Abstract
This is a retrospective case series to evaluate clinical variables, complications and outcome of 50 patients who underwent anterior lumbar interbody fusion (ALIF) supplemented with posterior percutaneous pedicle screw fixation for degenerative conditions of the lumbar spine. Twenty-four patients underwent single-level fusion and 26 patients had a two-level fusion for a total of 76 levels fused. The mean lengths of the anterior and posterior (including repositioning) portions of the procedure were 131 and 102 min, respectively. The mean estimated blood loss for the entire procedure was 288 ml. The overall adverse event rate was 12%. The mean VAS score for leg pain, VAS score for back pain and mean ODI all improved postoperatively. This study found that ALIF using allograft bone and rhBMP-2 combined with percutaneous pedicle screw fixation had a high fusion rate and a low incidence of perioperative complications. Patient outcomes showed significant improvements in back and leg pain and physical functioning.
Keywords: Lumbar arthrodesis, Anterior lumbar interbody fusion (ALIF), Percutaneous pedicle screw fixation
Introduction
Spinal arthrodesis is a useful treatment for a variety of degenerative conditions in the lumbar spine, including spondylolisthesis, scoliosis, pseudarthrosis, adjacent segment degeneration and painful degenerative disc disease. Various techniques of arthrodesis have been described, each with certain advantages and disadvantages. Anterior/posterior or 360° fusion surgery has been shown to have a high arthrodesis rate [1–3]. However, this type of combined procedure has been reported to have significant complication rates [4–8] and cost [9–12]. Recently, less invasive techniques such as percutaneous pedicle screw fixation have been introduced [13–15]. Percutaneous pedicle screw fixation provides rigid, three column fixation similar to traditional, open pedicle screw constructs. Percutaneous pedicle screws can be implanted through small, paramedian incisions with theoretically less trauma to the paraspinal muscles and less blood loss compared to open exposures of the lumbar spine. When combined with anterior lumbar interbody fusion (ALIF), percutaneous fixation offers a mechanically rigid construct, similar to a traditional 360° fusion, but with potentially less morbidity. Few studies to date have evaluated the outcome of ALIF combined with percutaneous fixation for lumbar degenerative conditions requiring arthrodesis. The purpose of this study was to retrospectively evaluate a cohort of patients following ALIF combined with posterior percutaneous pedicle screw fixation. This data should be useful to surgeons and patients when considering surgical options for lumbar conditions requiring arthrodesis.
Materials and methods
Following Institutional Review Board (IRB) approval, a prospectively maintained database was utilized to identify a consecutive series of patients who underwent ALIF with posterior percutaneous pedicle screw fixation. The database was searched for patients who had undergone this type of surgery between 2004 and 2008. A total of 116 patients were identified. All patients were operated on by a single surgeon (DGA). Exclusion criteria included: treatment for a non-degenerative diagnosis (deformity, trauma, tumor or infection), the presence of a prior fusion surgery in the lumbar spine or fusion of greater than two levels. A total of 83 patients met the inclusion criteria. Of these, 50 consecutive patients had completed a minimum of 12 months of clinical follow-up, and 45 patients had available anteroposterior (AP) and lateral plain radiographs at the 12-month time point.
All patients had undergone a primary lumbar fusion consisting of an ALIF using fresh frozen femoral ring allograft combined with recombinant human bone morphogenic protein-2 (rhBMP-2) followed by percutaneous pedicle screw fixation for the diagnosis of a degenerative disease of the lumbar spine.
Hospital charts and office medical records for this cohort patients were evaluated by an independent observer for clinically relevant variables including the length of surgery (time), estimated blood loss, length of hospital stay and the occurrence of any operative or postoperative complications. The AP and lateral radiographs taken at the 12-month time point following surgery were evaluated for the presence of a successful fusion by three observers using a standardized grading scale. A subset of 22 patients provided preoperative and 12-month postoperative Oswestry disability index (ODI) scores and visual analog scores (VAS) for back and leg pain.
