Abstract
Post laminectomy arachnoiditis has been shown by experiments with rats and post operative radiological imaging in humans. The purpose of this experimental study was to determine the efficacy of tenoxicam in preventing arachnoiditis in rats. Twenty-four Wistar rats were divided into two groups, and L3 laminectomy was performed. In the tenoxicam group, 0.5 mg/kg tenoxicam was applied intraperitoneally. Normal saline was applied intraperitoneally in the control group. Later, the rats were killed at weeks 3 and 6, and the laminectomy sites were evaluated pathologically for arachnoiditis. The results showed that 6 weeks after surgery, the tenoxicam group showed lowest arachnoiditis grades. However, statistically significant difference was not found in arachnoiditis between the control group and the tenoxicam group. Based on these findings it is concluded that application of the tenoxicam after lumbar laminectomy did not effectively reduce arachnoiditis. Performing the most effective surgical technique without damage around tissue in a small surgical wound and having meticulous hemostasis in surgery seem to be the key for preventing arachnoiditis effectively.
Keywords: Arachnoiditis, Laminectomy, Non-steroidal anti-inflammatory drugs, Tenoxicam
Introduction
Spinal adhesive arachnoiditis is a chronic nonspecific inflammatory condition involving the leptomeninges and intrathecal neural elements [1]. The possible etiologic factors were reported to be mechanical disorders and bacterial infections such as prolapsed intervertebral disc, degenerative spinal stenosis, spinal trauma, surgical intervention to the spine, and intrathecal injection of myelographic dyes or anesthetics [2, 3]. Following lumbar disc surgery, 4.6% of patients have lumbar adhesive arachnoiditis [4]. Symptomatic cases may present chronic low-back pain, motor and sensory loss, and reflex changes [3]. Previous clinical studies have demonstrated that adhesive spinal arachnoiditis in the caudal sac is very difficult to treat [5].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are at present the most widely used medications in the treatment of inflammation and pain [6]. NSAIDs are inhibitors of prostaglandin H synthase, more commonly referred to as cyclooxygenase (COX) [7]. Tenoxicam, one of the NSAIDs, has been tested in many experimental studies intraperitoneally to prevent the initial inflammatory stage of adhesion formation in a noninfectious environment [8, 9]. Previously, tenoxicam has not been used for the treatment of post laminectomy arachnoiditis. In the present study, we examined the effect of intraperitoneal tenoxicam application on the prevention of arachnoiditis in laminectomized rats.
Materials and methods
Twenty-four Wistar rats, each weighing 250–300 g, were used for this study. The protocols were approved by the Gazi University Institutional Animal Care and Use Committee for ethics in animal experiments.
Surgical procedure
Animal were anesthetized with intraperitoneal ketamine sulfate of 5 mg/kg (Ketalar, Parke Davis, Eczacibasi, Turkey) and 2% xylazine of 10 mg/kg (Rompun, Bayer, Istanbul, Turkey). The lumbosacral regions of the animals were first shaved; Later, the surgical area was prepared using povidine iodine (Betadine). Then, a midline skin incision from L2 to L4 was incised, and paraspinous muscles were dissected bilaterally. A total laminectomy was performed at L3 lamina. Care was taken not to damage the dura mater. In addition, hemostasis was achieved with electrocautery, and meticulous microsurgical technique was performed using loupe magnification (Easy loupe, Oculus, Germany). The wound was closed in layers after the operation.
Rats were randomly assigned to 2 groups of 12 rats each. Normal saline (control group) and 0.5 mg/kg tenoxicam (tenoxicam group) were applied intraperitoneally immediately after laminectomy. Both the control group and the tenoxicam group were further divided into two equal groups, each comprising six rats. Subsequently, the animals were killed with an overdose of intraperitoneally injected pentobarbital at 3 and 6 weeks of surgery. In the post-operative period, none of the rats showed the evidence of infection and neurological deficit.
