Abstract
Epithelioid hemangioendothelioma, an aggressive vascular tumor has the rarity of morbidity that arises in the spine. There were few cases reported in literatures in recent years, and little was known about this disease. A review study of the patient files in our constitutions between 1996 and 2006 showed that five patients were treated for spinal epithelioid hemangioendothelioma. Although only five patients, this study attempts to bring more informations about this rare lesion. This patient group included two males and three females. The lesions located in the cervical (case 1) or thoracic (case 2–4) or lumbar spine (case 5). Treatments included: laminectomy and cytoreductive surgery followed by external beam irradiation (one patient), expanded resection in piece meal with postoperative external beam irradiation (three patients), and total en bloc resection alone (one patient). Reconstruction of the spinal stability was performed in four patients. Follow-up period ranged from 25 to 72 months, averaged 47.4 months. The neurologic function of patients got a satisfactory progress except the paraplegic patient at diagnosis. The patient who received laminectomy and cytoreductive surgery followed by external beam irradiation still presented with tumor local progress, metastasis, and she died at 34 months after operation. No local recurrence or distant metastasis was detected in the other four patients. Epithelioid hemangioendothelioma of the spine is so rare in clinic as a primary aggressive vascular tumor. Based on our experience, a valid expanded resection of the tumor with adjunct radiation therapy or total en bloc excision may present with acceptable results.
Keywords: Epithelioid hemangioendothelioma, Spine, Clinical character, Outcomes, Surgical treatment
Introduction
There is a complex network of vascular tissue in the spine. The most common vascular tumors involving the spine are hemangiomas [19], which are benign and most cases are not need to be treated. Epithelioid hemangioendothelioma (EHE) is an angiocentric vascular tumor with metastatic potential. Behavior of EHE is intermediate between hemangiomas and conventional angiosarcomas. Primary EHE of the spine is rare [23].
This literature showed a series of five cases of this tumor. To our knowledge, there were few large series of spinal epithelioid hemangioendotheliomas, most probably because of the rarity of this disease; hence, not much was known [8]. The objective of the current study was to describe the clinical presentation and outcomes of the five patients with different surgical treatments.
Materials and methods
Epidemiology and clinical presentation
A review of the patient files from our institutions and oncology departments from 1996 to 2006 revealed that five patients were diagnosed and treated for the spinal epithelioid hemangioendothelioma. The patients included two males and three females. The patient’s age ranged from 20 to 50 years at diagnosis, with an average age of 34.8 years. The lesions located in the cervical (case 1) or thoracic (case 2–4) or lumbar spine (case 5). In our series, single vertebra tumor can be detected in three patients (patients 2, 4 and 5), and two vertebra tumors in two patients (patients 1 and 3).
Chronic, progressive pain in the neck or back was the most consistent complaint. This atypical symptom appearing before the diagnosis ranged from 3 months to 1 year, average about half a year. On physical examination, all patients had point tenderness in the area of involvement. Patient 5 had paresthesia in the groin due to the level of involvement. Patients 1 and 2 had the symptoms of numbness and inability in the limbs. Patient 4 was already paraplegia at diagnosis. The neurologic status of patients was evaluated according to the Frankel system before treatments (Table 1).
Table 1.
Summary of clinical data of 5 spinal EHE patients
| No./sex/age (years) | Level | Major symptoms/F-S (pre) | Treatment pre | Surgical treatment | F-S (post) | Follow-up (month) |
|---|---|---|---|---|---|---|
| 1/F/20 | C5–C6 | Neck pain/C | No | Expanded resection with APA, postoperative XIR | D | 72/NED |
| 2/M/42 | T3 | Back pain/C | CT-guided biopsy | Expanded resection with I-F; postoperative XIR | D | 58/NED |
| 3/M/50 | T8–T9 | Back pain/D | Embolization | En bloc resection with APA | D–E | 48/NED |
| 4/F/22 | T9 | Back pain/A | Embolization | Laminectomy and cytoreductive surgery with postoperative XIR | A–B | 34/metastasis dead |
| 5/F/40 | L1 | Back pain Paresthesia in the groin/D–E | Embolization | Expanded resection with APA; postoperative XIR | E | 25/NED |
M male, F female, F-S Frankel Score, Pre pre-operation, APA anteroposterior arthrodesis, I-F internal fixation, XIR external beam irradiation, Post post-operation, NED no evidence of disease
Radiological studies
The vertebral involvement was evident on plain radiographs in all patients. All patients had involvement of all the vertebral body with partial vertebral collapse, and case 4 presented with more than 50% collapse of the involved vertebral body. The site of tumor origin was the vertebral body with extension into the pedicle, and one lesion (case 2) was diffused with lamina involvement. Computed tomography scans revealed expansile and lytic process. All lesions appeared lytic with no matrix formation and had disruption of the posterior cortex of the vertebral body with varying amounts of soft tissue extension into the canal. MR imaging showed a mass with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Fig. 1).