Surgical technique
All cases were conducted at a university teaching hospital with the involvement of resident or fellow teaching as a component of the surgical procedure. The ALIF portion of the procedure was performed with the assistance of an exposure surgeon (MSW) with extensive experience in anterior spinal procedures. Under general anesthesia, patients were positioned supine on a standard operating table. Through a midline abdominal incision, a left-sided, retroperitoneal exposure of the disc level(s) was performed.
Following radiographic verification of the correct level, the intervertebral disc was removed, leaving only a thin rim of the lateral and posterior annulus. All cartilaginous material was removed from the endplates, but care was taken to avoid violation of the subchondral bony endplate. Normal disc height was re-established (compared to adjacent levels) and an appropriately sized machined, fresh frozen femoral ring allograft was selected. An Infuse™ kit (Medtronic Spine, Memphis, TN, USA) was prepared per the manufacturer’s recommendations and an approximately 1 by 3 cm portion of the sponge was placed into the central canal of the allograft ring. For single-level cases, a small Infuse™ kit was utilized; a medium kit was utilized for two-level procedures. After the allograft spacers were positioned, the remaining Infuse™ sponges were packed into the disc spaces lateral to the allograft spacers. For two-level procedures, the Infuse™ sponges were evenly divided between the two disc spaces. Radiographic verification of correct positioning of the allograft spacers within the disc space was then obtained, followed by routine wound closure.
Under the same general anesthetic, the patients were repositioned prone on a Jackson frame (MisohOSI, Union City, CA, USA). After prepping and draping, the C-arm was utilized to obtain true postero-anterior (PA) views of each vertebra in the fusion construct. Using small, bilateral paramedian incisions, Jamshidi needles were docked at the lateral radiographic borders of the pedicle on the true (PA) view. The Jamshidi needles were then seated to a depth of 20 mm into the bone of the pedicle and the position of the needle tip was documented to lie within the pedicle shadow between one-half to three-quarters of the distance from lateral to medial across the pedicle shadow on the true PA view. Guide wires were placed through the Jamshidi needles into the cancellous bone of the vertebral bodies. All pedicles were cannulated using this technique before switching to a lateral fluoroscopic view. Using the lateral view, the pedicles were tapped and cannulated screws were implanted (Viper system, DePuy Spine, Raynham, MA, USA). Rods were connected to the screws and the constructs were tightened. Final radiographic views were obtained to confirm the position of the final surgical construct. No formal fusion of the posterior column of the spine was performed.
Surgical variables
Estimated blood loss was recorded by the cell salvage technician based on a direct analysis of the suction losses and use of surgical sponges; these estimates were confirmed by the anesthesiology service. Hospital stay was calculated as the number of whole days following surgery until the patient was released from the hospital. Surgical time for the entire procedure was calculated as the number of minutes from the first surgical incision to placement of the final dressing (including repositioning). Surgical time for the anterior portion of the procedure was calculated as the number of minutes from the initial incision to the placement of the anterior dressing. Surgical time for the posterior portion of the procedure was calculated as the number of minutes from the placement of the anterior dressing to the placement of the final posterior dressing (including repositioning).
Complications were collected from a thorough review of the hospital charts and office notes and were defined as any medical event occurring during the operative or postoperative period (until final follow-up) with a negative or potentially negative impact on the patient.
