augmented cannulated pedicle screw instrumentation between October 2006 and February 2009. Revision surgery patients were excluded. Data were collected prospectively for all patients with a mean follow-up of 32 (range, 20–49) months.
A total of 16 patients (70%) were ASA II and 7 patients (30%) were ASA III. The number of comorbidity factors associated with high rates of complication following Elixhauser Index [15] is shown in Table 1.
Table 1.
Patient characteristics
| Patient | Gender | Age | Diagnosis | ASA | No. of comorbidities | Fused levels |
|---|---|---|---|---|---|---|
| 1 | Female | 79 | Stenosis | III | 3 | 2 |
| 2 | Female | 77 | Spondylolisthesis | III | 3 | 2 |
| 3 | Female | 79 | Stenosis | II | 2 | 5 |
| 4 | Female | 72 | Stenosis | II | 1 | 2 |
| 5 | Female | 73 | Spondylolisthesis | II | 0 | 1 |
| 6 | Female | 73 | Spondylolisthesis | II | 2 | 2 |
| 7 | Female | 79 | Spondylolisthesis | II | 3 | 1 |
| 8 | Female | 81 | Spondylolisthesis | III | 5 | 1 |
| 9 | Male | 79 | Stenosis | III | 7 | 1 |
| 10 | Female | 80 | Stenosis | II | 2 | 4 |
| 11 | Female | 76 | Stenosis | III | 4 | 2 |
| 12 | Female | 79 | Spondylolisthesis | II | 1 | 2 |
| 13 | Female | 75 | Spondylolisthesis | II | 0 | 2 |
| 14 | Male | 79 | Stenosis | II | 0 | 2 |
| 15 | Female | 78 | Stenosis | II | 1 | 2 |
| 16 | Female | 80 | Spondylolisthesis | II | 1 | 1 |
| 17 | Female | 75 | Spondylolisthesis | II | 2 | 1 |
| 18 | Male | 78 | Stenosis | III | 2 | 1 |
| 19 | Female | 77 | Stenosis | II | 1 | 2 |
| 20 | Female | 81 | Spondylolisthesis | II | 4 | 1 |
| 21 | Female | 70 | Stenosis | II | 1 | 2 |
| 22 | Female | 79 | Spondylolisthesis | III | 2 | 2 |
| 23 | Female | 74 | Spondylolisthesis | II | 2 | 3 |
All patients had osteoporotic fracture history or bone mineral density criteria for osteoporosis. Lumbar dual energy X-ray absorptiometry (DEXA) main T score was −2.4 (−1.8 to −4.1).
Surgical technique and care
A standard, open, posterior midline approach to the lumbar spine was made. Cell-saver was used for all patients. Laminectomy or hemilaminectomy, associated or not with facetectomy, was performed prior to fusion in patients with foraminal or central canal stenosis.
The optimal starting pedicle point for screws was confirmed with fluoroscopy and burr initiation was made. A standard rounded pedicle finder was fluoroscopically guided until the tip was anterior to 1/3 of the vertebral body. A bony wall palpation was made before taping with 5.5-mm tape. Cannulated pedicular screws (Omega21 cemented screw, Biomet Inc., Indiana, USA) were placed with concentric angulation and checked for correct positioning with fluoroscopy. Once all pedicle screws were placed, the instrumentation was completed and the screws were dynamometrically tightened.
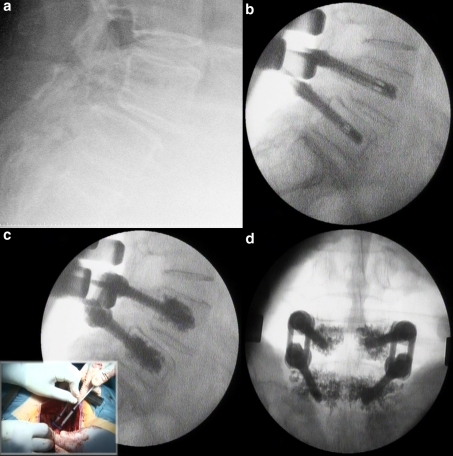
The optimal lateral view position of the fluoroscopy C-arm was obtained before cementation. Vertebroplasty cement was used for augmentation (Biomet V, Biomet Inc., Indiana, USA). The cement was mixed and managed in 1-mm3 sterile syringes. This syringe fits in the screw base and allows fine control of the cement flow. When the viscosity was like toothpaste, the cement was slowly injected through the screw under continuous fluoroscopy to control the distribution of the cement and leakage as done during standard vertebroplasty techniques. Cement injection was stopped and tried later if any cement leakage occurred (Fig. 1).
