Abstract
The aim was to study if postoperative rehabilitation improves functional outcome in lumbar spinal stenosis (LSS). Surgically treated LSS patients (n = 102) were randomized to rehabilitation-group (A) and “standard postoperative treatment”—group (B). Intervention for A-group started 3 months postoperatively, consisting of once a week outpatient visits for 12 weeks (1.5 h per visit; 1–6 patients per one physiotherapist). Physiotherapist guided stretching and strengthening exercises. A-group performed individually estimated exercises at those visits with guiding and at home up to 24-month postoperative follow-up. Physiotherapeutic guidance (12 times) was repeated after 12 months, in order to update exercises and motivate patients to keep training. For B-group, the “standard treatment” thus included normal postoperative treatment, or no treatment/self-management. Outcome measures were measured at the start and the end of the first physiotherapeutic intervention (3 and 6 months postoperatively), and at 12- and 24-month postoperative follow-ups. Oswestry Disability Index (ODI, 0–100%) was the main outcome measure. The other outcome measures were back- and leg pain separately (NRS-11); satisfaction (7-point scale) and treadmill test (0–1,000 m; not at 6 month). The intervention consisting of 12 + 12 physiotherapeutic sessions with further home exercises did not influence the course ODI in the 24-month postoperative follow-up (p = 0.95 for ODI; “as-rehabilitated” analysis). No influence on any other outcome measures was observed. After LSS surgery, routinely performed outpatient rehabilitation did not improve functional outcome compared to standard treatment. In addition, it had no impact on the back and leg pain, satisfaction and walking ability.
Electronic supplementary material
The online version of this article (doi:10.1007/s00586-011-1781-y) contains supplementary material, which is available to authorized users.
Keywords: Lumbar stenosis, Surgery, Outcome, Rehabilitation
Introduction
The surgical outcome in lumbar spinal stenosis (LSS) is good to excellent in 64% of cases [40]. Postoperative physiotherapy is often prescribed after LSS diagnosis [38], and also after LSS surgery. Postoperative rehabilitation (PR) has been reported to improve outcome after disc surgery [26]. According to Mannion et al. [24], supervised rehabilitation had no significant influence in pain and self-rated disability as compared to no treatment/self-management after decompressive lumbar surgery including disc herniation and LSS patients. However, there are no earlier studies of PR examining exclusively LSS patients. Our aim was to study if supervised PR, including strengthening and stretching exercises (Figs. 1–7 of supplementary material) guided and done at 12+12 physiotherapeutic visits and further at home, could improve the functional outcome compared to standard treatment.
Materials and methods
Patients
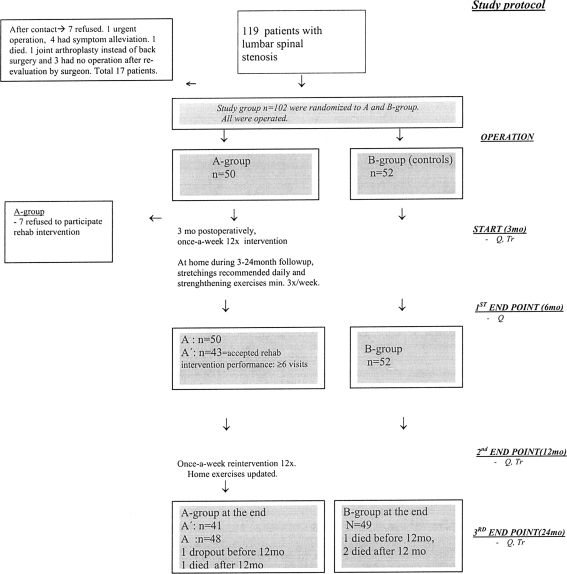
The study subjects were 102 patients with clinically and radiologically defined LSS [31–36]. Selection for surgery (secondary level care) was made by the orthopaedist or neurosurgeon between October 2001 and October 2004 in the University Hospital. The inclusion criteria were: (1) presence of back, buttock, and/or lower extremity pain, with radiographic evidence (computed tomography, magnetic resonance imaging (MRI), rhizography) of compression of the cauda equina and/or exiting nerve roots due to degenerative changes (ligamentum flavum, facet joints, osteophytes and/or disc material) [21]. (2) The surgeon’s judgement that the patient had clinically significant degenerative LSS as the main diagnosis indicative for operative treatment. A previous spine operation or co-existing disc herniation was permitted. The exclusion criteria included emergency or urgent spinal operation precluding recruitment and protocol investigations; cognitive impairment prohibiting completion of the questionnaires or other failures in co-operation; the presence of metallic particles in the body contra-indicating the MRI-investigation. The surgeons sent the information about eligible patients to be operated to the Department of Physical and Rehabilitation Medicine (DPRM), which organized the study. The study flowchart is presented in Fig. 1.
Fig. 1.
