Abstract
We investigate the anchorage of pedicle screws with different surface treatments in osteoporotic bone. Eight ewes were divided into two groups of four animals each: four sheep underwent bilateral ovariectomy (OVX Group), whereas the operation was simulated in the remaining group (SHAM Group). Eighteen months after the first operation, the Dynesys® System was fitted to the sheep using pedicle screws with three different surface treatments: untreated, rough blasted (uncoated) and bioactive coated (bioactive). Uncoated screws showed a significantly higher bone ingrowth value compared with the untreated screws in the OVX group (9.3%, p < 0.005) and a significantly lower bone ingrowth value in the SHAM group (−11.0%, p < 0.05). Furthermore, the bioactive pedicle screws had a significant lower bone ingrowth value than the untreated screws in the SHAM group (−12.1%, p < 0.05). These results suggest that both tested surface treatments of pedicular screws may provide an advantage in terms of bone quality and osseointegration, when implanted in osteoporotic vertebrae.
Keywords: Pedicle screws, Surface treatment, Osteoporosis, Osseointegration
Introduction
To overcome complications related to disc degeneration at the adjacent hypermobile segments occurring in spinal fusion surgery, non-fusion systems have been developed, such as dynamic stabilization devices, which are able to control the segment motion and share the load with the disc [1–7]. Dynamic stabilization devices are divided in two groups according to the way they are appended to the spinal segment: interspinous and pedicle-screw-based devices. Interspinous implants are inserted between the posterior processes, after posterior ligament removal and some designs are secured in place by laces. Pedicle screw systems are placed between the posterior processes and fixed by using screws that anchor the system onto the pedicles and vertebral body. Anchoring the screws to the bone is crucial for the success of these implants. Screw loosening was particularly evident during the instrumentation of vertebral bodies with low mineral density or patients with osteoporosis [8–10].
Due to the demographic development and the increasing average age of the population, treatment of these conditions often involves instrumentation of osteoporotic spinal segments and the indications for placing instrumentation in patients with osteoporosis will likely continue to broaden [11]. Cancellous bone is more affected by osteoporosis than cortical bone, and it is not surprising that pedicle screw fixation is significantly less effective in patients with low bone mineral density, as its anchorage relies also on the properties of the cancellous bone within the vertebral body [12]. A variety of methods have been employed clinically to improve pedicle screw fixation [12–14], including the use of cannulated pedicle screws allowing cement injection, and bone cement augmentation, but no technique has shown to provide a substantial improvement in fixation strength without the risk of neural injury or anterior body penetration and possible vascular or visceral injury [12]. However, an appropriate surface design (e.g. roughness, porosity) and a coating with bioactive materials (e.g. hydroxyapatite, fluorohydroxyapatite) have been recognized to improve the rate of osseointegration in both healthy and osteoporotic conditions and both the axial and appendicular skeleton [13, 15–19]. The use of screws with surface properties capable of stimulating bone ingrowth at the bone–screw interface, which leads to good accelerated osseointegration, can obviously reduce the number of implant failures.
The dynamic neutralization system for spine stabilization—Dynesys® (Dynesys®—Zimmer Spine, Winterthur, Switzerland) is a pedicle screw-based system allowing flexible stabilization of the spine, thus restoring the biomechanics of the posterior annulus and facet joints [20]. It consists of titanium alloy screws connected by an elastic synthetic compound that stabilize the affected joints at rest, in flexion and in extension. The aim of the present study was to evaluate the performance of different pedicular screw designs, implanted in both healthy and osteoporotic bone. In particular, a preliminary evaluation of new surface treatments for the pedicle screws of the Dynesys® system was performed by placing them in ovariectomized and sham-operated sheep. Bone–screw osseointegration and the quality of the implanted host bone were measured by histomorphometry of pedicular trabecular bone and iliac crest bone.
Materials and methods
Pedicle screw surface treatments
Ninety pedicle screws adapted from the Dynesys screw design, 4.4 mm in diameter and 25 mm in length, made of Ti-6Al-7Nb (PROTASUL® 100), were used. Thirty of them were used as controls (untreated), whereas the others were used as substrates for two different surface treatments: rough blasting (uncoated) and bioactive coating (bioactive) [21, 22]. The chemical treatment consisted of passivation in 35 wt% HNO3 (25°C for 30 min) and alkali etching in 5 M NaOH (60°C for 24 h). The heat treatment was performed by dwelling the samples at 600°C for 1 h. The uncoated screws were characterized by the Ra value = 4–6 μm. The main characteristics of the treated surfaces are reported elsewhere [21, 24, 25].