Postoperative care
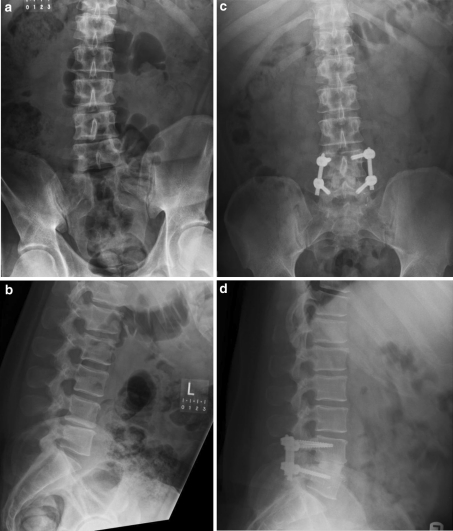
Postoperatively, the patients were mobilized by the physical therapist on postoperative day #1. All patients were started on enoxaparin (Sanofi Aventis, Bridgewater, NJ 08807) on postoperative day #1 for thromboembolic prophylaxis; this was continued for the first 30 days at a dose of 40 mg injected subcutaneously one time per day. A clear liquid diet was started immediately and advanced to solid food as tolerated. Patients were discharged from the hospital when they were independent with ambulation and activities of daily living and were comfortable on oral analgesics and tolerating a regular diet. Patients were evaluated postoperatively at the 2-week, 3-, 6- and 12-month time points for a clinical examination and to obtain radiographs of the lumbar spines. Any complications or complaints were noted in the medical record at each follow-up time point. Plain radiographs were used to judge the status of the fusion. Patients were allowed to progress to unrestricted activities after the 3-month time point and were referred for outpatient rehabilitation with a physical therapist (Fig. 1a, b, c, d).
Fig. 1.
a Preoperative anteroposterior X-ray. b Preoperative lateral X-ray. c Anteroposterior (AP) X-ray shows anterior lumbar interbody fusion (ALIF) supplemented with posterior percutaneous pedicle screw fixation at the L4–L5 level at the 6 months follow-up. d Lateral X-ray shows ALIF supplemented with posterior percutaneous pedicle screw fixation at the L4–L5 level at the 6 months follow-up
Radiographic analysis
Anteroposterior and lateral plain radiographs, taken at the 12-month time point (±2 months), were graded by three observers for the presence of a solid fusion of the interbody space(s). The observers included a spinal surgeon, an experienced spine practice-based physician’s assistant and an experienced spine research physician. The grading system proposed by Bridwell et al. [16] was utilized, which includes four discrete categories: “definitely fused”, “probably fused”, “probably not fused” and “definitely not fused” (Table 1). The observers underwent a training session, using radiographic example of each grading category, prior to commencing with study grading. A disc space was graded as “definitely fused” if there was complete remodeling of the interbody graft with trabeculation across the disc space. A disc space was said to be “probably fused” if both the upper and lower graft junctions showed no radiolucent line at the junction of the allograft and host endplate but complete remodeling was not present. A disc space was said to be “probably not fused” if there was any radiolucent line present at the junction of the allograft and host endplate. A disc space was said to be “definitely not fused” if there was evidence of radiolucency along an entire surface of the allograft, or any lucency around a pedicle screw or any subsidence of the allograft. The observers were blinded to each other grades and to patient identity and clinical status of the patient.
Table 1.
The fusion grading system used for the study which included four discrete categories
| Fusion grade | |
|---|---|
| Definitely fused | Allograft completely remodeled with trabeculae across disc space |
| Probably fused | Graft intact with no lucent lines seen between graft and adjacent endplates |
| Probably not fused | Graft intact, but a radiolucent line is seen between the graft and an adjacent endplate |
| Definitely not fused | Lucency along an entire border of the graft, or lucency around a pedicle screw or subsidence of the graft |
Patient-based clinical outcome measures
A subset of patients (n = 22) provided preoperative and postoperative outcome data at the 12-month time point following the procedure including ODI (V1.3 Survey) and a modified visual analog scale (VAS) (version V1-ordinate) in which they were asked to separately grade their average daily back pain and average daily leg pain.
Statistics
A two-tailed student’s t test was used to compare the change in ODI and VAS for back and leg pain between the preoperative and 12-month postoperative time point. Kappa statistics were used to evaluate the degree of variation between the three observers for fusion grading. Multivariate analysis was used to compare the variables of age, gender, diagnosis, number of surgical levels, fusion rate and complication rate for the subset of patients with ODI and VAS data to the whole patient cohort.