Fig. 1.
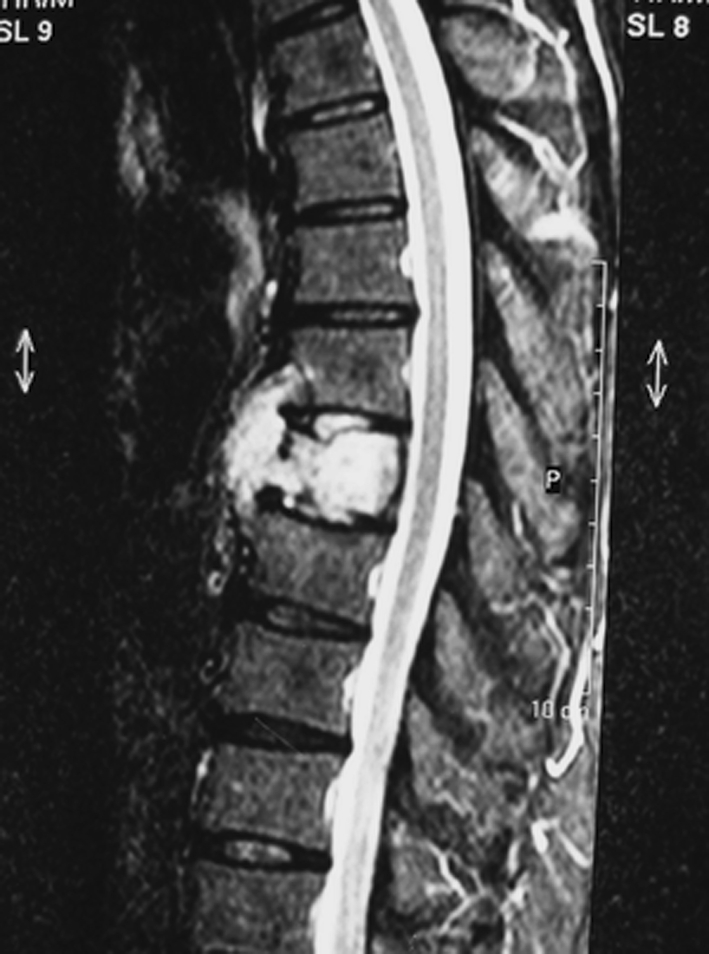
A 50-year-old male (patient 3). A part of T8 and total T9 vertebra tumor involvement with paravertebral mass on T2-weighted sagittal MR images
Treatment
In the current study, patients 2 received CT-guided biopsy before surgery. Angiographic evaluation and selective embolization of the involved vertebras was performed in patients 3–5. Different treatments were applied in the five cases. Laminectomy and cytoreductive surgery were performed in patient 4 because of extensive involvement of T9 and paraplegia at diagnosis as well as poor physical condition that does not tolerate a long-term surgery. External beam irradiation was given to her after operation. Expanded resection (one or two sectors expanded according to the WBB grade) in piece meal with postoperative external beam irradiation was performed in patient 1, 2 and 5. Total en bloc resection alone of T8–T9 involvement columns was performed in patient 3 through single posterior approach. Reconstruction included a T7–T10 interbody fusion with a titanium mesh filling with autologous iliac crest and T6–T12 transpedicular screw stabilization (Figs. 2, 3). The total dose of radiotherapy ranged from 4,000 to 6,000 rads. Reconstruction for the stability of spine by internal fixation was performed in four patients. The vertebral body replaced by titanium mesh filled with autogenous chips of bone was preferred (case 1, 3, and 5). Bone cement was applied in patient 2.
Fig. 2.