Surface morphology and composition on five screws for each type of surface treatment were assessed by scanning electron microscopy (SEM Philips 525 M) and energy dispersion spectrometry (EDS Philips-EDAX 9100) The bioactivity of the treated samples was tested by soaking in standard simulated body solution (SBF) for 5–30 days at 37°C. The soaked samples were analyzed by SEM–EDS, to verify the precipitation of hydroxyapatite on their surfaces (n = 5).
All the implants were cleaned and degreased using a detergent solution in an ultrasound bath (30°C for 30 min), rinsed in distilled water (30°C for 30 min), air-dried at 50°C for 30 min and then at 180°C for 60 min. Finally all the implants were stored in individual gamma-sterilized surgical packs that were opened just before their insertion in the bone.
Animal study
The study was performed in compliance with European and Italian Law on animal experimentation: the animal experimental protocol was approved by the Ethical Committee of Rizzoli Orthopedic Institute and by the Italian Ministry of Health.
After a quarantine period, eight crossbred adult ewes, 75.5 ± 4.5 kg b.w., aged 7 ± 1 years at the beginning of the study, were randomly divided into two groups of four animals each and submitted to surgery under general anesthesia: four sheep underwent bilateral ovariectomy (OVX group), while the operation was simulated in the remaining group (SHAM group).
The sheep were premedicated with an i.m. injection of 10 mg/kg ketamine and 0.3 mg/kg xylazine, and with an s.c. injection of 0.0125 mg/kg atropine sulfate. General anesthesia was induced with 10 mg/kg i.v. sodium thiopentone (2.5% solution) and maintained with 60%/40% O2/N2O and 1.5–2% fluothane. Postoperatively, antibiotics and analgesics (cephalosporin 1 g/day for 5 days and ketoprofen 500 mg/day for 3 days) were administered i.m.
Implantation surgery
At least 18 months after the first surgery, the sheep underwent a second operation to place the Dynesys® system. After incising the skin and sectioning the fasciae, the lumbar vertebrae (L1 to L6) were exposed. The cortical bone of both pedicles of L2, L3, L4 and L5 were pre-drilled and Dynesys® pedicle screws were implanted by placing one design per vertebra, according to the scheme shown in Table 1. Then, Universal Spacers (SULENE®-PCU) and Cords (SULENE®-PET) were applied bilaterally between L2 and L3 and between L4 and L5 using Dynesys® specific equipment. Postoperatively, the sheep received the same antibiotics and analgesics treatment previously adopted.
Table 1.
List of pedicle screws implanted bilaterally in lumbar vertebrae of OVX and SHAM sheep divided by sheep and vertebrae id
| Group | Vertebrae | |||
|---|---|---|---|---|
| L2 | L3 | L4 | L5 | |
| OVX | ||||
| 1 | Untreated | Bioactive | Uncoated | Untreated |
| 2 | Bioactive | Untreated | Bioactive | Uncoated |
| 3 | Uncoated | Bioactive | Untreated | Untreated |
| 4 | Uncoated | Uncoated | Bioactive | Bioactive |
| SHAM | ||||
| 1 | Untreated | Bioactive | Uncoated | Untreated |
| 2 | Bioactive | Untreated | Bioactive | Uncoated |
| 3 | Uncoated | Untreated | Untreated | Uncoated |
| 4 | Uncoated | Bioactive | Bioactive | Untreated |
The different pedicle screws were randomized to eliminate the influence of the single vertebra. Those underscored or in italic bold showed signs of infections (Escherichia coli–Morganella Morganii; Enterococcus–Bacillus species) or aseptic osteolysis, respectively, in only one pedicle per vertebra
Bioactive bioactive coating, Uncoated uncoated blasting, Untreated no surface treatments
Finally, 4 months after the implantation surgery the animals were anesthetized and then euthanized with an i.v. injection of 1 ml Tanax (Hoechst AG, Frankfurt-am-Main, Germany). The lumbar spines L1–L5 were removed and stripped of soft tissue. X-rays of the lumbar spines were taken to check the presence of osteolysis and the maintenance of the correct position of the transpedicular screws. The L1 lumbar vertebrae were removed and used for densitometric investigations. Then, the spines were processed for histology and histomorphometry.
Bone quality assessment
Immediately before ovariectomy or sham surgery and at the moment of the second surgery (pedicle screw implantation) a right and left transiliac biopsy, respectively, was vertically performed in all of the animals. Biopsies were fixed in 4% buffered paraformaldehyde and processed for histology.