Results
Overall, 50 patients were included in the study. There were 24 females (48%) and 26 males (52%) with a mean age of 48.2 years (age range of 32–84). All patients had a preoperative diagnosis of a degenerative spine disease with the subcategories listed in Table 2. A total of 24 patients (48%) underwent a single-level fusion, 26 patients (52%) had a fusion at two levels for a total of 76 levels fused with the specific levels and combinations of levels shown in Table 3. The overall surgical time from the initial incision to the placement of the final surgical dressing (including repositioning) ranged from 127 to 389 min (mean 265 min). When analyzed on a per level basis, the time for the whole procedure averaged 176 min per level. The anterior portion of the procedure (from the induction of anesthesia to the placement of the anterior dressing) ranged from 74 to 200 min (mean 131 min). Overall, the time for the anterior procedure averaged 87 min per level.
Table 2.
The preoperative diagnoses with the number of patients in each category
| Preoperative diagnoses | Number of patients |
|---|---|
| Spondylolisthesis | 23 |
| Painful degenerative disc disease | 24 |
| Recurrent herniated disc disease | 2 |
| Painful spondylolysis | 1 |
Table 3.
The number of each level fused and the combinations of level for each patient
| Specific levels | # | Level combinations | # |
|---|---|---|---|
| L2–L3 | 1 | L2–L3, L3–L4 | 1 |
| L3–L4 | 3 | L3–L4, L4–L5 | 2 |
| L4–L5 | 30 | L4–L5 | 5 |
| L5–S1 | 42 | L4–L5, L5–S1 | 23 |
| Total | 76 | L5–S1 | 19 |
The posterior procedure (from the beginning of the repositioning to the placement of the posterior surgical dressing) ranged from 79 to 279 min (mean 102 min). When analyzed on a per level basis, the time for the posterior procedure averaged 95 min per level. The estimated blood loss for the entire operation (anterior and posterior) ranged from 50 to 900 ml (mean 288 ml). The length of hospitalization ranged from 1 to 7 days (mean 4 days).
The presence of a solid fusion at the operative levels was evaluated in a blinded fashion by three observers using a modified scale proposed by Bridwell et al. [16] Overall, the mean fusion grading was 61% “definitely fused”, 31% “probably fused”, 8% “probably not fused” and 0% “definitely not fused”. Comparison of the interobserver variation of fusion grades produced a kappa value of 0.6 indicating a moderate degree of agreement between observers (Table 4).
Table 4.
The fusion grading for each of the three observers
| Fusion grade | Observer #1 (%) | Observer #2 (%) | Observer #3 (%) | Mean of observer grading (%) |
|---|---|---|---|---|
| Definitely fused | 49 (70) | 44 (62.8) | 41 (58.6) | 61.1 |
| Probably fused | 18 (25.7) | 21 (30) | 22 (31.4) | 30.7 |
| Probably not fused | 3 (4.3) | 5 (7.2) | 7 (10) | 8.2 |
Complications were collected from the hospital records and office charts. The overall complication rate (all postoperative complications) was 12%. See Table 5 for a list of complications observed in this clinical series. When analyzed further, the rate of intraoperative complications was 0%. The rate of postoperative complications was 12%. The most common postoperative complication was a urinary tract infection. Notably, there were no cases of wound infection, thromboembolic disease, symptomatic pseudarthrosis, hardware loosening or hardware failure in the study cohort. No patient required return to the OR for hardware repositioning.
Table 5.
The complications encountered in the study
| Complications | # of patients |
|---|---|
| Ileus requiring an NG tube for 2 days | 1 |
| Scrotal edema | 1 |
| Tachycardia, transient hypotension with trace pericardial effusion (medically managed) | 1 |
| Urinary retention | 1 |
| Urinary tract infection | 2 |
For the 22 patients with complete preoperative and postoperative ODI and VAS data, there were 13 males and 9 females with average age of 47.0 years (range 31–73). The mean modified VAS score for back pain preoperatively was 8.0 ± 1.8, which improved to a mean of 3.0 ± 2.5 postoperatively at the 12-month time point (p < 0.0001). The mean VAS score for leg pain was 6.0 ± 2.5 preoperatively and improved to a mean of 2.0 ± 2.1 postoperatively at the 12-month time point (p < 0.0001). The mean ODI score was 47.0 ± 17.5 preoperatively; this improved to a mean of 28.0 ± 10.2 postoperatively at the 12-month time point (p < 0.001). The improvements in VAS scores for both back and leg pain and the improvement in physical functioning as measured by ODI scores were highly statistically significant. When patient variables were compared between the subset of patients providing VAS and ODI data (outcome subset) to the entire study cohort, only the gender distribution demonstrated a significant difference in the outcome subset, due to a larger proportion of males in the outcome subset compared to the entire study cohort.