Flowchart of the study, A A-group, B B-group, A′ patients who had acceptable ≥6/12 physiotherapy visits between 3 and 6 months: they were included in “as-rehabilitated” analysis (A′ vs. B). Seven patients refused (4 had health related reasons) to participate in 12× physiotherapy (3–6 months): they were included intention-to-treat analysis (A vs. B). MO months; Start start of rehabilitation intervention. Stages in study protocol are italicized in the right. Q questionnaire; Tr treadmill test
The patients received an account of the study during their outpatient visit to the DPRM and provided informed consent. The information of 119 eligible patients was sent to the DPRM; 17 patients were excluded due to different reasons [10], concluding 102 patients to final study population (Fig. 1). MRI of the entire lumbar spine was performed with a 1.5-T imager (Vision; Siemens Medical Solutions, Erlangen, Germany) for study purposes. T1 and T2-weighted magnetic resonance images were obtained (T12–S1 sagittal images including parasagittal imaging of all of the neural foramina bilaterally; transverse images from the L1 to S1). The type of stenosis (1. central only 2. lateral only 3. mixed) and dural sac area (mm2) of the most affected level were determined once by one neuroradiologist without knowledge of the patients’ clinical symptoms. The Ethics Committee of Kuopio University Hospital approved the study design. Patients’ demographics, information of type and severity of stenosis and excision rates of co-existent disc herniation are presented in Table 1.
Table 1.
Preoperative demographics of study groups and the total population
| A′ (n = 43) | A (n = 50) | B (n = 52) | All (range; SD) | |
|---|---|---|---|---|
| Age (years at operation, mean) | 63.8 | 62.8 | 62.3 | 62.5 (34–86; 11.1) |
| Female/male (n) | 24/18 | 29/21 | 30/22 | 59/43 |
| BMI (kg/m2) | 28.7 | 29.0 | 29.9 | 29.5 (20.1–38.2; 4.0) |
| Type of stenosis (n) | ||||
| Central and lateral | 35 | 40 | 44 | 84 |
| Lateral only | 7 | 10 | 8 | 18 |
| Dural sac area (mean; mm2) | ||||
| the most stenotic level | 64.7 | 67.5 | 69.7 | 68.6 (12–170; 33.9) |
| Previous lumbar operation (n) | 2** | 6 | 10** | 16 |
| Operation | ||||
| Disc excision (any level) | 2 | 2 | 5 | 7 |
| L2–3 | 1 | 1 | ||
| L3–4 | 1 | 1 | ||
| L4–5 | 1 | 3 | 4 | |
| L5–S1 | 1 | 1 | 1 | |
| Fusion performed (n) | 10 | 10 | 9 | 19 |
| Comorbidity | ||||
| BDI-21 (mean) | 8.8 | 9.6 | 10.8 | 10.2 (1–36; 6.0) |
| Number of comorbidities | 5.3 | 5.5 | 5.3 | 5.4 (0–14; 3.2) |
A′ patients who performed acceptably at the 3–6 month intervention, A total A-group, B total B-group, BDI Beck Depression Inventory
** p = 0.038 Mann–Whitney U test between A′- and B-groups. Other differences of descriptives between groups are non-significant
Randomization
In the presence of two authors, the two-block randomization was performed to the rehabilitation group (A) and “standard treatment” group (B). Gender and age under/over 60 were adjusted in randomization.
Surgical technique and normal postoperative “standard treatment” for all patients
All the patients had open or microscopic decompression of the affected level(s), i.e. laminotomy, hemilaminectomy or laminectomy, with undercutting facetectomy. In seven cases, additional disc excision was done. Nineteen patients had additional fusion, two of them with instrumentation (Table 1). All the patients received routine (not-study-related) preoperative information at the hospital about immediate postoperative mobilisation. They were advised “to stay active” with no restrictions in normal daily living. Patients had routine operation-related control in the orthopaedic or neurosurgical clinic at 2–3 months postoperatively. At this time point, the surgeon also confirmed that there were no restrictions prohibiting rehabilitation. Since there were no restrictions with other postoperative treatment by the study protocol, the surgeons and also GP could prescribe other possible treatments postoperatively if needed (analgetics, physiotherapy etc.). If no specific need for further treatments existed, then either no treatment or self-management represented the only “standard treatment” for patients in B-group.
Postoperative rehabilitation for A-group
The A-group had thus PR in addition to “standard treatment”. The more detailed description of PR is presented in supplementary material. Three months postoperatively, once a week supervised exercise training sessions (90 min each, lasting 12 weeks) were started at the DPRM, in the university hospital. At the first and second visit, a physiotherapist supervised the stretching and strengthening exercises. Exercising continued at home and at the next training sessions. In physiotherapeutic training session, there were maximum six members of A-group per one physiotherapist enabling individually estimated exercises. On the 6th and 12th visits, the volume and/or type of exercises, if needed, were progressively increased (or decreased/modified if there were complaints of pain or other symptoms) to optimize the effective training. This 12-week intervention was repeated at the one-year follow-up in order to check the appropriateness of the home exercises and to motivate patients to keep training. The strengthening exercises were intended to maintain and improve muscle strength and endurance of the hip and thigh muscles, as well abdominal and back muscles. There were minimum three different types of strengthening exercises. The recommended frequency of these exercises was at least 3–5 times a week. Each type of exercises was performed 3 to 5 times a day, with repetition frequency of 60% of maximum.
The aim of the minimum of four different stretching exercises was to increase stretchability and decrease muscular tightness if they were present in hip flexor- and hamstring muscles, as well as in the back muscles. The recommended frequency was once a day. Each type of stretching lasted for 20–30 s [4, 29] and was repeated 3 to 5 times after a 5–10 min warm-up.
Four trained physiotherapists (personnel of DPRM) were involved in supervising and monitoring the exercise sessions, where each participant undertook their own programmed sessions. Patients were verbally instructed on how to perform the exercises, given pictures illustrating the positions and an “exercise-diary” to improve their motivation to perform the exercises at home. We presumed a minimum 6/12 visits (50%) in first 12-week intervention as meaning that the patient had adopted an effective home exercise program (A’-group = “as-rehabilitated group”). We did not set minimum amounts but only provided recommendations about minimum amounts of stretching and strengthening exercises to be done at home.