Bone mineral content (BMC, g) and density (BMD, g/cm2) of the vertebral pedicles of L1 were measured using dual X-ray absorptiometry (Norland XR 26 Mark II densitometer, Norland Corp. Fort Atkinsons, USA) and its research mode. Before taking the measurements, the instrument was calibrated in a Norland phantom. Vertebrae were scanned in an anterior to posterior direction. A tubular Plexiglas device was used to hold the bones in the position for scanning and the region of interest (ROI) of the pedicles was determined using the same anatomical landmarks for each bone. To avoid any positioning error, the repeatability of the densitometric protocol was assessed on five occasions on one of these bones; after each scan, the bone was repositioned for the next scan. The intraoperator precision of DXA techniques was 2.0%.
Finally, to check the quality of bone at the implantation site, histomorphometric evaluations were also performed in a defined area of the pedicles of the implanted vertebrae (L2–L5) as described below.
Histology and histomorphometry
The lumbar vertebral containing the screws were sectioned using the system EXAKT (EXAKT Apparatus GmbH, Norderstedt, Germany). The bone specimens were fixed in 4% buffered paraformaldehyde for 48 h.
Undecalcified iliac crest biopsies and vertebrae were dehydrated in graded series of alcohols until absolute and processed for methacrylate embedding. Iliac crest and vertebrae blocks were sectioned transversally along a plane parallel to the long axis of the implant using the same cutting–grinding system. Sections (20-μm thick) were stained with Solochromo-cyanine (iliac crest biopsies) or with fuchsin acid and fast green (vertebral pedicles) and then used for histomorphometric analysis.
Bone histomorphometric measurements were performed semi-automatically in specific regions of interest (ROIs) of iliac crest and vertebrae sections (3 sections for each site) by using an optic microscope (BX41, Olympus Optical Co. Europa GmbH, Germany) connected to an image analyzer system (Qwin, Leica Imaging Systems Ltd., United Kingdom). The ROIs of 2,776 × 2,074 pixels were grabbed at a magnification of 4×; the ROIs of iliac crest were located between the two cortexes, while those of vertebrae were located in an area corresponding to the implant profile, periimplant region and adjacent host bone, comprising pedicles.
To evaluate the screw osseointegration the following measurements were made [23]:
Bone ingrowth (%): the amount of bone growth inside the gap between the implant and the host bone, measured in an area located between the root and the top of the threads independently of direct bone-to-implant contact;
Bone-to-implant contact (%): the amount of bone contact at the interface, defined as the percentage of implant length showing a direct bone-to-implant contact without any intervening soft-tissue layers.
For the evaluation of bone quality, the following measurements were performed and calculated in the iliac crest and in vertebral pedicle specimens by following nomenclature approved by the American Society of Bone and Mineral Research (ASBMR) [24]: cortical thickness (Ct.Th, μm) of vertebral pedicles; trabecular bone volume (BV/TV, %); trabecular thickness (Tb.Th, μm); trabecular number (Tb.N, /mm); trabecular separation (Tb.Sp, μm).
Statistical analysis
Statistical analysis was performed using the SPSS v.12.1 SOFTware (SPSS Inc., Chicago, Illinois, USA). Data are reported at a significance level of p < 0.05. After having verified the normal distribution of data, the one-way ANOVA followed by the post hoc tests were performed to analyze histomorphometric data between groups. Student’s t test was used to compare histomorphometric data of vertebral pedicle cortical and cancellous bone around pedicle screws between SHAM and OVX sheep.
Results
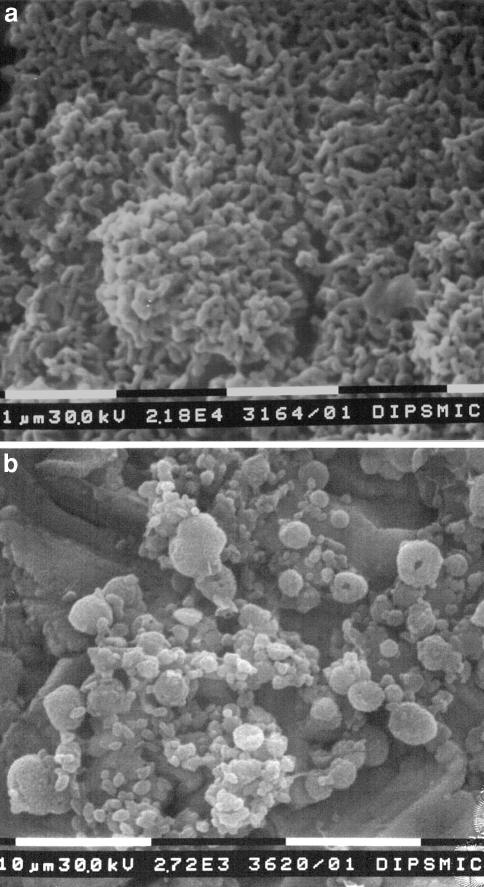
The chemical treatment modifies the morphology of the implant surface, producing a micro-porous layer (diameter of micro-pores about 0.1 μm). The heat treatment at 600°C caused a densification of the porous surface and the growth of spherical and thickened particles (Fig. 1a). Their diameter is about 80 nm. No evident compositional changes were seen by EDS after the treatments. Thus, the thermo-chemical process produced a homogeneous nanometric textured layer. The bioactivity of the surface was tested in vitro by soaking in SBF. Precipitates, shown by EDS analysis to be rich in calcium, appear on the surface of the treated samples after soaking for 5 days in SBF. They evolve into bigger apatite crystals after more time in SBF (14–30 days) (Fig. 1b).