Fig. 1.
Surgical technique. Preoperative patient radiograph (a). Pedicle screws placed in position prior to cementation (b). Continuous fluoroscopy control of cementation (c). Final PA Rx control (d)
Grafting was made with local laminar bone from decompression combined with allograft or Polibone (macroporous biphasic calcium phosphate, Biomatlante ZA, Vigneux de Bretagne, France) enriched with blood extracted through the screws.
Most patients were allowed to walk beginning from the second postoperative day with a soft lumbar brace for their comfort, which was removed 6–8 weeks later. Prophylactic intravenous antibiotics were used preoperatively and 24 h postoperatively. Patients were admitted to the Bone Metabolism Unit to manage their osteoporosis treatment.
Clinical and radiologic follow-up
Outpatient revisions were made 1, 3, 6 and 12 months after surgery and then annually thereafter. Plain AP and lateral standing radiographs were obtained. Radiologic analysis and measurements were taken to look for adjacent fractures, adjacent disc failure, screw-cement or bone-cement radiolucency, posterior or posterolateral bony bridges, hardware mobilization and screw pullout or failure.
Six months postoperatively, all patients underwent a control 3D CT scan. If there was any doubt about malpositioning of the instrumentation or concern about leakage of cement in the radiographic control, another CT scan was performed during the immediate postoperative period. We evaluated the position of the screws and the leakage of cement with Yeom’s classification [16].
Plain radiographs were classified into fused, doubtfully fused or non-union. Radiographs showing an evident bony bridge were classified as fused. Radiographs with definable cleft in the bony bridge, hardware mobilization or failure were classified as non-union. The remaining radiographs were classified as doubtfully fused.
Fusion signs were reviewed in reconstruction images of 6-month control CT scans. For a segment to be categorized as fused in CT, there had to be a continuous bony bridge between the transverse processes or at the lateral side of the facet joints. If there was only unilateral facet joint fusion, questionable bilateral facet fusion or the possible presence of cleft in the bony bridge, the fusion was categorized as doubtfully fused. Segments with a clearly definable cleft in the bony bridge, questionable fusion in one facet joint and none in the contralateral or with desorption of most of the fusion mass were classified as non-union [17].
Lumbar and leg visual analog scale, Oswestry Disability Index, and items 2 and 3 of the Core validated scales were selected to evaluate pain, functional evolution and quality of life [18, 19]. Values were compared with the Wilcoxon test for paired samples. For statistical analyses, we used Stata v10 (College Station, Texas, USA).
Results
Eight patients (35%) were instrumented at one level, 12 (52%) at two levels, and 3 (13%) at three levels or greater. We augmented 103 cannulated screws of 58 cemented vertebrae in 23 patients. The average cement volume injected was 1.8 cc. Vertebra distribution was as follows: S1 17%, L5 21%, L4 35%, L3 17%, L2 8% and L1 2%. The mean surgical time was 147 min (range, 96–225 min). Average differences between preoperative and postoperative hemoglobin and hematocrit were 3.6 g/dl (1.7–6.2) and 10.8% (4.9–20), respectively. The average hospital stay was 8 days (4–23 days).