Discussion
This study was performed to assess a specific approach for lumbar fusion in degenerative conditions of the lumbar spine: ALIF using rhBMP-2 and allograft combined with percutaneous posterior pedicle screw fixation. To our knowledge, this is the first clinical series of this type to evaluate pertinent clinical outcome measures using this surgical technique. Overall, there was a high fusion rate with over 92% of patients achieving a fusion grade of either “definitely fused” or “probably fused” at the 12-month postoperative time point.
Fusion success was assessed using the previously reported grading system of Bridwell et al. [16] To control for potential variation between observers, kappa statistics were used to define the magnitude of agreement between observers. Kappa statistics are appropriate to measure variation between observers when two or more independent observers evaluate the same parameters. [17] Moderate agreement was found between the observers in the current study, which is consistent with other studies employing a clinical fusion grading scheme [18]. Other authors have studied fusion success following anterior/posterior arthrodesis procedures. El Masry et al. [19] reported an overall fusion success of 97% in 47 patients treated by ALIF, using autogenous iliac crest bone combined with posterior pedicle fixation. Moore et al. [20] studied 58 patients treated with an anterior arthrodesis and posterior instrumentation and found a solid fusion in 95%. Sarwat et al. [21] in a study of 43 patients who underwent ALIF with allograft bone followed by a posterior instrumented fusion, reported a radiographic fusion rate of 100% for single-level procedures and 93% fusion in two-level procedures. Finally, Liljenqvist et al. [22] reported the overall fusion rate to be 95.2% in a study of 41 patients who were treated with ALIF, using a cortical femoral ring allograft filled with autologous iliac crest bone, and posterior arthrodesis with translaminar facet screws. The present fusion rate of 92% is comparable to other reports of circumferential (anterior/posterior) lumbar arthrodesis procedures, despite the fact that the grading scheme we used for fusion was more rigorous than those used in most of the prior reports.
The high rate of successful fusion in this study is most likely due to the benefits of an ALIF procedure using the osteoinductive agent rhBMP-2 combined with rigid stabilization provided by percutaneous pedicle screw fixation. ALIF provides a large surface area for fusion and places the graft under compression, while the adjacent endplates provide a rich blood supply. rhBMP-2 has been shown to be strongly osteoinductive, leading to a high rate of fusion in mechanically stable ALIF constructs [23, 24]. The combination of allograft bone and rhBMP-2 appears to provide rapid radiographic remodeling of the allograft bone, as shown by the fact that over 60% of the cases demonstrated complete remodeling of the allograft by the 12-month time point.
The findings of this series are starkly different from those reported by Pradhan et al. [25], who reported a high rate of graft collapse and pseudarthrosis following “stand-alone” ALIF procedures using femoral ring allograft and rhBMP-2. The difference, of course, is that in the Pradhan series, no posterior fixation was utilized, thus the mechanical stability of the construct was insufficient to prevent collapse of the allograft during the rapid remodeling created by rhBMP-2. Therefore, stable fixation is important to the success of a lumbar interbody fusion using structural allograft bone and rhBMP-2.