Fig. 1.
SEM microphotograph of the treated implant surface before (a) and after (b) soaking in SBF (30 days). Apatite crystals are observable. In the presence of body fluids, this treated surface induces apatite nucleation at the bone implant interface, resulting in a dense and homogenous calcium-phosphate layer at the surface and a graded structure down to the substrate metal with no distinct implant-coating interface
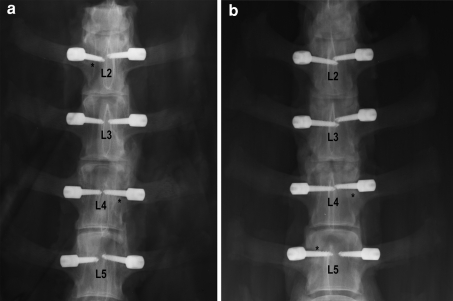
No postoperative clinical complications were observed. When the lumbar spines were retrieved, macroscopic signs of osteolysis or infection were found around some implants confirmed also by radiography (Fig. 2). To clarify the origin of these signs a biopsy of tissue around the screws was taken for microbiological tests and bacterial culture. Of the entire pedicle screws retrieved, those shown in Table 1 presented complications due to infection or signs of aseptic osteolysis observed macroscopically.
Fig. 2.
Anteroposterior X-rays of lumbar spines of OVX_3 (a) and SHAM_1 (b) sheep. Asterisks indicate pedicle screw loosening
Histomorphometric analysis performed on the iliac crest biopsies during implantation surgery showed marked generalized trabecular bone rarefaction in both groups due to aging for the SHAM group and estrogen deficiency for the OVX group (Table 2). As expected, bone rarefaction was more severe in estrogen deficient animals than that observed in physiological aging. In more detail, BV/TV in the OVX group decreased significantly when compared to the Baseline (−29%, p < 0.0005) and SHAM (−19%, p < 0.005) groups. Tb.Th decreased significantly by −22% (p < 0.0005) and −15% (p < 0.005) in the OVX group in comparison with the Baseline and SHAM groups, respectively. Finally, Tb.Sp increased significantly in the OVX group by 27% (p < 0.0005) in comparison with the Baseline group, and by 14% (p < 0.005) when compared to the SHAM group.
Table 2.
Histomorphometric results of iliac crest biopsies at the beginning of the study (Baseline) and at the second operation for SHAM and OVX sheep (Mean ± SD)
| Parameter | Unit | Baseline (n = 8) | SHAM (n = 4) | OVX (n = 4) |
|---|---|---|---|---|
| BV/TV | % | 34.9 ± 3.2 | 30.8 ± 4.1** | 24.8 ± 5.6***,°°° |
| Tb.Th | μm | 112.9 ± 20.7 | 103.9 ± 8.8* | 88.5 ± 21.3***,°° |
| Tb.N | /mm | 3.2 ± 0.6 | 3.0 ± 0.3 | 3.1 ± 0.3**,° |
| Tb.Sp | μm | 210.7 ± 33.8 | 234.9 ± 22.4** | 267.9 ± 37.9***,°°° |
Cortical thickness (Ct.Th, μm) the thickness of pedicular cortical bone; Trabecular bone volume (BV/TV, %): the whole spongy bone area expressed as a percentage of the total tissue area at the sampling site and converted to a volume; Trabecular thickness (Tb.Th, μm) given by 2/(BS/BV), where BS/BV is the ratio B. Pm/B. Ar multiplied by a factor of 1.199; Trabecular number (Tb.N,/mm) [(BV/TV)/Tb. Th]; Trabecular separation (Tb. Sp, μm) 1/Tb. N–Tb. Th
Dunnet’s t test between SHAM and OVX versus Baseline: * p < 0.05; ** p < 0.005; *** p < 0.0005