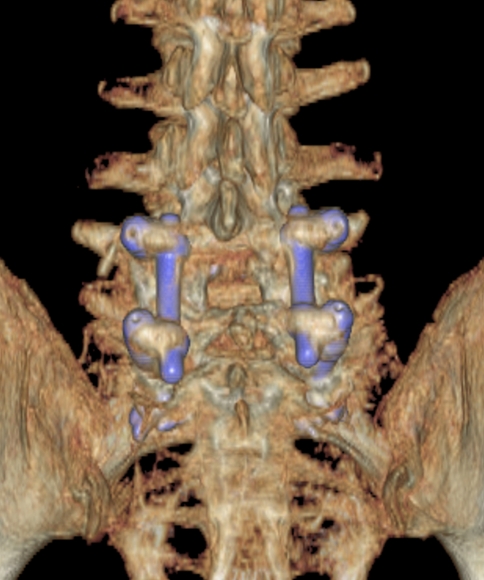
During the follow-up, 17 (74%) patients had Plain Rx radiologic criteria categorizing them as fused and 6 other patients (26%) had doubtfully fused criteria. All patients had an evident posterior and/or posterolateral bone fusion mass in the 6-month CT control (Fig. 2). No patient had CT signs of non-union. In three patients, radiolucency in the cement–screw interface was observed in one screw from the beginning. No bone–cement radiolucency was observed. No cases of pullout or hardware failure were found. There were no cases of adjacent vertebra fractures, although one patient experienced a mid-thoracic osteoporotic vertebral fracture six levels above a lumbosacral fusion performed 8 months previously and was treated with vertebroplasty.
Fig. 2.
A 3D CT reconstruction with an evident posterolateral bone fusion mass in a 79-year-old patient with rheumatoid arthritis and severe osteoporosis
Four patients (17%) presented with progressive adjacent disc degeneration with collapse and low grade spondylolisthesis. All of them presented with associated deterioration of pain and function scales, but none accepted revision surgery at the time of this report.
Cement leakage was observed in 29.3% of cemented vertebrae. We found type-B leakage (epidural leakage) in 8 vertebrae (13.8%), type-S leakage (lateral venous leakage) in 12 vertebrae (20.7%) and type-C leakage in 3 vertebrae. Two foraminal (3.4%) and one extravertebral leakages (3.4%) were found. No cases of discal leakage were identified. Both patients with a foraminal leakage from S1 and L1 screws (2% of all screws) had no root symptoms. Malpositioning of two screws, one medial (S1 with foraminal leakage) and one lateral, was confirmed, although none had neural or vascular contact and no revision was needed.
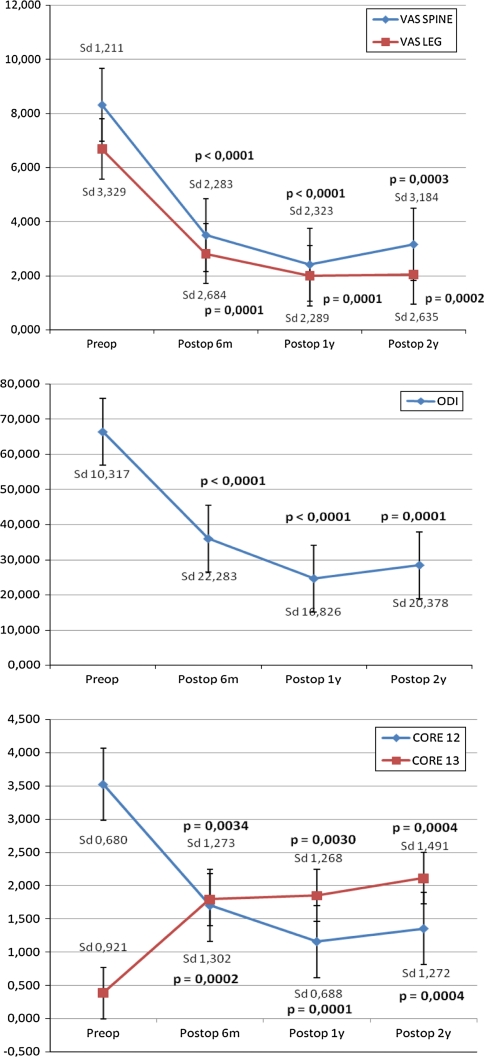
Pain and functional test results are shown in Fig. 3. Patient no. 17 had a previous cervical myelopathy of Nurick 3 grade and was excluded from the statistical analysis. Results showed a statistical improvement in all the parameters studied since the first follow-up, and these were maintained over time. Following FDA criteria for significant functional improvement (upgrade ≥ 15 points in ODI scale), 90% of patients had a satisfactory result. Based on the last two items of the Core Outcome Measures Index, 86% of patients were satisfied or very satisfied at the last follow-up.
Fig. 3.