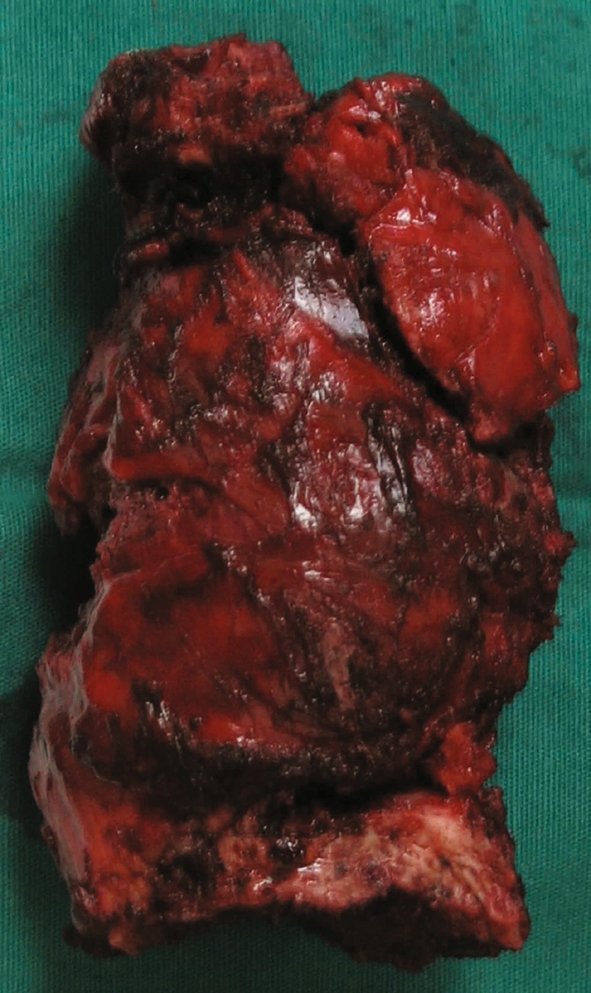
En bloc excision tumor specimen in T8–T9 of patient 3
Fig. 3.
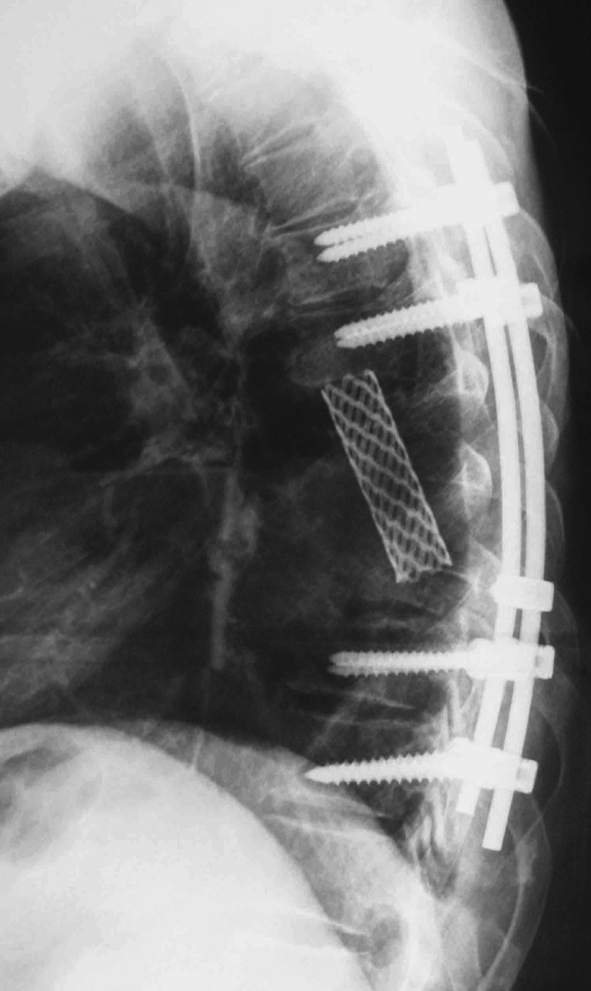
Transpedicular screw and a titanium mesh were used in the reconstruction of the stability of the spine in patient 3
Pathology
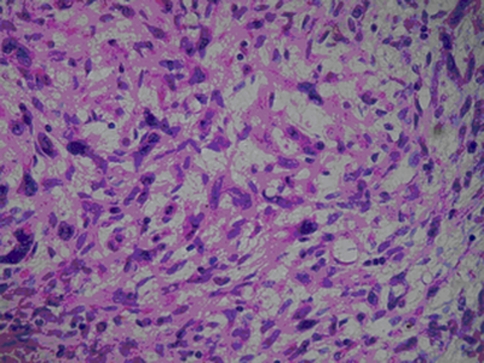
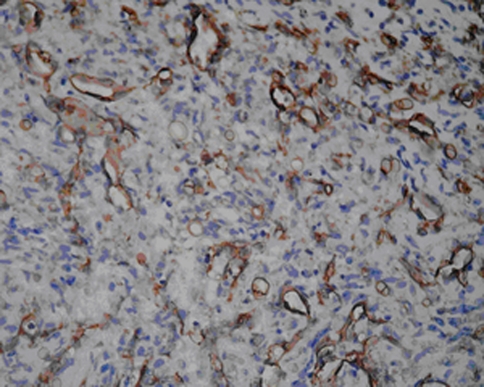
Microscopically, epithelioid hemangioendothelioma cells composed of cords, short strands, and solid nests (Fig. 4). In the tumor cells endothelial differentiation primarily appeared at the cellular level as evidenced by intracytoplasmic vacuoles containing erythrocytes which distort or blister their contours. Usually the cells appeared quite bland with little or no mitotic activity. Minimal nuclear atypia could be seen in two of these five cases. The neoplastic epithelioid endothelial cells are embedded in a myxoid matrix. Immunohistochemistry: Vascular antigens CD31, CD34 and Factor VIII-related protein were identified as sensitive within epithelioid hemangioendothelioma. All the tumors stained diffusely and intensely with antibodies to vimentin. In three of the five cases examined, the tumor cells were positive for antibodies for Factor VIII-related protein in both the cytoplasm and cell membranes (Fig. 5). The intensity of immunoreactivity varied from mild to moderate. Four of the five tumors stained for CD31, predominantly in the cell. In two of the membranes in five tumors, the tumor cells were positive for CD34. None of them showed immunoreactivity for cytokeratin or S-100.
Fig. 4.
The typical appearance of a hemangioendothelioma: cords of epithelioid endothelial cells in a myxohyaline background. H&E ×400
Fig. 5.
Diffuse Factor VIII-related protein immunoreactivity characterizing the endothelial nature of the tumor cells (×400)
All tumor specimens underwent pathological studies after the surgical removal, and the final diagnosis was EHE. Tissue on the tumor margins was also sent to pathological study to confirm a valid excision of lesions in patients under expanded resection.
Result
Intraoperative blood loss ranged from 1,000 to 4,800 ml, averaged 2,800 ml. The rate of fusion for bone grafts of the three patients was 100%, and all the internal instruments of four patients fuse well. No instability of spine could be seen in the cases which received reconstruction of the spine.