Scheffé’s multiple comparison test of OVX versus SHAM groups: ° p < 0.05; °° p < 0.005; °°° p < 0.0005
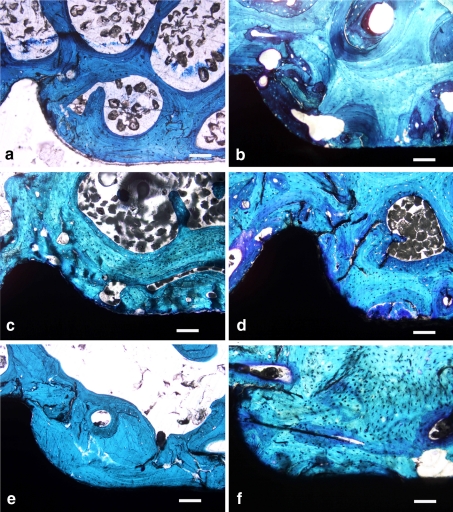
The histological appearances of the pedicle screws in aged and osteopenic bone are shown in Fig. 3. Except for infected screws (only the contralateral one in the same vertebra was considered) all the implanted screws were used for histological and histomorphometric assessment by analyzing the average values of the screws implanted in the same vertebra. The trabecular bone around the screws showed signs of rarefaction in the OVX group and, particularly, the BV/TV (−36%, p < 0.005) and Tb.Sp (43%, p < 0.005) parameters were significantly different from those of the SHAM group (Table 3). These results confirmed the development of osteopenia at vertebral body and pedicle level and that it was worse than that found in the iliac crest in the second operation.
Fig. 3.
Histologies of bioactive coated (a, b), rough blasted (c, d) and untreated (e, f) pedicle screws implanted in the vertebral pedicles (L2–L5) of OVX (a, c, e) and SHAM (b, d, f) sheep at a magnification of ×10 (Fuchsin acid and Fast green) (Scale bar 400 μm). All tested screws are in direct contact with peri-implant bone. Signs of bone rarefaction are evident in OVX sheep. The presence of coatings improves the screw–biomaterial interface even in the presence of osteopenia and no areas of biomaterial not in direct contact with bone are visible
Table 3.
Histomorphometric results of vertebral pedicle cortical and cancellous bone around pedicle screws for SHAM and OVX sheep (Mean ± SD)
| Parameter | Unit | SHAM (n = 4) | OVX (n = 4) |
|---|---|---|---|
| Ct.Wi | μm | 772.7 ± 153.9 | 788.6 ± 175.5 |
| BV/TV | % | 42.2 ± 6.6 | 27.1 ± 7.7* |
| Tb.Th | μm | 109.8 ± 46.5 | 98.9 ± 32.6 |
| Tb.N | /mm | 3.0 ± 1.2 | 2.5 ± 1.4 |
| Tb.Sp | μm | 262.3 ± 48.6 | 374.5 ± 56.9* |
Student’s t test: ** p < 0.005
Densitometric analysis performed at the end of the study on the retrieved L1 vertebral pedicles showed that BMD of OVX group (0.77 ± 0.14 g/cm2) was significantly reduced by 22% (p < 0.01) in comparison to SHAM group (0.99 ± 0.18 g/cm2), whereas no significant difference was found for BMC (OVX group: 4.25 ± 1.37 g; SHAM group: 5.50 ± 1.76 g).
One-way ANOVA highlighted significant differences in terms of bone ingrowth between the pedicle screws implanted in the OVX sheep (F = 29.2, p < 0.0005) and those in the SHAM sheep (F = 17.3, p < 0.005) (Table 4). In particular, uncoated pedicle screws showed a significantly higher bone ingrowth than that of the untreated screws in the OVX group (9.3%, p < 0.005) and significantly reduced bone ingrowth in the SHAM group (−11.0%, p < 0.05). Bioactive pedicle screws also showed significantly lower bone ingrowth than that of untreated screws in the SHAM group (−12.1%, p < 0.05). When comparing the behavior of the same screws inside the OVX and SHAM groups, significant differences were highlighted in terms of bone ingrowth for untreated screws (OVX lower than SHAM: −18.6%, p < 0.05).
Table 4.