Results of VAS, ODI and Core Outcome Measures Index during the follow-up period compared to preoperative values
Three (13%) deep subacute infections were registered during the follow-up. All of these required revision surgery but not instrumentation removal, and the patients were treated with intravenous antibiotics for at least 6 weeks with good evolution. One rheumatoid arthritis patient on immunosuppressive treatment for years experienced surgical wound dehiscence, although the immunosuppressant was withdrawn 6 weeks preoperatively. She needed negative pressure wound therapy (V.A.C.® Therapy, Kinetic Concepts Inc. TX, USA) and a free skin graft for wound closure. Three patients developed mild leg pain without palsy in the immediate postoperative period with full resolution within the first 6 months. Two urinary infections were treated with antibiotics, but were not related to wound infections. Two patients developed bronchospasm in the recovery room without pulmonary complications.
Discussion
Increased life expectancy and decreased death rates have contributed to a rapid increase in the number of individuals 65 years and older. The elderly population in developed countries is projected to increase by 60% during the next three decades [20]. Currently, advanced age is not a contraindication to surgical treatment and rates of surgical procedures in the elderly have increased dramatically in the past two decades [21].
Degenerative spine disease leading to lumbar stenosis and spondylosis is a major cause of morbidity among the elderly [22]. Back and leg pain resulting from nerve root compression in these patients may cause loss of function and an inability to perform activities required to meet basic daily needs [23].
Anatomical degenerative changes associated with spinal stenosis often require wide decompression with partial or total facet joint removal, and iatrogenic destabilization may occur [24]. Other patients experience primary instability for mobile spondilolysthesis. Instrumentation is indicated to restore stability and to achieve good graft integration.
Age-related bone loss causes elderly patients to have a high rate of poor bone quality. Loosening and backing out of the pedicle screws resulting from failure of screw fixation remains a significant clinical problem in osteoporotic patients [12]. Many methods to improve the pullout strength of pedicle screw fixation have been reported [11]. Less rigid instrumentation techniques and pedicle screw augmentation have been proposed with good clinical results published [25]. The injection of PMMA was found to be the most effective augmentation method, increasing the pullout strength to approximately 150% of the initial strength [9, 10].
Chang et al. [14] reviewed 291 PMMA-augmented pedicle screws in 41 osteoporotic patients with a median of 22-months follow-up. They used direct PMMA cement injection in the pedicle tract before inserting the screw. No screw migration or loosening was reported in the series, and all cement leakages were asymptomatic.
Widespread use of vertebroplasty has provided experience and consistent data about low risks of cement leakage when done in a controlled fashion. Cannulated pedicular screws allow performing screw augmentation once the screws are inserted, and precisely control the consistency, rhythm and volume of the cement injected into each screw. Continuous fluoroscopy performed during cementation makes identification of the trabecular pattern of cementation possible and stops cementation at the beginning of cement leakage. Therefore, high rates of leakage found in our series are correlated with the high sensibility of 3D CT, even in minimal volume cement leakage, and are comparable with vertebroplasty results in the literature [26]. No clinical complication observed was linked with cement leakage, even in the foraminal cases.
Some authors suggest a relationship between cementation and adjacent vertebral fracture [27], but no cases occurred in our series. One distant osteoporotic vertebral fracture (mid-thoracic) was found in a lumbosacral fusion 8 months after surgery and treated with vertebroplasty.
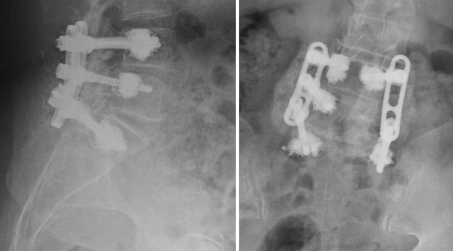
High rates of fusion in the radiological and CT controls confirm the good stability reached with rigid instrumentation when good bone anchorage is achieved (Fig. 4). Clinical result analysis demonstrated a significant improvement in function and lumbar and leg pain relief with a high rate of satisfaction similar to other series of degenerative lumbar surgery in younger patients [28].
Fig. 4.