Complications
Three patients adopted selective complete embolization of the involved vertebras, but no correlating complication occurred. Patient 3 presented with persistent intercostal neuralgia after removal of the thoracic vertebral tumor, and oral administration of pain-killer could solve the problem. Cerebrospinal fluid fistula also occurred in this patient, and the cut got delayed union. Four patients received radiation therapy after operation, and there was no radio-induced sarcoma detected in this current study.
Neurologic status
Tumor expansion formed a slow process of cord compression relatively because of the rate of tumor growth, so in most cases, a significant improvement of the neurological deficit followed the removal of the lesions or decompression of the vertebral canal (Table 1). The only dissatisfactory case was the paraplegic patient, because no evident improvement of paralysis symptom could be observed till half a year after decompression.
Local control and survival
Follow-up of the five patients ranged from 25 to 72 months, averaged 47.4 months. Patient 4 received laminectomy and cytoreductive surgery combined with external beam irradiation, but tumor local progress still could be detected in follow-up period. Metastasis of the lung was found in this patient 24 months after operation and she died of the tumor in the next 10 months. No tumor recurrence or distant metastasis could be observed in the other four patients (Table 1).
Discussion
Most vascular tumors in the spine are benign in nature, such as hemangiomas. In the past, the concept of hemangioendothelioma (HE) included various kinds of benign, intermediate, and malignant vascular lesions. According to the new WHO classification of tumors of soft tissue and bone which was published in 2002, there has been some effort to classify most HE into intermediate malignant tumors, which, a low-grade tumor rarely associated with distant metastasis [13]. But, as a family member of HE, epithelioid hemangioendothelioma (EHE) is an exception. Even the distant metastasis rate of this tumor is not as frequent as angiosarcoma, it is now appreciated that 20–30% of epithelioid hemangioendothelioma may give rise to distant metastases and 10–20% of this tumor may lead to death of patients. Such features correlate EHE with a more aggressive course.