Bone Ingrowth (%) results of the different type of pedicle screws implanted in L2–L5 lumbar vertebrae of OVX and SHAM sheep and loaded by Dynesys®: all valuable implanted screws (not infected)
| Group | Bioactive | Uncoated | Untreated |
|---|---|---|---|
| OVX | 75.5 ± 0.2 | 80.4 ± 2.2** | 71.1 ± 2.0° |
| SHAM | 77.6 ± 1.8* | 78.7 ± 2.4* | 89.7 ± 4.7 |
Mean ± SD, n = 4
Bone ingrowth (%): the amount of bone growth inside the screw threads measured in an area located between the bottom and the top of the threads and expressed as a percentage
In the Dunnett’s t test, SHAM group: bioactive and uncoated versus untreated pedicle screws (* p < 0.05); OVX group: uncoated versus untreated pedicle screws (** p < 0.005)
Student’s t test between OVX and SHAM groups: ° p < 0.05
Regarding bone-to-implant contact results, significant differences were found between the pedicle screws implanted in the OVX sheep (F = 7.3, p < 0.05) (Table 5). Significant lower bone-to-implant contact values were observed for all tested screws in the OVX group when compared with the SHAM Group (bioactive: −13.3%, p < 0.05; uncoated: −23.1%, p < 0.005; untreated: −29.6%, p < 0.05). Bioactive pedicle screws showed significantly increased bone-to-implant contact compared with that of untreated screws in the OVX group (16.4%, p < 0.05).
Table 5.
Bone-to-Implant Contact (%) results of the different type of pedicle screws implanted in L2–L5 lumbar vertebrae of OVX and SHAM sheep and loaded by Dynesys®: all valuable implanted screws (not infected)
| Group | Bioactive | Uncoated | Untreated |
|---|---|---|---|
| OVX | 72.9 ± 3.7*,° | 66.6 ± 4.0°° | 56.5 ± 8.6° |
| SHAM | 86.2 ± 3.9 | 89.7 ± 1.4 | 86.1 ± 3.9 |
Mean ± SD, n = 4
Bone-to-implant contact (%): the interface contact between bone and implant calculated on the best three consecutive threads and considered as the length of the bone profile directly opposite the implant and beyond the length of the bone implant
In the Dunnett’s t test, OVX Group: bioactive versus untreated pedicle screws (* p < 0.05); SHAM group: ns
Student’s t test between OVX and SHAM groups: ° p < 0.05; °° p < 0.005
Discussion
The target of the present study was to evaluate the performance of different pedicle screw designs when implanted in osteoporotic bone. Specifically, an evaluation was performed of two surface treatments (rough and bioactive) versus no surface treatment (untreated) for the pedicle screws of the Dynesys® system. The screws were placed in ovariectomized and sham-treated sheep. Unsurprisingly, on the whole the screws gave better results in terms of bone quality in the SHAM model with respect to the OVX model (Table 3). The osseointegration of treated screws in terms of bone ingrowth was similar in both groups; only untreated screws showed increased levels compared with that of the treated screws in the SHAM model. When considering osseointegration in terms of bone-to-implant contact, all tested screws presented significantly reduced levels in the OVX model compared to the SHAM model (Table 5), but the bioactive screws performed better than the uncoated screws. Based on the results of the present in vivo study in the presence of osteoporotic bone, surface treatment of the screws seems to give an advantage in terms of osseointegration levels.
Despite the wide use of polymethylmethacrylate for pedicle screw fixation in osteoporotic patients, the use of surface treatment to enhance osseointegration seems to be a good choice. In fact, cement augmentation has been used to improve the fixation of different types of devices to be implanted in various anatomical regions. Problems of osteolysis and subsequent implant failure have been reported, thus highlighting the need for an alternative solution. Furthermore, in a fusion system, the majority of the load is borne by the healed bone, i.e., the screw withstands loads only until fusion occurs. In a dynamic stabilization system like the one presented here, the pedicle screws bear the majority of the load for the lifetime of the system [25, 26]. As a consequence, screw loosening is more critical for the function of the system when it occurs [27–29]. Load sharing between the screw and the bone depends on the design of the screw itself, thus surface treatments may play an important role. Current results showed that 5 out of 64 (8%) and 3 out of 64 (5%) screws had been affected by infection or aseptic loosening, respectively (Table 1). Data were in agreement with those reported in the literature associated with this type of surgery and device [27, 28]. In an experimental study Meyer et al. [30] did not find a significant correlation between T scores and the magnitude of the moment in the pedicle screw. Despite this, the authors stated that failure at the screw–bone interface would be expected to occur more rapidly in osteoporotic bone. The results of the present study may encourage this hypothesis.