Main and secondary outcome measures
The functional outcome was evaluated using Oswestry Disability Index (ODI), which was the main outcome measure. Four secondary clinical outcome measures based on pain, satisfaction and objective walking ability were also used. Outcome measures were evaluated at the start of the intervention (3 months postoperatively) and at primary end points (6-, 12- and 24-month postoperatively; treadmill test was not done at 6 months).
ODI (0–100%) was measured by the validated Finnish version, where 0% represents no disability and 100% extreme debilitating disability [8, 9, 11].
Back pain at rest (during last week) and leg pain at walking (during last week) were measured separately with a numeric rating scale 0–10 (NRS-11) [6]. The questions about pain were anchored on the left (0) with the phrase “No pain” and on the right (10) with the phrase “Intolerable pain”. Satisfaction for surgery was assessed using with a seven category scale as follows: −3 = surgery was a total failure, −2 = condition considerably worse −1 = condition slightly worse, 0 = no change, 1 = condition slightly improved 2 = condition considerably improved 3 = totally cured [32]. Physiotherapist supervised treadmill test. The patient was asked to keep a straight upright position during walking (a zero degree ramp). The starting speed was 0.67 m/s, for the first 10 min (400 m), then 1 m/s next 10 min (600 m); maximum result thus 1,000 m in 20 min. If the patient was not able to start with a speed of 0.67 m/s, another test with a starting speed of 0.5 m/s was applied. The overall back and leg pain (at the moment, in the sitting position during study physician visits) was recorded with a Visual Analogue Scale (VAS 0-100 mm) at the start of the intervention, in order to adjust overall pain [27].
Other descriptives measured preoperatively (Table 1). The physiotherapist measured height and weight. Body mass index was computed (kg/m2). The age was determined at the time of the operation. Depression was assessed with the Finnish version of the 21-item Beck Depression Inventory (BDI), with scores ranging from 0 to 63 [5, 28]. The number of comorbidities was recorded with one modified item (item number 3) of the Work Ability Index (WAI) questionnaire [39]; the self-reported number of current or recurring diseases diagnosed by a physician (range 0–49). The number of diseases was then recorded as a sum score.
Back problem related preoperative conservative treatment was also asked with a preoperative questionnaire, as well as in postoperative follow-ups between 3- and 24-month follow-up as follows: number of physiotherapy visits or guided gym/training (NPG) was recorded before operation, and in follow-up, in order to describe the postoperative not-study-related treatment in both study groups. Self-acting exercises were recorded also before the operation and in 6-, 12- and 24-month postoperative follow-ups with question “Do you have had self-acting back exercises (yes/no)?” The frequency ofback exercise training (FBE) was asked with questions “How often have you done the exercises during the last 3 months?” (6-month questionnaire) and “How often have you done the exercises during the last month?” (preoperative, 12- and 24-month questionnaires). Score was 0 = daily training, 1 = 3–5 times/week, 2 = 1–2 times/week, 3 = less than once a week. The patients who reported not having self-acting exercises were coded as 4 = no back exercises.
Statistical analysis
Power calculation was carried out for main outcome measure (ODI) only, to attain power at least equal to 0.80 at the 0.05 significance level (two-sided). A clinically significant change (effect size) was considered to be 12 points. Postoperative standard deviation (SD) was estimated to be 18 based on the earlier works [15–17]. Thus, 37 patients per group would be sufficient to detect a 12 point clinically significant difference in ODI. The final population over 100 was chosen to ensure power even with a 20% dropout rate.
The general linear regression model (GLM; repeated measures; SPSS, Version 14.0.1) was used to compare the differences between A- and B-groups 3–24 months postoperatively. For each outcome measure, three separate GLM models from the start of the intervention for each three primary end points were created (3- to 6-month; 3-, 6-, 12-month and 3-, 6-, 12-, 24-month), to detect potential effect of intervention in any primary end point. Three covariates were used. The ODI and overall Visual Analogue Scale (at the start of the intervention) as they considered being confounders [41, 42]. The cumulative FBE from the start of the intervention to the 6- to 24-month primary end points was taken into account (summing the codes 0–4) to adjust the cumulated effect of self-acting training during intervention. The missing data of FBE (each 6- to 24-month questionnaire separately) were replaced with the group mean before summing the score.
The analyses were performed “as-rehabilitated analysis” (A′ vs. B-group) and also “intention-to-treat-analysis” (A vs. B-group).
Imputationsof outcome measures for the first postsurgery assessment (3 months), the values were imputed by using the patient’s preoperative value and assuming the same % change from presurgery to postsurgery as obtained in the whole group. At all other follow-ups, the last value was carried forward [19, 24]. Missing data about satisfaction at 3-month postoperatively were imputed using the 6-month value.
Results
The preoperative questionnaire indicated that a minimum 50% of the study population had physiotherapy or guided gym/training related to back problem before the operation, on average 14 times (Table 2). Furthermore, about 2/3 reported that they had undertaken self-acting exercises (any source) and over 46% reported doing self-acting training minimum 1–2 times/week preoperatively.
Table 2.