The overall surgical time for this combined anterior and posterior procedure is longer than that reported by others for either anterior-alone or posterior-alone procedures [26], but is similar to that reported by other authors for anterior/posterior (360°) fusion procedures. Thalgott et al. [1] reported a mean operative time of 183 min for a single-level fusion (anterior 97 min, posterior 86 min) and a mean operative time of 215 min for two-level fusions (anterior 130 min, posterior 85 min) despite the use of translaminar facet screws for the posterior instrumentation, which tends to be more rapid to implant compared to pedicle screws. When performing anterior and posterior procedures, additional time is required for repositioning, and prepping and draping of the second operative field. However, the exposure and closure time for the posterior portion of the procedure is reduced with percutaneous pedicle screw fixation when compared with a traditional, open approach for pedicle screw placement.
The overall complication rate of 12% was consistent with prior reports [4–8], although certain complications such as wound infection, construct failure or pseudarthrosis were not seen [27]. The lack of posterior wound infections is most likely due to the use of small percutaneous incisions in the posterior column of the spine, which are relatively less likely to develop a wound infection compared with traditional, open exposures. Low infection rates in percutaneous spinal approaches have been previously described [28, 29]. Anterior infections, in our study, as with other studies, were not common [30].
No approach-related complications, such as vascular injury, were encountered in the present study. However, vascular complications are a known, significant risk with ALIF procedures. Oskouian et al. [4] reported an incidence of vascular complications following anterior thoracolumbar surgery of 5.8%, with a resultant mortality of 1% in their series.
Garg et al. [5] reported vascular injuries in 6.1% of cases; all were treated without perioperative mortality. In the opinion of the authors, the assistance of a skilled exposure surgeon is beneficial in avoiding and managing vascular complications with anterior spinal exposures. Patient outcome is the most important issue in evaluating the utility of a therapeutic procedure. The preferred method of evaluating patient outcome is to use validated, patient-based outcome measures such as the ODI and VAS. In our study, a subset of 22 patients provided preoperative and postoperative ODI and VAS scores for both back and leg pain. The results suggested that these patients sustained highly significant improvements in back and leg pain and in physical functioning. The subset of patients who provided ODI and VAS data were statistically similar in age, diagnosis, number of surgical levels, fusion rate and complication rate when compared with the overall study cohort, although there was a higher ratio of males in this subset compared to the overall cohort.
Others have studied the outcome following anterior/posterior lumbar arthrodesis including Matejka et al. [31], who prospectively studied a non-randomized cohort of 40 patients undergoing ALIF with tantalum cages followed by posterolateral stabilization. In this study, a 100-mm VAS scale was used. Patients reported a mean initial back pain score of 58 mm prior to surgery, which improved to 18 mm at the 1-year postoperative time point. In the same study, the VAS scores for leg pain improved from 54 mm preoperatively to 9 mm at the 1 year time point. McKenna et al. [32] reported 83 prospectively studied patients who underwent ALIF followed by posterior stabilization and also found a significant improvement in VAS at the 6-, 12- and 24-month postoperative time points. Park et al. [33] studied 29 patients with degenerative spinal disease who underwent ALIF followed by percutaneous translaminar facet screw fixation and reported improvement in VAS scores from 6.6 to 1.5 for back pain and 7.5 to 1.8 for leg pain.
Certain limitations of the current study must be acknowledged. The retrospective data analysis and lack of a control group limit a direct comparison of this approach to other operative techniques for lumbar arthrodesis. However, these factors are partially offset by the fact that the present patient cohort represents a consecutive series performed by a single surgeon, reducing the variable of surgical technique. A second limitation of the present study is the lack of complete ODI and VAS data for the entire study cohort.
Although most surgical variables were similar between the outcome data subset and the entire study cohort, there was a higher proportion of males in the outcome data subset. Also, plain radiographs were used in conjunction with a standardized grading scheme to assess the presence of fusion in this study. Fine-cut CT scanning has recently been the modality of choice when assessing fusion rates; however, CT scans were not available as they were not part of our normal “standard of care” when following patients after lumbar fusion. Despite this, it should be acknowledged that the grading of an anterior arthrodesis when using allograft bone is straightforward using plain radiographs, as the graft/endplate junction and bony remodeling can be directly visualized.