Light microscopy evaluation
The surgical area, including the laminectomy site and paraspinal musculature, was removed en bloc including distal and proximal sites of lesion and fixed in 10% buffered formaldehyde solution. After fixation, the specimens were decalcificated and dehydrated. Then, the spine was further sawn to 8 mm axial sections and embedded in paraffin. The sections were stained with hematoxylin and eosin. Pathological sections were evaluated by a pathologist who was blinded to the animals. Since post laminectomy arachnoiditis has not been evaluated quantitatively previously in the literature, we have used a new grading system for arachnoiditis evaluation which is a modified form of the system defined by He [10]. The extent of arachnoiditis was graded according to the following classifications:
- Grade 1
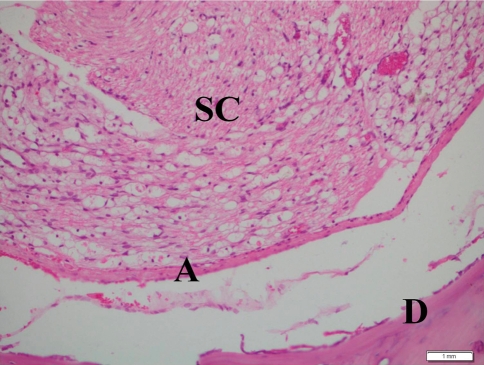
Without spinal cord compression, when the dura mater was free from the arachnoid mater (Fig. 1).
- Grade 2
Without spinal cord compression, when only thin adhesion region between the dura mater and arachnoid mater was observed.
- Grade 3
Without spinal cord compression, when continuous adherence was observed between the dura mater and leptomeninx.
- Grade 4
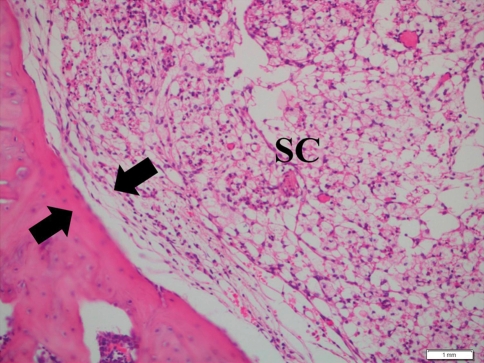
When spinal cord compression and continuous adherence were observed between the dura mater and leptomeninx.
Fig. 1.
Pathologic section (hematoxylin and eosin) of the laminectomy site demonstrating Grade 1 arachnoiditis in the tenoxicam group at week 6. No direct contact was observed between the dura mater (D) and the underlying arachnoid mater (A). SC Spinal cord. Scale bar 1 mm
The grades of arachnoiditis were determined for each section of groups.
Statistical analysis
Comparison of arachnoiditis grades of the control group and the experimental groups was performed with Chi-square test. Statistical significance was determined at p < 0.05.
Results
There was no evidence of any systemic and/or local adverse effect due to the application of tenoxicam. None of the rats developed neurological deficits during the experiment. Summary of the pathological grades of the control and the tenoxicam groups is presented in Table 1. The control group and the tenoxicam group did not show Grade 3 and Grade 4 arachnoiditis in 3 week evaluation. Grade 4 arachnoiditis was observed only in one animal of the control group killed 6 weeks after the surgery (Fig. 2). In addition, none of these control group animals (killed at 6 weeks of the experiment) showed Grade 1 arachnoiditis. There were no statistically significant differences between groups and weeks when the results of arachnoiditis were analyzed (p > 0.05). Even though no statistically significant differences between the groups were found, grades of arachnoiditis were highest in the control group and lowest in the tenoxicam group in 6 week evaluation.
Table 1.
Results of the arachnoiditis grades in the control group and the tenoxicam group
| Group | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Control Group, 3 weeks | 3 | 3 | 0 | 0 |
| Tenoxicam Group, 3 weeks | 2 | 4 | 0 | 0 |
| Control Group, 6 weeks | 0 | 3 | 2 | 1 |
| Tenoxicam Group, 6 weeks | 3 | 1 | 2 | 0 |
Fig. 2.