Radiologic control after 2 years of follow-up in a 76-year-old patient with evident posterolateral bony bridge
Many authors describe a high incidence of complications with instrumented fusion in elderly patients, especially when they are over 69 years of age [5]. Associated comorbidities in these patients were also correlated with complications and adverse outcomes after lumbar surgery [3, 4]. The high rate of complications in our series did not depend on the instrumentation, but rather on the surgical procedure and the patient’s profile. Carefully explaining to patients the risks of this surgery is of utmost importance.
Some patients (17%) developed degenerated disc disease adjacent to the level of fusion with some functional impairment. These findings correlate with previous results in elderly patients, even when less stress dynamic stabilization is used [29]. Factors associated with adjacent disc disease should be studied in the future.
The present study has several strengths and limitations. One strength is that this is one of the few clinical studies published to date assessing cemented pedicle screw instrumentation augmented with PMMA for spinal fusions. Additionally, prospective data were collected from a homogeneous population of elderly patients with degenerative spinal disease. Nevertheless, several limitations of this study remain. A greater number of patients and a larger period of follow-up are needed to evaluate adjacent disc disease evolution and its clinical significance.
Conclusion
The results of our study are encouraging. The use of cemented rigid instrumentation in patients with lumbar instability results in clinical improvement and radiologic stability over time. PMMA pedicle screw augmentation is a safe and effective technique, and may be a good alternative to other methods to improve fixation.
Conflict of interest
None.
References
- 1.Bederman SS, Coyte PC, Kreder HJ, Mahomed NN, McIsaac WJ, Wright JG. Who’s in the driver’s seat? The influence of patient and physician enthusiasm on regional variation in degenerative lumbar spinal surgery: a population-based study. Spine (Phila Pa 1976) 2011;36(6):481–489. doi: 10.1097/BRS.0b013e3181d25e6f. [DOI] [PubMed] [Google Scholar]
- 2.Recommendation CM/Rec (2009) 6 of the Committee of Ministers to member states on ageing and disability in the 21st century: sustainable frameworks to enable greater quality of life in an inclusive society (Adopted by the Committee of Ministers on 8 July 2009 at the 1063rd meeting of the Ministers’ Deputies)
- 3.Li G, Patil CG, Shivanand PL, et al. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine. 2008;33(11):1250–1255. doi: 10.1097/BRS.0b013e3181714a44. [DOI] [PubMed] [Google Scholar]
- 4.Raffo CS, Lauerman WC. Predicting morbidity and mortality of lumbar spine arthrodesis in patients in their ninth decade. Spine. 2006;31(1):99–103. doi: 10.1097/01.brs.0000192678.25586.e5. [DOI] [PubMed] [Google Scholar]
- 5.Daubs MD, Lenke LG, Cheh G, et al. Adult spinal deformity surgery. complications and outcomes in patients over age 60. Spine. 2007;32(20):2238–2244. doi: 10.1097/BRS.0b013e31814cf24a. [DOI] [PubMed] [Google Scholar]
- 6.Essens S, Sacs BL, Drezyin V. Complications associated with the technique of pedicle screw fixation: a selected survey of ABC members. Spine. 1993;18:2231–2239. doi: 10.1097/00007632-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Polly DW, Jr, Orchowski JR, Ellenbogen RG. Revision pedicle screws. Bigger, longer shims—what is best? Spine. 1998;23(12):1374–1379. doi: 10.1097/00007632-199806150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Strempel A, Kühle J, Plitz W. Stability of pedicle screws. 2: maximum pullout force with reference to bone density. Z Orthop Ihre Grenzgeb. 1994;132(1):82–86. doi: 10.1055/s-2008-1039824. [DOI] [PubMed] [Google Scholar]
- 9.Wittenberg RH, Lee KS, Shea M, White AA, Hayes WC., 3rd Effect of screw diameter, insertion technique, and bone cement augmentation of pedicular screw fixation strength. Clin Orthop Relat Res. 1993;296:278–287. [PubMed] [Google Scholar]