The most frequent complaints of patients with spinal EHE were pain in the neck and the back, and numbness in the extremities. Some times, spinal EHE may give rise to severe symptom of cord compression. Brennan et al. [5] presented an EHE of vertebrae C5–C6 that caused evident cord compression symptom presenting as cervical myelopathy. Gokhan et al. [14] reported an incomplete paralysis patient of EHE originating from L1 level. Also one of five cases in the current study had paralysis.
Radiographic findings of EHE are not specific. On the plain X-ray image, lytic process with solitary or multiple lesions has no diagnostic modality, because it would be difficult to make differential diagnosis with other vascular tumors. Although Computed tomography scanning or MRI scans detected bony destruction, the scale of involvement and evidence of cord compression due to tumors, it was not useful for the prebiopsy diagnosis. According to the literatures, EHE has the tendency to present with multifocal lesions. Bone scan of all over the body should be used as a protocol of routine examinations. All of the cases in this study had bone scan examination before treatments, and no multifocal lesion could be observed.
During surgical treatment for spinal EHE, how to control the intraoperative blood loss is a critical problem that must be faced. Because, as a vascular tumor, HEs have a substantial potential for intraoperative blood loss [2]. To solve this problem, angiographic evaluation and selective embolization of the involved vertebrae should be applied if possible. Sybert et al. [20] reported a case of spinal HEs who did not have embolization, and the surgery had to be terminated due to perfuse bleeding. Three of the five patients in the current study had selective embolization to decrease intraoperative blood loss, even though the intraoperative blood loss can still be a challenge to the surgical team in the process of tumor resection.
According to some reports in literatures, the result of external beam irradiation alone for spinal HEs was acceptable. While, as an aggressive tumor, the treatment of spinal EHE needs a more positive plan. Patient 4 in this current study, who received laminectomy and cytoreductive surgery combined with external beam irradiation (total dose was 5,000 rads) had to face the problem of tumor local progression. But tumor recurrence could not be detected after valid, expanded removal of the tumors with adjunct external beam irradiation (3 cases) or en bloc resection alone (one case) with a follow-up on average of 50.8 months. Campanacci et al. [6] advocated the use of postoperative external beam irradiation to lessen the risk of local recurrence after surgical resection. The risk of postirradiation sarcoma is related directly to the total dosage of radiation therapy. With the dose range of 4,000–7,000 rads, the risk of radiation-induced sarcoma is less than 1% [21]. In the current series, four of the five patients were treated with radiation therapy after operation, and no such complication occurred. But the findings in these cases underscore the difficulty in predicting the uncertainty of the roles of surgery and radiotherapy in the oncological management of a spinal tumor for which clinical data are very limited.
EHE have been diagnosed in bone [4, 16, 20] and many other sites by several literatures, such as the parotid salivary gland, nasal cavity, testis, lung, brain and so on [7, 9, 10, 18, 22]. When localized in the bones, multifocal involvement is common [15]. Spine involvement is much less often encountered. To our knowledge, there were about ten original spinal cases reported in recent years [1, 3, 5, 8, 11, 12, 14, 17]. Among them, two metastatic cases were found, and three died of the tumors. Tumor metastasis to lung in our study was only observed in Patient 4, and the patient died of the tumor.
Conclusion
Although the limitation of the case amount restricts us to draw any definitive conclusion, it is, to our knowledge, a comparatively larger series of patients of spinal epithelioid hemangioendothelioma under surgical treatments and with middle and long-term follow-up. Based on our experience, a valid surgical resection of the lesions with adjunct radiation therapy or total en bloc resection may present with acceptable results.
Acknowledgments
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Conflict of interest None.