Pathologic section (hematoxylin and eosin) of the laminectomy site demonstrating Grade 4 arachnoiditis in the control group at week 6. The dura mater was adhered to the underlying leptomeninx (arrows) with spinal cord (SC) compression. Scale bar 1 mm
Discussion
Spinal arachnoiditis is a condition of fibrous invasion of the pia mater. The fibrosing process may be secondary to a deformity of the spinal column, to the sequelae of physical injury to the spine, or to toxic effects of introduced noxious substances [11]. Following the spinal surgeries, the exact mechanism of the spinal arachnoiditis promoted by epidural inflamation is currently unknown [12]. Three distinctive pathological stages of arachnoiditis were defined: arachnoiditis, adhesive arachnoiditis and arachnoiditis ossificans [1]. It has been hypothesized that the pathological process starts with fibrin deposition and a mild inflammatory response followed by collagen deposition and granulomatous reaction [3]. Burton et al. [11] reported that it could result from an inflammatory focus (a herniated lumbar disc), foreign body (especially Pantopaque myelography) and autoimmune reaction.
Reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical and hydrogen peroxide are crucial in inflammatory responses where they participate in physiological processes such as arachidonic acid cascade and phagocytosis [13, 14]. As the inflammatory process is typically a situation of increased ROS production that may aggravate the pathological manifestations of the diseases [15], several commonly used or potential NSAIDs have been tested as potential free radical scavengers. Their capacity to react with some of these species and therefore to prevent oxidative damage in vivo has also been comparatively determined [16, 17].
Tenoxicam 4 hydroxy-2-methyl (N-C2 pyridyl) 2H-thieno-(2-3e)-1,2-thiazine carboxamide 1,1 dioxide is a new non-steroidal anti-inflammatory and analgesic agent of the oxicam class useful in the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis and gout [18]. Previously, Naziroğlu et al. [14] reported that tenoxicam has protective effects on the oxidative stress by inhibiting free radicals and supporting antioxidant redox system in rat brain. In addition, Galvão et al. [19] reported that tenoxicam prevented ischemia-induced cerebral eicosanoid formation, and caused significant reduction of the damaged area. Furthermore, Ezberci et al. reported that tenoxicam inhibited the formation of postoperative intra-abdominal adhesions without compromising wound healing. In our study, the tenoxicam group showed lowest arachnoiditis grades in the 6 week evaluation.
Arachnoiditis has been treated by drug therapy, physiotherapy, surgical dissection of scar tissue from the cauda equina, transcutaneous nerve stimulation and injection of corticosteroids, but frequently with little benefit [20] because it was reported that tenoxicam had beneficial effects on central nerve system previously [14, 19]; we hypothesise that it may be useful as an arachnoiditis preventing agent. The results of this study showed that at 6 weeks of surgery, formation of arachoiditis was inhibited in the tenoxicam group. However, statistically significant difference was not found regarding the arachnoid formation between the control group and the tenoxicam group.
In the English literature till date, none of the treatment modality such as chemical agents or surgical technique has been successful to prevent arachnoiditis. Because of this situation, it has been necessary to investigate new chemicals or surgical interventions to achieve effective arachnoiditis treatment. In this report, tenoxicam has shown better grades than control group. However, statistically significant difference could not been found. We believe that the best way to prevent arachnoiditis following lumbar surgeries is performing the operation carefully with minimally surgical wound, decreased muscle and laminectomy damage as well as a meticulous hemostasis using bipolar cautery.
Conclusion
The findings of this study propose that the application of the tenoxicam did not prevent arachnoiditis formation. The findings of this study propose that performing the most effective surgical technique without damage around tissue in a small surgical wound and having meticulous hemostasis in surgery may be the key for preventing arachnoiditis effectively.
Conflict of interest
None.