To investigate the anchorage of pedicle screws with different surface treatments in osteoporotic bone, an ovariectomized sheep model was used [13, 31, 32]. As far as the age of the model is concerned (about 7 years at the beginning of the study), Turner and Villanueva found that measurements of bone volume, osteoid volume and mineral apposition rate in 9- to 10-year-old ewes are comparable with those of men and women in their 6–7th decade of life, suggesting that aged sheep may make suitable models for human osteopenic and osteoporotic bone [33]. Another aspect of the osteopenic model is that the bone mineral density in sheep is different from that of humans, which might influence screw osseointegration. However, when BMD is assessed in the adult spine of a human without osteoporosis the ‘at risk’ value is decreased by about 25%, which corresponds to that observed in our sheep model (22–25%) [9, 34]. Finally, current results suggest that this kind of model is suitable for studying osseointegration and fixation in the osteoporotic spine.
The sheep is also considered to be a good model for assessing spinal anatomic and mechanical characteristics, especially because of its size, the location of the transverse processes and diameter of the pedicles [35]. However, quadrupedal models differ from humans in the loads applied to the ambulating spine. Posterior elements of the lumbar portions of the spine are under tension in the quadruped as opposed to compression in human beings. As a consequence, vertebral motion might be more limited than in humans, thus decreasing the stresses transmitted to the bone–screw interface [10] and affecting bone quality and osseointegration levels. Lower stress levels in the bone–screw interface may have two different consequences: (1) lower stimulus for bone growth, hence poorer bone quality or (2) less damage caused by the lower stresses, hence better bone quality.
Despite these limitations, the results of the present study suggest that surface treatment of pedicle screws may offer an advantage in terms of bone quality and osseointegration levels when implanted in osteoporotic vertebrae. Finally, the untreated surface is a clinically well-established standard surface and provides good secondary stability in normal bone.
Acknowledgments
The Authors wish to thank Mr. Keith Smith for his assistance in language supervision.
Conflict of interest None.
References
- 1.Boden SD. Bone repair and enhancement of clinical trial design: spine applications. Clin Orthop. 1998;355:336–346. doi: 10.1097/00003086-199810001-00033. [DOI] [PubMed] [Google Scholar]
- 2.Stoll TM, et al. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11:S170–S178. doi: 10.1007/s00586-002-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulholland RC. The myth of lumbar instability: the importance of abnormal loading as a cause of low back pain. Eur Spine J. 2008;17(5):619–625. doi: 10.1007/s00586-008-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beastall J, et al. The Dynesys lumbar spinal stabilization system: a preliminary report on positional magnetic resonance imaging findings. Spine. 2007;32:685–690. doi: 10.1097/01.brs.0000257578.44134.fb. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel JD, et al. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970–981. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Galbusera F, et al. Design concepts in lumbar total disc arthroplasty. Eur Spine J. 2008;17:1635–1650. doi: 10.1007/s00586-008-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002;11:S198–S205. doi: 10.1007/s00586-002-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel BM, et al. Segmental polymethylmethacrylate augmented pedicle screw fixation in patients with bone softening caused by osteoporosis and metastatic tumor involvement: a clinical evaluation. Neurosurgery. 2007;61:531–537. doi: 10.1227/01.NEU.0000290899.15567.68. [DOI] [PubMed] [Google Scholar]
- 9.Halvorson TL, et al. Effects of bone mineral density on pedicle screw fixation. Spine. 1994;19:2415–2420. doi: 10.1097/00007632-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Upasani VV, et al. Pedicle screw surface coatings improve fixation in nonfusion spinal constructs. Spine (Phila Pa 1976) 2009;34(4):335–343. doi: 10.1097/BRS.0b013e318194878d. [DOI] [PubMed] [Google Scholar]
- 11.Carmouche JJ, et al. Effects of pilot hole preparation technique on pedicle screw fixation in different regions of the osteoporotic thoracic and lumbar spine. J Neurosurg Spine. 2005;3:364–370. doi: 10.3171/spi.2005.3.5.0364. [DOI] [PubMed] [Google Scholar]