Preoperative physiotherapy or guided gym/training (NPG) and back exercises
| A′ (n = 42) | A (n = 50) | B (n = 52) | All | |
|---|---|---|---|---|
| Physiotherapy visits or guided gym/training related to back problem (%) | ||||
| Yes (%) | 61.9 | 56.0 | 44.3 | 50.0 |
| No (%) | 26.2 | 26.0 | 28.8 | 27.5 |
| Not answered/missing | 11.9 | 18.0 | 26.9 | 22.5 |
| Back problem related NPG (mean (range)) | 14.8 (0–200) | 16.5 (0–200) | 11.2 (0–100) | 14 (0–200) |
| Self-acting back exercises, from any source | ||||
| Yes (%) | 69 | 66 | 67.3 | 66.7 |
| Frequency of back exercise training | ||||
| daily training | 11.9% | 12% | 11.5% | 11.8% |
| 3–5 times/week | 14.3% | 12% | 13.5% | 12.7% |
| 1–2 times/week | 16.7% | 18% | 25.0% | 21.6% |
| less than once a week | 19.0% | 18% | 15.4% | 16.7% |
| Not get/not done back exercises | 26.2% | 26.0% | 23.1% | 24.5% |
| Not answered/missing | 11.9% | 14.0% | 11.5% | 12.7% |
In A- and B-groups, all outcome measures significantly (p < 0.05) improved after the operation [31–36]. Figure 1 reveals that 7/50 patients in A-group were not able to undertake the anticipated (6/12) physiotherapy visits (3–6 months postoperatively), and there was one dropout leaving 42 patients in the “as-rehabilitated-group” (A′-group) for statistical analysis. A-group had significantly more postoperative physiotherapy visits or guided gym/training than B-group (22.10 vs. 9.17 times, respectively; p < 0.0001 t test). The frequency of self-acting exercises was also significantly higher in A-group during 3- to 24-month follow-up (p = 0.028, Mann–Whitney U test). In A′-group, the differences were even higher: 83.4% reported at least 3 to 5 times a week training frequency in a 3- to 6-month period, compared to 42.3% in B-group. At 12-month follow-up, the same figures were 47.6% (A′) and 28.8% (B) (Table 3). Total loss was 4.9% (four died due to not-study-related reasons; one drop-out).
Table 3.
All postoperative rehabilitation (physiotherapy and/or self-acting exercises) in treatment groups during intervention (3- to 24-month follow-up)
| Intervention + any other treatments in A and A′-groups | “Standard treatment” | |||
|---|---|---|---|---|
| A′ (n = 42) | A (n = 50) | B (n = 52) | All | |
| NPG during 3–24 month (mean) | 28.10 | 22.89 | 9.17 | 17.07 |
| FBE (mean summative score between 3–24 month) | 5.19 | 5.21 | 5.71 | 5.47 |
| Self-acting back exercise, from any source (yes, %) | 100% | 100% | 88.5% | 93.1% |
| FBE between 3 and 6 months | ||||
| Daily training | 54.8% | 48.0% | 23.1% | 35.3% |
| 3–5 times/week | 28.6% | 28.0% | 19.2% | 23.5% |
| 1–2 times/week | 11.9% | 12.0% | 26.9% | 19.6% |
| Less than once a week/not answered | 4.8% | 12.0% | 30.8% | 21.6% |
| FBE at 12 month (mean during last month) | ||||
| Daily training | 21.4% | 22.0% | 17.3% | 19.6% |
| 3–5 times/week | 26.2% | 22.0% | 11.5% | 16.7% |
| 1–2 times/week | 35.7% | 36.0% | 28.8% | 32.4% |
| Less than once a week/not answered | 16.7% | 20% | 42.3% | 31.4% |
| FBE at 24 month (mean during last month) | ||||
| Daily training | 4.8% | 6% | 7.7% | 6.9% |
| 3–5 times/week | 14.3% | 14% | 13.5% | 13.7% |
| 1–2 times/week | 40.5% | 40% | 32.7% | 36.3% |
| Less than once a week/not answered | 40.5% | 38% | 46.2% | 43.1% |
NPG number of physiotherapy visits or guided gym/training, FBE the frequency of back exercise training
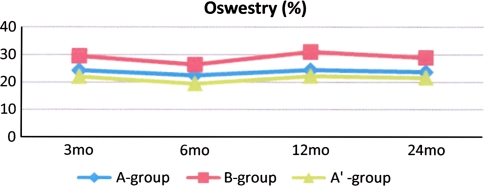
The study intervention did not improve the outcome compared to the standard treatment (Fig. 2; Table 4) in any points at 6-, 12- and 24-month follow-ups according ODI (p values were 0.74, 0.43 and 0.95 in “as-rehabilitated analyses”, respectively). The course of ODI was stable in the A′- (A) and in the B groups during the entire follow-up. The secondary outcome measures exhibited also stable courses (Table 4). Outcome measures appeared to be worse in B-group after randomization, but the levels were non-significant (p > 0.08, t test and Mann–Whitney U test depending on the distribution of the outcome measure).
Fig. 2.
Mean values of Oswestry Disability Index during intervention in treatment groups
Table 4.