- 10.Pfeifer BA, Krag MH, Johnson C. Repair of failed transpedicle screw fixation. A biomechanical study comparing polymethylmethacrylate, milled bone, and matchstick bone reconstruction. Spine. 1994;19(3):350–353. doi: 10.1097/00007632-199402000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Tan JS, Kwon BK, Dvorak MF, et al. Pedicle screw motion in the osteoporotic spine after augmentation with laminar hooks, sublaminar wires, or calcium phosphate cement: a comparative analysis. Spine. 2004;29(16):1723–1730. doi: 10.1097/01.BRS.0000134569.63542.49. [DOI] [PubMed] [Google Scholar]
- 12.Renner SM, Lim T, Kim WJ, et al. Augmentation of pedicle screw fixation strength using an injectable calcium phosphate cement as a function of injection timing and method. Spine. 2004;29(11):E212–E216. doi: 10.1097/00007632-200406010-00020. [DOI] [PubMed] [Google Scholar]
- 13.Yi X, Wang Y, Lu H, Li C, Zhu T. Augmentation of pedicle screw fixation strength using an injectable calcium sulfate cement: an in vivo study. Spine. 2008;33(23):2503–2509. doi: 10.1097/BRS.0b013e318184e750. [DOI] [PubMed] [Google Scholar]
- 14.Chang MC, Liu CL, Chen TH. Polymethylmethacrylate augmentation of pedicle screw for osteoporotic spinal surgery: a novel technique. Spine. 2008;33(10):E317–E324. doi: 10.1097/BRS.0b013e31816f6c73. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. 2003;85(1):83–89. doi: 10.1302/0301-620X.85B1.13026. [DOI] [PubMed] [Google Scholar]
- 17.Carreon LY, Glassman SD, Djurasovic M. Reliability and agreement between fine-cut CT scans and plain radiography in the evaluation of posterolateral fusions. Spine. 2007;7(1):39–43. doi: 10.1016/j.spinee.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Flórez García M, García Pérez MA, García Pérez F. Adaptación transcultural a la población española de la escala de incapacidad por dolor lumbar de Oswestry. Rehabilitación. 1995;29:138–145. [Google Scholar]
- 19.Ferrer M, Pellisé F, Escudero O, Alvarez L, Pont A, Alonso J, Deyo R. Validation of a minimum outcome core set in the evaluation of patients with back pain. Spine 20. 2006;31(12):1372–1379. doi: 10.1097/01.brs.0000218477.53318.bc. [DOI] [PubMed] [Google Scholar]
- 20.Jackson SA (1999) The epidemiology of aging.In: Hazzart WR, Blass JP, Ettinger WH, Halter JB, Ouslander JP (eds) Principles of geriatric medicine and gerontology, 4th edn. McGraw-Hill Book Co 4, New York, pp 203–225
- 21.Shabat S, Leitner Y, Nyska M, et al. Surgical treatment of lumbar spinal stenosis in patients aged 65 years and older. Arch Gerontol Geriatr. 2002;35:143–152. doi: 10.1016/S0167-4943(02)00016-X. [DOI] [PubMed] [Google Scholar]
- 22.Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14:491–504. [PubMed] [Google Scholar]
- 23.Jonsson B, Stromqvist B. Symptoms and signs of degeneration of the lumbar spine: a prospective, consecutive study of 300 operated patients. J Bone Joint Surg [Br] 1993;75:381–385. doi: 10.1302/0301-620X.75B3.8496204. [DOI] [PubMed] [Google Scholar]
- 24.Fischgrund JS, Mackay M, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cavagna R, Tournier C, Aunoble S, Bouler JM, Antonietti P, Ronai M, Le Huec JC. Lumbar decompression and fusion in elderly osteoporotic patients: a prospective study using less rigid titanium rod fixation. J Spinal Disord Tech. 2008;21(2):86–91. doi: 10.1097/BSD.0b013e3180590c23. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez L, Pérez-Higueras A, Granizo JJ, Miguel I, Quiñones D, Rossi RE. Predictors of outcomes of percutaneous vertebroplasty for osteoporotic vertebral fractures. Spine. 2005;30(1):87–92. doi: 10.1097/00007632-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 27.Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 28.Videbaek TS, Christensen FB, Soegaard R, Hansen ES, Høy K, Helmig P, Niedermann B, Eiskjoer SP, Bünger CE. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine. 2006;31(25):2875–2880. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 29.Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine. 2008;33(18):E636–E642. doi: 10.1097/BRS.0b013e31817d2435. [DOI] [PubMed] [Google Scholar]