- 12.Cook SD, et al. Lumbosacral fixation using expandable pedicle screws. an alternative in reoperation and osteoporosis. Spine J. 2001;1:109–114. doi: 10.1016/S1529-9430(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 13.Nicoli Aldini N, et al. Pedicular fixation in the osteoporotic spine: a pilot in vivo study on long-term ovariectomized sheep. J Orthop Res. 2002;20:1217–1224. doi: 10.1016/S0736-0266(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 14.Fransen P. Increasing pedicle screw anchoring in the osteoporotic spine by cement injection through the implant. Technical note and report of three cases. J Neurosurg Spine. 2007;7:366–369. doi: 10.3171/SPI-07/09/366. [DOI] [PubMed] [Google Scholar]
- 15.Fini M, et al. Biological assessment of the bone–screw interface after insertion of uncoated and hydroxyapatite-coated pedicular screws in osteopenic sheep. J Biomed Mater Res. 2003;66:176–183. doi: 10.1002/jbm.a.10605. [DOI] [PubMed] [Google Scholar]
- 16.Fini M, et al. Biomechanical and histomorphometric investigations on two morphologically differing titanium surfaces with and without fluorohydroxyapatite coating: an experimental study in sheep tibiae. Biomaterials. 2003;24:3182–3192. doi: 10.1016/s0142-9612(03)00164-9. [DOI] [PubMed] [Google Scholar]
- 17.Giavaresi G, et al. Mechanical and histomorphometric evaluations of titanium implants with different surface treatments inserted in sheep cortical bone. Biomaterials. 2003;24:1583–1594. doi: 10.1016/S0142-9612(02)00548-3. [DOI] [PubMed] [Google Scholar]
- 18.Giavaresi G, et al. Different diagnostic techniques for the assessment of cortical bone on osteoporotic animals. Biomed Pharmacother. 2004;58:494–499. doi: 10.1016/S0753-3322(04)00127-1. [DOI] [PubMed] [Google Scholar]
- 19.Nicoli Aldini N, et al. Osseointegration of bioactive glass-coated and uncoated zirconia in osteopenic bone: an in vivo experimental study. J Biomed Mater Res. 2004;68A(2):264–272. doi: 10.1002/jbm.a.20057. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzenbach O, et al. Posterior dynamic stabilization systems: DYNESYS. Orthop Clin N Am. 2005;36:363–372. doi: 10.1016/j.ocl.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim HM, et al. Formation of bioactive functionally graded structure on Ti-6Al-4 V alloy by chemical surface treatment. J Mater Sci Mater Med. 2000;11:555–559. doi: 10.1023/A:1008924102096. [DOI] [PubMed] [Google Scholar]
- 22.Spriano S, et al. Characterisation of surface modified Ti-6Al-7Nb alloy. J Mater Sci Mater Med. 2005;16:301–312. doi: 10.1007/s10856-005-0628-7. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 24.Spriano S, et al. Surface properties and cell response of low metal ion release Ti-6Al-7Nb alloy after multi-step chemical and thermal treatments. Biomaterials. 2005;26:1219–1229. doi: 10.1016/j.biomaterials.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Chappard D, et al. The early remodeling phases around titanium implants: a histomorphometric assessment of bone quality in a 3- and 6-month study in sheep. Int J Oral Maxillofac Implants. 1999;14:189–196. [PubMed] [Google Scholar]
- 26.Spriano S, et al. New chemical treatment for bioactive titanium alloy with high corrosion resistance. J Mater Sci Mater Med. 2005;16:203–211. doi: 10.1007/s10856-005-6681-4. [DOI] [PubMed] [Google Scholar]
- 27.Iannuzzi A, et al. In vivo deformation, surface damage, and biostability of retrieved Dynesys systems. Spine. 2010;35:E1310–E1316. doi: 10.1097/BRS.0b013e3181d6f84f. [DOI] [PubMed] [Google Scholar]
- 28.Ko CC, et al. Screw loosening in the Dynesys stabilization system: radiographic evidence and effect on outcomes. Neurosurg Focus. 2010;28:E10. doi: 10.3171/2010.3.FOCUS1052. [DOI] [PubMed] [Google Scholar]
- 29.Liu CL, et al. Influence of Dynesys system screw profile on adjacent segment and screw. J Spinal Disord Tech. 2010;23:410–417. doi: 10.1097/BSD.0b013e3181b63d89. [DOI] [PubMed] [Google Scholar]
- 30.Meyer CM, et al. Discrepancies in T-score readings between patients with asymmetrical gait. J Am Geriatr Soc. 2008;56:758. doi: 10.1111/j.1532-5415.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 31.Fini M, et al. The ovariectomized sheep as a model for testing biomaterials and prosthetic devices in osteopenic bone: a preliminary study on iliac crest biopsies. Int J Artif Organs. 2000;23:275–281. [PubMed] [Google Scholar]
- 32.Giavaresi G, et al. The ovariectomized ewe model in the evaluation of biomaterials for prosthetic devices in spinal fixation. Int J Artif Organs. 2001;24:814–820. [PubMed] [Google Scholar]
- 33.Turner et al (1993) Static and dynamic histomorphometric data in 9-to 11-years old ewes. Poster Session Abstracts-ACVS: 413
- 34.Rocca M, et al. Osteointegration of hydroxypapatite-coated and uncoated titanium screws in long-term ovariectomized sheep. Biomaterials. 2002;23:1017–1023. doi: 10.1016/S0142-9612(01)00213-7. [DOI] [PubMed] [Google Scholar]
- 35.Wilke HJ, et al. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;2:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]