Outcome of rehabilitation intervention
| Means of outcome measures of rehab-intervention | ||||
|---|---|---|---|---|
| At baseline | At primary end points | |||
| Postoperative stage | 3 mo | 6 mo | 12 mo | 24 mo |
| Oswestry (0–100%) | ||||
| A′(SD) p (A′vs. B) | 22.0 (15.3) | 19.4 (15.8) 0.74 | 22.2 (17.9) 0.43 | 22.4 (18.3) 0.95 |
| A (SD) p (A vs. B) | 24.3 (15.9) | 22.5 (17.8) 0.70 | 24.8 (19.1) 0.46 | 23.9 (19.1) 0.88 |
| B (SD) | 29.7 (20.5) | 26.4 (19.1) | 31.0 (20.1) | 28.9 (19.6) |
| Back pain (0–10) | ||||
| A′(SD) p (A′vs. B) | 1.3 (1.7) | 1.2 (1.7) 0.29 | 1.4 (1.7) 0.27 | 1.1 (1.8) 0.47 |
| A (SD) p (A vs. B) | 1.6 (1.9) | 1.4 (1.8) 0.33 | 1.6 (2.0) 0.27 | 1.5 (2.1) 0.61 |
| B (SD) | 2.0 (2.6) | 2.0 (2.6) | 2.4 (2.6) | 2.2 (2.5) |
| Leg pain (0–10) | ||||
| A′(SD) p (A′vs. B) | 2.4 (2.4) | 2.1 (2.3) 0.10 | 2.6 (2.8) 0.30 | 2.6 (2.9) 0.43 |
| A (SD) p (A vs. B) | 2.7 (2.6) | 2.4 (2.4) 0.11 | 2.8 (2.9) 0.26 | 2.8 (2.9) 0.69 |
| B (SD) | 3.2 (2.8) | 3.5 (2.9) | 3.5 (3.1) | 3.8 (2.8) |
| Satisfaction [3–(−3)] | ||||
| A′(SD) p (A′vs. B) | 1.7 (0.9) | 1.9 (1.0) 0.50 | 1.5 (1.0) 0.29 | 1.7 (1.1) 0.30 |
| A (SD) p (A vs. B) | 1.7 (0.9) | 1.8 (1.0) 0.49 | 1.5 (1.1) 0.44 | 1.7 (1.0) 0.50 |
| B (SD) | 1.3 (1.2) | 1.4 (1.0) | 1.3 (1.4) | 1.4 (1.1) |
| Treadmill (0–1,000 m) | ||||
| A′(SD) p (A′vs. B) | 758 (392) | – | 778 (372) 0.86 | 787 (354) 0.86 |
| A (SD) p (A vs. B) | 762 (381) | – | 759 (388) 0.70 | 782 (367) 0.58 |
| B (SD) | 718 (411) | – | 751 (390) | 738 (375) |
A′ patients who performed acceptably at the 3–6 month intervention (as-rehabilitated analyses), A all A-group (intention to treat analyses), B standard treatment group, mo month(s)
p values in General Linear Models (repeated measures; tests of within subjects effects, Huynh–Feldt) between study groups at 6-, 12- and 24 month primary end points are italicized
Discussion
Main findings and methodological considerations
This is the first prospective study of PR with LSS patients only. The result indicates that routinely performed active physiotherapy with strengthening and stretching home exercises do not improve functional outcome (ODI) as compared with standard treatment.
In the statistical analysis, the focus was on identifying the real impact of postoperative 3- to 24-month study intervention compared to standard treatment. This was done, first, by adjusting the real effect of the fulfilled self-acting training (FBE). Secondly, the functional ability and overall pain at the start of rehab-intervention were adjusted, since both were potential factors influencing the training capability. Finally, the data was analyzed in “as-rehabilitated analysis” according the fulfilled physiotherapy study-related visits between 3 and 6 months (A′ vs. B-group), in addition to comparing original the A- and B-groups (intention-to-treat -analysis). The presented group sizes and observed SD (18.5) enabled sufficient statistical power.
The intervention
The scientific basis for intervention was thus “conventional active physiotherapy”, in order to improve muscle strength and endurance, as well as stretchability, this being based on a training program with individually estimated volume and, if needed, quality of exercises. In the literature, the frequency for undertaking stretching exercises in different diseases and among older patients has varied from 2 to 7 days/week [2, 25]. We provided a daily recommendation to ensure the best effect to increase flexibility, with sufficient stretching time [3, 4, 29] and frequency [3, 13]. The recommended minimum frequency for muscle strengthening exercises was 3–5 days/week. According to the ACSM/AHA recommendations 2007, every adult should perform activities that maintain or increase muscular strength and endurance on a minimum of 2 days every week [12], this also being the case for older adults [25].
There are results in patients with lumbar disorders indicating that impaired abdominal muscle function in low back pain can benefit from abdominal muscle training in rehabilitation trials [18]. In addition, the benefits of physiotherapy in conservative treatment of LSS have been reported [23, 42, 43]. There was little evidence available concerning the effect of abdominal muscle training in a clinical setting when planning this study [14]. According to Simotas 2001 [30] and clinical experience, LSS patients have often a kyphotic posture, adopted to reduce the pincer effect of lordosis. This may cause a secondary shortening of the hip flexors. Hip flexor tightness (shortening) can aggravate the condition by forcing the patient to effectively increase lordosis. Lumbar hyperlordosis would increase the symptoms of LSS [7]. Maintaining better posterior tilt with better lumbopelvic muscular stabilisation has been claimed to relieve the symptoms in LSS [30]. Thus, the stability of lumbopelvic and abdominal muscles, as well as the flexibility of hip flexors, hamstrings and lumbar paraspinals was regarded as an important issue in the exercises, which were provided here in the postoperative setting.