Conclusion
Anterior lumbar interbody fusion using rhBMP-2 and structural allograft bone, combined with percutaneous posterior pedicle screw fixation, is a useful surgical strategy for degenerative conditions of the lumbar spine requiring arthrodesis including spondylolisthesis and painful degenerative disc disease. The present study illustrates the high fusion rate and favorable clinical improvement that can be obtained with this approach. This approach has certain disadvantages that must be considered, including potentially longer surgical times, the need for a competent exposure surgeon and experience with percutaneous pedicle screw placement. Future studies prospectively comparing the clinical outcome and cost between various methods of lumbar fusion would be useful.
Acknowledgments
The authors wish to thank Andrew Miller, a medical doctor candidate, for assistance in the statistical analysis of this paper.
Conflict of interest None.
References
- 1.Thalgott JS, Chin AK, Ameriks JA, Jordan FT, Giuffre JM, Fritts K, Timlin M. Minimally invasive 360 degrees instrumented lumbar fusion. Eur Spine J. 2000;9(Suppl 1):S51–S56. doi: 10.1007/PL00010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vargas-Soto HA, Mehbod A, Mullaney KJ, Dykes D, Schwender J, Transfeldt E, Akesen B, Wroblewski J. Salvage procedures for pseudarthrosis after transforaminal lumbar interbody fusion (TLIF)- anterior-only versus anterior–posterior surgery: a clinical and radiological outcome study. J Surg Orthop Adv. 2009;18(4):200–204. [PubMed] [Google Scholar]
- 3.Jang JS, Lee SH. Clinical analysis of percutaneous facet screw fixation after anterior lumbar interbody fusion. J Neurosurg Spine. 2005;3(1):40–46. doi: 10.3171/spi.2005.3.1.0040. [DOI] [PubMed] [Google Scholar]
- 4.Oskouian RJ, Jr, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96(Suppl 1):1–5. doi: 10.3171/jns.2002.96.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Garg J, Woo K, Hirsch J, Bruffey JD, Dilley RB. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 2010;51(4):946–950. doi: 10.1016/j.jvs.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Scaduto AA, Gamradt SC, Yu WD, Huang J, Delamarter RB, Wang JC. Perioperative complications of threaded cylindrical lumbar interbody fusion devices: anterior versus posterior approach. J Spinal Disord Tech. 2003;16(6):502–507. doi: 10.1097/00024720-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J. 2002;2(3):216–223. doi: 10.1016/S1529-9430(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 8.Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(1 Suppl):60–64. doi: 10.3171/spi.1999.91.1.0060. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech. 2002;15(5):355–361. doi: 10.1097/00024720-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Nichols TA, Yantzer BK, Alameda S, Johnson WM, Guiot BH. Augmentation of an anterior lumbar interbody fusion with an anterior plate or pedicle screw fixation: a comparative biomechanical in vitro study. J Neurosurg Spine. 2003;6(3):267–271. doi: 10.3171/spi.2007.6.3.267. [DOI] [PubMed] [Google Scholar]
- 11.Alt V, Chhabra A, Franke J, Cuche M, Schnettler R, Le Huec JC. An economic analysis of using rhBMP-2 for lumbar fusion in Germany, France and UK from a societal perspective. Eur Spine J. 2009;18(6):800–806. doi: 10.1007/s00586-009-0935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyer RD, Tromanhauser SG, Regan JJ. An economic model of one-level lumbar arthroplasty versus fusion. Spine J. 2007;7(5):558–562. doi: 10.1016/j.spinee.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Kang BU, Jeon SH, Park JD, Maeng DH, Choi YG, Choi WC. Revision surgery of the lumbar spine: anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation. J Neurosurg Spine. 2006;5(3):228–233. doi: 10.3171/spi.2006.5.3.228. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Choi WG, Lim SR, Kang HY, Shin SW. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J. 2004;4(6):644–649. doi: 10.1016/j.spinee.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Saraph V, Lerch C, Walochnik N, Bach CM, Krismer M, Wimmer C. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13(5):425–431. doi: 10.1007/s00586-004-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20(12):1410–1418. [PubMed] [Google Scholar]