Footnotes
J. Ma and L. Wang contributed equally to this work.
References
- 1.Aquilina K, Lim C, Kamel MH, et al. Epithelioid hemangioendothelioma of the spine. Report of two cases. J Neurosurg Spine. 2005;3(5):393–399. doi: 10.3171/spi.2005.3.5.0393. [DOI] [PubMed] [Google Scholar]
- 2.Arbelaez A, Castillo M, Williams L, et al. Vertebral hemangioendothelial sarcoma: MR findings. J Comput Assist Tomogr. 1999;23:981–983. doi: 10.1097/00004728-199911000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Bollinger BK, Laskin WB, Knight CB. Epithelioid hemangioendothelioma with multiple site involvement Literature review and observations. Cancer. 1994;73(3):610–615. doi: 10.1002/1097-0142(19940201)73:3<610::AID-CNCR2820730318>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Boutin RD, Spaeth HJ, Mangalik A, et al. Epithelioid hemangioendothelioma of bone. Skeletal Radiol. 1996;25:391–395. doi: 10.1007/s002560050102. [DOI] [PubMed] [Google Scholar]
- 5.Brennan JW, Midha R, Ang LC, et al. Epithelioid hemangioendothelioma of the spine presenting as cervical myelopathy: case report. Neurosurgery. 2001;48(5):1166–1169. doi: 10.1097/00006123-200105000-00045. [DOI] [PubMed] [Google Scholar]
- 6.Campanacci M, Boriani S, Giunti A. Hemangioendothelioma of bone: a study of 29 cases. Cancer. 1980;46:804–814. doi: 10.1002/1097-0142(19800815)46:4<804::AID-CNCR2820460427>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Chan YL, Ng HK, Poon WS, et al. Epithelioid hemangioendothelioma of the brain: a case report. Neuroradiology. 2001;43(10):848–850. doi: 10.1007/s002340100579. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulou A, Symeonidis PD, Kapoutsis D, et al. Primary epithelioid hemangioendothelioma of the lumbar spine. Spine. 2008;8(2):385–390. doi: 10.1016/j.spinee.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Cronin P, Arenberg D. Pulmonary epithelioid hemangioendothelioma: an unusual case a review of the literature. Chest. 2004;125(2):789–793. doi: 10.1378/chest.125.2.789. [DOI] [PubMed] [Google Scholar]
- 10.Di Girolamo A, Giacomini PG, Coli A, et al. Epithelioid hemangioendothelioma arising in the nasal cavity. J Laryngol Otol. 2003;117(1):75–77. doi: 10.1258/002221503321046711. [DOI] [PubMed] [Google Scholar]
- 11.Evans HL, Raymond AK, Ayala AG. Vascular tumors of bone: a study of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epithelioid hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Human Pathol. 2003;34(7):680–689. doi: 10.1016/S0046-8177(03)00249-1. [DOI] [PubMed] [Google Scholar]
- 12.Faust J, Schmidt M, Eysel P, et al. Epithelioid hemangioendothelioma of the spine. Med Klin (Munich) 2001;96(12):740–744. doi: 10.1007/PL00002171. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CDM. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48:3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 14.Gokhan GA, Akyuz M, Gurer IE, et al. Epithelioid hemangioendothelioma derived from the spine region: case report and review of the literature. Wien Klin Wochenschr. 2006;118(11–12):358–361. doi: 10.1007/s00508-006-0582-5. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M. (2003) Epithelioid hemangioendothelioma. In: Diagnostic soft tissue pathology. Armed Forces Institute of Pathology, editor. Churchill Livingtone, New York, pp 323–324
- 16.Kleer CG, Unni KK, McLeod RA. Epithelioid hemangioendothelioma of bone. Am J Surg Pathol. 1996;20:1301–1311. doi: 10.1097/00000478-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kopniczky Z, Tsimpas A, Lawson DD, et al. Epithelioid hemangioendothelioma of the spine: report of two cases and review of the literature. Br J Neurosurg. 2008;22(6):793–797. doi: 10.1080/02688690802158694. [DOI] [PubMed] [Google Scholar]
- 18.Pigadas N, Mohamid W, McDermott P. Epithelioid hemangioendothelioma of the parotid salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(6):730–738. doi: 10.1067/moe.2000.106299. [DOI] [PubMed] [Google Scholar]
- 19.Smith J. Tumors of Vascular Origin. In: Taveras JM, Ferrucci JT, editors. Radiology: diagnosis, imaging, intervention. Philadelphia: JB Lippincott Co.; 1988. pp. 1–8. [Google Scholar]
- 20.Sybert DR, Steffee AD, Keppler L, et al. Seven-year follow up of vertebral excision and reconstruction for malignant hemangioendothelioma of bone. Spine. 1995;20:841–844. doi: 10.1097/00007632-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Tountas AA, Fornasier VL, Harwood AR, et al. Postirradiation sarcoma of bone: a perspective. Cancer. 1979;43:182–187. doi: 10.1002/1097-0142(197901)43:1<182::AID-CNCR2820430127>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Tsolos C, Polychronidis A, Sivridis E, et al. Epithelioid hemangioendothelioma of the testis. J Urol. 2001;166(5):1834. doi: 10.1016/S0022-5347(05)65692-3. [DOI] [PubMed] [Google Scholar]
- 23.Wenger DE, Wold LE. Benign vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol. 2000;29:63–74. doi: 10.1007/s002560050012. [DOI] [PubMed] [Google Scholar]