Our results are in line with the randomized controlled trial by Mannion et al. [24] where a 12 week physiotherapeutic intervention (2 × 30 min/week with home exercises) did not lead to any differences between three groups (1. Controls, 2. stabilization exercises and 3. mixed techniques). Our intervention was more uniform, since our preplanned exercises were supervised by the same trained physiotherapists. Our intervention started 1 month later to minimize possible operation-related problems. We included also patients with fusion. Posterolateral fusion has been reported to be the most invasive procedure of the paraspinal muscles in various lumbar back surgery procedures. Paraspinal muscle injury during lumbar back surgery may be one of the most important factors that causes atrophy of the muscles [37]. The aim to rehabilitate after lumbar fusion is supported also by the finding that back muscle strength and endurance has been reported decline after lumbar fusion [22]. We believed that functional outcome after decompression with fusion could be improved by means of “conventional active physiotherapy” in the same way as after decompression only. This is because the intervention included training also for other muscles, i.e. it was not restricted to the back muscles. LSS patients with fusion have similar postoperative problems as tightness of hip flexors and hamstring and/or muscle weaknesses as patients with decompression only. The similar aspect in inclusion was when we did not want to exclude patients with isolated lateral stenosis, or patients who had additional disc herniation. We wanted also ensure that surgeon could feel free to undertake all the necessary procedures (fusion, disc excision) according to preoperative findings in operation, i.e. we ensured that our study could not possibly affect the “normal operative treatment of LSS”.
The specific home exercises of intervention were performed in the period 3–24 months postoperatively, which was considered to be long enough to reveal any improvement in the study groups, at any of 6- to 24-month follow-ups. The 12 week physiotherapeutic intervention was repeated at 12 months to motivate A-group to keep training, because in patients with chronic low back pain, an active progressive treatment program was more successful in the short term follow-up but the group difference in lumbar endurance tended to decline at the 1-year follow-up [20]. A′/A-group had clearly more guiding for self-training during the intervention by trained physiotherapist, who had also several years′ experience of treatment of postoperative back patients in the university DPRM clinic. Based on the remarkable greater amount of the NPG and the FBE in the A′-group, the study intervention should indicate the change in outcome measures at least in the first primary end point if the intervention had had any effect. Here, A′/A-group had had significantly more NPG and they had done 3 months intensively home exercises up to the 6 months follow-up. The FBE diminished in A-group at the end of the follow-up to the same level as noted in B-group, indicating that the 12 times “booster” intervention had no effect on frequency of the training and outcome at the end of the follow-up.
According to the latest study, 59% of patients had been prescribed physiotherapy after the LSS diagnosis by the spine surgeon; according to the therapists, 87% of them had been given flexibility-, 86% stabilization- and 83% strengthening exercises [38]. Thus, it is likely that LSS patients received supervised exercises in some course of the disease, which is supported by findings in B-group (67.3%) in preoperative phase (Table 3) but also in the postoperative phase (88.5%; Table 2).
Limitations
Due to several shortcomings, our results need to be interpreted with caution. It should be noted that this study could not determine whether our study intervention was better than “no exercises” or “no physiotherapy or guided gym” because of substantial activity in the B-group. The effect of the patients’ own activity on outcome in control group, or the natural postoperative course of the disease was also highlighted in the study of Mannion et al. [24].
The second weakness was that we permitted 10 of 102 patients to participate as controls without a true randomization. This decision was done after start of the study, when a patient did not have the opportunity to participate in the rehab-intervention if randomized to A-group, but he/she could participate as a control. Some patients had a long (even >100 km) distance to travel to DPRM, and some patients had also limited transport opportunities. To avoid potential drop-outs from rehab-intervention and to optimize recruiting time which took still 3 years, we made the compromise decision to allow totally ten patients to participate as controls (since they fulfilled all the other inclusion criteria of this study project). Thus, this was not a true randomization study. When comparing, however, A′-group (n = 42) for patients who were truly randomized in B-group (n = 42), there were no significant differences with ODI (6 month: p = 0.69; 12 month: p = 0.80; 24 month: p = 0.90).
The third weakness was that after randomization at the start of the intervention, the B-group had worse though not statistically significant worse symptoms and functional ability, and this situation lasted throughout the follow-up (Table 4). Referring Tables 1 and 3, the groups were balanced in terms of many parameters. The only difference was that in B-group there were more patients with previous lumbar operation (PLO) than in A-group (10 vs. 2). Patient with PLO had statistically significant worse disability and satisfaction after surgery (data not shown).
Contrary, those ten patients who were admitted to B-group without randomization had indeed less symptoms and disability than others in B-group. This indicates that bias in outcome measures between A- and B-groups should be coincidental, and these ten not-truly randomized patients in B-group did not explain the differences. Furthermore, it should be noted that outcome measures and PLO were not taken into account in the randomization. This emphasizes the importance of the adjustment of functional ability and pain at the start of the intervention in comparing unbalanced study groups [42]. Nonetheless, the most important point is that the courses of ODI (and also secondary outcome measures) were stable during the follow-up, indicating that intervention had no effect on their course.
Our result cannot rule out the possibility that individual physiotherapy for selected LSS patients, multidisciplinary intervention, earlier start of intervention, in-patient or other more specific rehabilitation intervention would be beneficial postoperatively in LSS.