- 17.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 18.Christensen FB, Laursen M, Gelineck J, Eiskjaer SP, Thomsen K, Bunger CE. Interobserver and intraobserver agreement of radiograph interpretation with and without pedicle screw implants: the need for a detailed classification system in posterolateral spinal fusion. Spine. 2001;26(5):538–543. doi: 10.1097/00007632-200103010-00018. [DOI] [PubMed] [Google Scholar]
- 19.El Masry MA, Badawy WS, Rajendran P, Chan D. Combined anterior interbody fusion and posterior pedicle screw fixation in patients with degenerative lumbar disc disease. Int Orthop. 2004;28(5):294–297. doi: 10.1007/s00264-004-0587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KR, Pinto MR, Butler LM. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine. 2002;27(15):1680–1686. doi: 10.1097/00007632-200208010-00018. [DOI] [PubMed] [Google Scholar]
- 21.Sarwat AM, O’Brien JP, Renton P, Sutcliffe JC. The use of allograft (and avoidance of autograft) in anterior lumbar interbody fusion: a critical analysis. Eur Spine J. 2001;10(3):237–241. doi: 10.1007/s005860000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liljenqvist U, O’Brien JP, Renton P, et al. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J. 1998;7(2):125–131. doi: 10.1007/s005860050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16(2):113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28(4):372–377. doi: 10.1097/01.BRS.0000048469.45035.B9. [DOI] [PubMed] [Google Scholar]
- 25.Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31(10):E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 26.Freudenberger C, Lindley EM, Beard DW, Reckling WC, Williams A, Burger EL, Patel VV. Posterior versus anterior lumbar interbody fusion with anterior tension band plating: retrospective analysis. Orthopedic. 2009;32(7):492. doi: 10.3928/01477447-20090527-12. [DOI] [PubMed] [Google Scholar]
- 27.Jutte PC, Castelein RM. Complications of pedicle screws in lumbar and lumbosacral fusions in 105 consecutive primary operations. Eur Spine J. 2002;11(6):594–598. doi: 10.1007/s00586-002-0469-8. [DOI] [PubMed] [Google Scholar]
- 28.Rauzzino MJ, Shaffrey CI, Nockels RP, Wiggins GC, Rock J, Wagner J. Anterior lumbar fusion with titanium threaded and mesh interbody cages. Neurosurg Focus. 1999;7(6):e7. doi: 10.3171/foc.1999.7.6.10. [DOI] [PubMed] [Google Scholar]
- 29.O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine. 2009;11(4):471–476. doi: 10.3171/2009.5.SPINE08633. [DOI] [PubMed] [Google Scholar]
- 30.Sasso RC, Best NM, Mummaneni PV, Reilly TM, Hussain SM. Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine. 2005;30(6):670–674. doi: 10.1097/01.brs.0000155423.18218.75. [DOI] [PubMed] [Google Scholar]
- 31.Matejka J, Zeman J, Belatka J. Mid-term results of 360-degree lumbar spondylodesis with the use of a tantalum implant for disc replacement. Acta Chir Orthop Traumatol Cech. 2009;76(5):388–393. [PubMed] [Google Scholar]
- 32.McKenna PJ, Freeman BJ, Mulholland RC, Grevitt MP, Webb JK, Mehdian SH. A prospective, randomised controlled trial of femoral ring allograft versus a titanium cage in circumferential lumbar spinal fusion with minimum 2-year clinical results. Eur Spine J. 2005;14(8):727–737. doi: 10.1007/s00586-005-1034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SH, Park WM, Park CW, Kang KS, Lee YK, Lim SR. Minimally invasive anterior lumbar interbody fusion followed by percutaneous translaminar facet screw fixation in elderly patients. J Neurosurg Spine. 2009;10(6):610–616. doi: 10.3171/2009.2.SPINE08360. [DOI] [PubMed] [Google Scholar]