References
- 1.Day P (2001) Arachnoiditis: a brief summary of the literature. New Zealand Health Technology Assessment Report
- 2.Vloeberghs M, Herregodts P, Stadnik T, Goossens A, D’Haens J. Spinal arachnoiditis mimicking a spinal cord tumor: a case report and review of the literature. Surg Neurol. 1992;37:211–215. doi: 10.1016/0090-3019(92)90233-D. [DOI] [PubMed] [Google Scholar]
- 3.Long DM. Chronic adhesive spinal arachnoiditis: pathogenesis prognosis and treatment. Neurosurg Q. 1992;2:296–319. [Google Scholar]
- 4.Fitt GJ, Stevens JM. Postoperative arachnoiditis diagnosed by high resolution fast spin-echo MRI of the lumbar spine. Neuroradiology. 1995;37:139–145. doi: 10.1007/BF00588631. [DOI] [PubMed] [Google Scholar]
- 5.Carroll SE, Wiesel SW. Neurologic complications and lumbar laminectomy. A standardized approach to the multiply-operated lumbar spine. Clin Orthop Relat Res. 1992;284:14–23. [PubMed] [Google Scholar]
- 6.Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5:19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni SK, Jain NK, Singh A. Cyclooxygenase isoenzymes and newer therapeutic potential for selective COX-2 inhibitors. Methods Find Exp Clin Pharmacol. 2000;22:291–298. doi: 10.1358/mf.2000.22.5.796648. [DOI] [PubMed] [Google Scholar]
- 8.Celebioglu B, Eslambouli NR, Olcay E, Atakan S. The effect of tenoxicam on intraperitoneal adhesions and prostaglandin E2 levels in mice. Anesth Analg. 1999;88:939–942. doi: 10.1097/00000539-199904000-00048. [DOI] [PubMed] [Google Scholar]
- 9.Yilmazlar T, Kaya E, Gürpinar E, Emiroğlu H. Efficacy of tenoxicam on intra-abdominal adhesion prevention in a rat model. J Int Med Res. 1996;24:352–357. doi: 10.1177/030006059602400406. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Revel M, Loty B. A quantitative model of post-laminectomy scar formation. Effects of a nonsteroidal anti-inflammatory drug. Spine. 1995;20:557–563. doi: 10.1097/00007632-199503010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Burton CV. Lumbosacral arachnoiditis. Spine. 1978;3:24–30. doi: 10.1097/00007632-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kroin JS, Buvanendran A, Cochran E, Tuman KJ. Characterization of pain and pharmacologic responses in an animal model of lumbar adhesive arachnoiditis. Spine. 2005;30:1828–1831. doi: 10.1097/01.brs.0000174276.73908.f0. [DOI] [PubMed] [Google Scholar]
- 13.Naziroğlu M, Uğuz AC, Gokçimen A, Bülbül M, Karatopuk DU, Türker Y, Cerçi C. Tenoxicam modulates antioxidant redox system and lipid peroxidation in rat brain. Neurochem Res. 2008;33:1832–1837. doi: 10.1007/s11064-008-9643-7. [DOI] [PubMed] [Google Scholar]
- 14.Eren I, Naziroğlu M, Demirdaş A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res. 2007;32:1188–1195. doi: 10.1007/s11064-007-9289-x. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B, Gutteridge JMC. Free radicals, other reactive species and disease. In: Halliwell B, Gutteridge JMC, editors. Free radicals in biology and medicine. 3. New York: Oxford University Press; 1999. pp. 639–645. [Google Scholar]
- 16.Ezberci F, Bulbuloglu E, Ciragil P, Gul M, Kurutas EB, Bozkurt S, Kale IT. Intraperitoneal tenoxicam to prevent abdominal adhesion formation in a rat peritonitis model. Surg Today. 2006;36:361–366. doi: 10.1007/s00595-005-3137-x. [DOI] [PubMed] [Google Scholar]
- 17.Ozgocmen S, Ardicoglu O, Erdogan H, Fadillioglu E, Gudul H. In vivo effect of celecoxib and tenoxicam on oxidant/anti-oxidant status of patients with knee osteoarthritis. Ann Clin Lab Sci. 2005;35:137–143. [PubMed] [Google Scholar]
- 18.Thadikonda KP, Lau-Cam CA, Thadikonda VL, Theofanopoulos V. The intranasal absorption of the quaternary ammonium compounds neostigmine bromide and tubocurarine chloride in the rat. Drug Dev Ind Pharm. 1995;21:461–473. doi: 10.3109/03639049509026635. [DOI] [PubMed] [Google Scholar]
- 19.Galvão RI, Diógenes JP, Maia GC, Filho EA, Vasconcelos SM, Menezes DB, Cunha GM, Viana GS. Tenoxicam exerts a neuroprotective action after cerebral ischemia in rats. Neurochem Res. 2005;30:39–46. doi: 10.1007/s11064-004-9684-5. [DOI] [PubMed] [Google Scholar]
- 20.Bourne IH. Lumbo-sacral adhesive arachnoiditis: a review. J R Soc Med. 1990;83:262–265. doi: 10.1177/014107689008300418. [DOI] [PMC free article] [PubMed] [Google Scholar]