The strengths of this study
The study population had LSS as the main diagnosis. We conducted both intention-to-treat- and “as-rehabilitated” analyses. The first physiotherapeutic guidance was 12 times (minimum 6 times) to adopt effective home exercises. A-group received significantly more guidance about the exercises at the university clinic with trained and experienced therapists (Table 4). The self-acting exercises were well performed during first year, especially during 3- to 6-month postoperative period. This kind of quality of physiotherapy and amount of training would be anticipated to improve the outcome, if the intervention were to have had any effect. The outcome measures were relevant, and have been used in surgical studies of LSS [1, 23, 24, 42]. By used covariates, the effect of initial pain, disability and accumulation of fulfilment of self-acting exercises on outcome could be controlled. This study is in line with the earlier literature [23], strengthening the concept that “treatment as usual” is sufficient postoperatively in LSS. With increasing life expectancy, the incidence and prevalence of LSS will increase in aging population, meaning increase also in rates of LSS surgery. To optimize resources and costs, postoperative physiotherapy should not be prescribed routinely in LSS to improve functional outcome.
Conclusion
Routinely performed outpatient rehabilitation, consisting of physiotherapy guidance with strengthening and stretching muscle exercises at home, did not improve subjective functional outcome during a postoperative two-year follow-up compared to standard treatment. In addition, there were no impact on pain, satisfaction and walking ability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
TJ Aalto was supported for this study a EVO grant by a Kuopio University Hospital, and a research grant from the Finnish Cultural Foundation (Hulda Tossavainen found. 2003; Aili and Leo Davidsson found. 2009; St. Michel Central Hospital 200-year Fund 2010). The authors thank for the associate personnel of the Kuopio University Hospital, especially secretaries and nurses of all associate clinics and DPRM enabling the fluent patient flow during study. Special thanks for physiotherapists Leena Hersio, Paula Heiskanen, Minna Siitari and Jaana Tervo. Also, of statistical advices we thank for Vesa Kiviniemi and prof. Seppo Sarna.
Conflict of interest None.
References
- 1.Aalto TJ, Malmivaara A, Kovacs F, et al. Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis: systematic review. Spine. 2006;31:E648–E663. doi: 10.1097/01.brs.0000231727.88477.da. [DOI] [PubMed] [Google Scholar]
- 2.American Geriatrics Society Exercise prescription for older adults in osteoarthritis pain: consensus practise recommendations. A supplement to the AGS Clinical Practise Guidelines on the management of chronic pain in older adults. J Am Geriatr Soc. 2001;49:808–823. doi: 10.1046/j.1532-5415.2001.00496.x. [DOI] [PubMed] [Google Scholar]
- 3.Bandy WD, Irion JM, Briggler M. The effect of time and frequency of static stretching on flexibility of the hamstring muscles. Phys Ther. 1997;77:1090–1096. doi: 10.1093/ptj/77.10.1090. [DOI] [PubMed] [Google Scholar]
- 4.Bandy WD, Irion JM, Briggler M. The effect of static stretch and dynamic range of motion training on the flexibility of the hamstring muscles. J Orthop Sports Phys Ther. 1998;27:295–300. doi: 10.2519/jospt.1998.27.4.295. [DOI] [PubMed] [Google Scholar]
- 5.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 6.Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16:22–28. doi: 10.1097/00002508-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Dong G, Porter R. Walking and cycling tests in neurogenic and intermittent claudication. Spine. 1989;14:965–969. doi: 10.1097/00007632-198909000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 9.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 10.Fergusson D, Aaron SD, Guyatt G, et al. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–654. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grönblad M, Hupli M, Wennerstrand P, et al. Intercorrelation and test-retest reliability of the Pain Disability Index (PDI) and the Oswestry Disability Questionnaire (ODQ) and their correlation with pain intensity in low back pain patients. Clin J Pain. 1993;9:189–195. doi: 10.1097/00002508-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR et al (2007) American College of Sports Medicine; American Heart Association. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116:1081–1093 [DOI] [PubMed]
- 13.Canada’s Physical Activity Guide to Healthy Active Living for Older Adults. Ontario: Ottawa; 1999. [Google Scholar]
- 14.Helewa A, Goldsmith CH, Lee P, et al. Does strengthening the abdominal muscles prevent low back pain-a randomized controlled trial. J Rheumatol. 1999;26:1808–1815. [PubMed] [Google Scholar]
- 15.Herno A, Airaksinen O, Saari T, et al. Lumbar spinal stenosis: a matched-pair study of operated and non-operated patients. Br J Neurosurg. 1996;10:461–465. doi: 10.1080/02688699647087. [DOI] [PubMed] [Google Scholar]
- 16.Herno A, Airaksinen O, Saari T, et al. The effect of prior back surgery on surgical outcome in patients operated on for lumbar spinal stenosis A matched-pair study. Acta Neurochir. 1996;138:357–363. doi: 10.1007/BF01420296. [DOI] [PubMed] [Google Scholar]
- 17.Herno A, Airaksinen O, Saari T, et al. Pre- and postoperative factors associated with return to work following surgery for lumbar spinal stenosis. Am J Ind Med. 1996;30:473–478. doi: 10.1002/(SICI)1097-0274(199610)30:4<473::AID-AJIM13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Hodges PW. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Exp Brain Res. 2001;141:261–266. doi: 10.1007/s002210100873. [DOI] [PubMed] [Google Scholar]
- 19.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kankaanpää M, Taimela S, Airaksinen O, et al. The efficacy of active rehabilitation in chronic low back pain. Effect on pain intensity, self- experienced disability, and lumbar fatigability. Spine. 1992;24:1034–1042. doi: 10.1097/00007632-199905150-00019. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Lipson SJ, Brick GW, et al. Clinical correlates of patient satisfaction after laminectomy for degenerative lumbar spinal stenosis. Spine. 1995;20:1155–1160. doi: 10.1097/00007632-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Keller A, Brox JI, Gunderson R, Holm I, Friis A, Reikerås O. Trunk muscle strength, cross-sectional area, and density in patients with chronic low back pain randomized to lumbar fusion or cognitive intervention and exercises. Spine. 2004;29:3–8. doi: 10.1097/01.BRS.0000103946.26548.EB. [DOI] [PubMed] [Google Scholar]
- 23.Malmivaara A, Slätis P, Heliövaara M, Finnish Lumbar Spinal Research Group et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1–8. doi: 10.1097/01.brs.0000251014.81875.6d. [DOI] [PubMed] [Google Scholar]
- 24.Mannion AF, Denzler R, Dvorak J, et al. A randomised controlled trial of post-operative rehabilitation after surgical decompression of the lumbar spine. Eur Spine J. 2007;16:1101–1117. doi: 10.1007/s00586-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson ME, Rejeski WJ, Blair SN et al (2007) American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116:1094–1105 [DOI] [PubMed]
- 26.Ostelo RW, Costa LO, Maher CG, et al. Rehabilitation after lumbar disc surgery. An update Cochrane Review. Spine. 2009;34:1839–1848. doi: 10.1097/BRS.0b013e3181abbfdf. [DOI] [PubMed] [Google Scholar]
- 27.Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;24:57–65. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 28.Raitasalo R (1977) Depression and its association with the need for psychotherapy (article in Finnish). The Social Insurance Institute of Finland Publications A:13, Helsinki
- 29.Roberts JM, Wilson K. Effect of stretching duration on active and passive range of motion in the lower extremity. Br J Sports Med. 1999;33:259–263. doi: 10.1136/bjsm.33.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simotas AC. Nonoperative treatment for lumbar spinal stenosis. Clin Orthop Relat Res. 2001;384:153–161. doi: 10.1097/00003086-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Sinikallio S, Aalto TJ, Airaksinen O, et al. Depressive burden in the preoperative and early recovery phase predicts poorer surgery outcome among lumbar spinal stenosis patients: a 1-year prospective follow-up study. Spine. 2009;34:2573–2578. doi: 10.1097/BRS.0b013e3181b317bd. [DOI] [PubMed] [Google Scholar]
- 32.Sinikallio S, Aalto TJ, Airaksinen O, et al. Lumbar spinal stenosis patients are satisfied with short-term results of surgery - younger age, symptom severity, disability and depression decrease satisfaction. Disabil Rehabil. 2007;29:537–544. doi: 10.1080/09638280600902646. [DOI] [PubMed] [Google Scholar]
- 33.Sinikallio S, Aalto TJ, Airaksinen O, et al. Depression is associated with poorer outcome of lumbar spinal stenosis surgery. Eur Spine J. 2007;16:905–912. doi: 10.1007/s00586-007-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinikallio S, Aalto TJ, Airaksinen O, et al. Somatic comorbidity and younger age are associated with life dissatisfaction among patients with lumbar spinal stenosis before surgical treatment. Eur Spine J. 2007;16:857–864. doi: 10.1007/s00586-006-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinikallio S, Aalto TJ, Airaksinen O, et al. Depression and associated factors in patients with lumbar spinal stenosis. Disabil Rehabil. 2006;28:415–422. doi: 10.1080/09638280500192462. [DOI] [PubMed] [Google Scholar]
- 36.Sinikallio S, Aalto TJ, Koivumaa-Honkanen H, et al. Life dissatisfaction is associated with a poorer surgery outcome and depression among lumbar spinal stenosis patients: a 2-year prospective study. Eur Spine J. 2009;18:1187–1193. doi: 10.1007/s00586-009-0955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suwa H, Hanakita J, Ohshita N, Gotoh K, Matsuoka N, Morizane A. Postoperative changes inparaspinal muscle thickness after various lumbar back surgery procedures. Neurol Med Chir. 2000;40:151–154. doi: 10.2176/nmc.40.151. [DOI] [PubMed] [Google Scholar]
- 38.Tomkins CC, Dimoff KH, Forman HS, Gordon ES, McPhail J, Wong JR, Battié MC. Physical therapy treatment options for lumbar spinal stenosis. J Back Musculoskelet Rehabil. 2010;23:31–37. doi: 10.3233/BMR-2010-0245. [DOI] [PubMed] [Google Scholar]
- 39.Tuomi K, Ilmarinen J, Jahkola A et al (1998) An approved version of the Work Ability Index. Occupational health—series 19. Finnish Institute of Occupational Health, Helsinki
- 40.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Viljanen M, Malmivaara A, Uitti J, et al. Effectiveness of dynamic muscle training, relaxation training, or ordinary activity for chronic neck pain: randomised controlled trial. BMJ. 2003;327:475. doi: 10.1136/bmj.327.7413.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the spine patient outcomes research trial. Spine. 2010;35:1329–1338. doi: 10.1097/BRS.0b013e3181cc52ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitman JM, Flynn TW, Childs JD, et al. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: a randomized clinical trial. Spine. 2006;31:2541–2549. doi: 10.1097/01.brs.0000241136.98159.8c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.