Abstract
Various mechanical stresses in vivo induce disc cell apoptosis and intervertebral disc (IVD) degeneration, but the underlying molecular mechanism is not fully known. The aim of this study was to investigate the role of endoplasmic reticulum stress in cyclic stretch-induced apoptosis of rat annulus fibrosus (AF) cells. Flexercell Tension Plus system was used to apply cyclic stretch to rat annulus fibrosus cells at a frequency of 0.5 Hz with 20% elongation for 12, 24, 36, or 48 h. Apoptosis was detected by flow cytometry, and nuclei morphologic changes were visualized by Hoechst 33258 staining and caspase-8, 9 activity assays. The expression of the markers of endoplasmic reticulum stress including CHOP, GRP78, and caspase-12 were determined by RT-PCR and Western blot. Mitochondrial membrane potential change was observed by JC-1 staining in situ. In addition, the levels of the nitric oxide (NO) were determined with the Griess reaction and fluorescence staining. The results indicated that cyclic stretch at a frequency of 0.5 Hz with 20% elongation-induced apoptosis in rat AF cells. Prolonged exposure of the unphysiologically cyclic stretch to AF cells caused NO overproduction, up-regulation of endoplasmic reticulum stress markers including CHOP, GRP78, and caspase-12, depolarization of mitochondria and activation of caspase-9. However, cyclic stretch at this level had no effect on caspase-8 activity. In addition, specific inhibitor of caspase-12 (Z-ATAD-FMK) and caspase-9 (Z-LEHD-FMK) partly suppressed cyclic stretch-induced AF cell apoptosis and the anti-apoptotic effects of the caspase inhibitors were additive. Our data suggest that endoplasmic reticulum stress, likely mediated by NO, contributes to the AF cell apoptosis induced by cyclic stretch in addition to the mitochondrial pathway. These findings could be helpful to understand the mechanism of disc cell apoptosis, the root cause of IVD degeneration.
Keywords: Intervertebral disc, Nitric oxide, Endoplasmic reticulum stress, Cyclic stretch, Apoptosis
Introduction
Excessive apoptosis of disc cells plays a pivotal role in the development of IVD degeneration, which frequently contributes to neck or low back pain [43]. Mechanical stimuli in physiologic ventilation have been shown a protective signal by stimulating cell proliferation of the nucleus pulposus [25], anabolic metabolism of ECM in annulus fibrosus (AF) cells [37]. However, mechanical stimuli, such as cyclic stretch, also leads to disc cell apoptosis and disc degeneration, as shown in both animal models of overloading-induced disc degeneration and cultured disc cells or tissues subjected to unphysiologically static compression or cyclic stretch [1, 10, 22]. Previous study has suggested that cyclic stretch-induced AF cell apoptosis was mediated through the mitochondrial pathway [34]. Interestingly, blocking the mitochondrial apoptotic pathway with inhibitors of caspase-8 or caspase-9 failed to completely suppress the apoptosis of disc cells [31, 34], indicating that other pathways may participate in the process of apoptosis in AF cells, in addition to the death receptor and mitochondrial pathways.
Activation of endoplasmic reticulum stress-induced apoptosis pathway has been shown to contribute to various degenerative diseases including Alzheimer’s disease [36], Parkinson’s disease [38] and osteoarthritis [19]. Endoplasmic reticulum is a major storage organelle for calcium and the site of synthesis and folding of proteins, designated for secretion, cell membrane, Golgi apparatus, and lysosomes. Moderate endoplasmic reticulum stress seen under various cellular stresses triggers unfolded protein response (UPR) to protect cells, whereas severe endoplasmic reticulum stress disturbs endoplasmic reticulum function and threaten cell survival, and then activate endoplasmic reticulum stress-mediated apoptosis pathway [6].
The endoplasmic reticulum stress marker GRP78 (a molecular chaperone) facilitates the restoration of proper protein folding within the endoplasmic reticulum. When misfolded proteins accumulate in the state of endoplasmic reticulum stress, GRP78 releases from the stress sensors, thus leading to UPR activation. Caspase-12 belongs to the ICE (caspase-1) subfamily of caspase cysteine protease family, and exists as inactive pro-forms and is specifically activated by endoplasmic reticulum stressors such as tunicamycin, thapsigargin, or calcium ionophores. Activated caspase-12 cleaves procaspase-9 and consequently leads to the activation of the caspase cascade and cell apoptosis, which was independent of mitochondrial-triggered signal [27, 28]. The caspase-12-deficient mice are partially resistant to endoplasmic reticulum stress-induced apoptosis [28]. CHOP (C/EBP homologous protein), also known as growth-arrest and DNA-damage inducible gene 153 (GADD153), is another protein strongly induced by endoplasmic reticulum stress as part of the UPR [29, 39]. Notably, besides chemical stressors such as hypoxia, lipopolysaccharide, calcium ionophore, and nutrient deprivation [35], application of high amplitude cyclic stretch induces CHOP expression and cell apoptosis through endoplasmic reticulum stress pathway in rat vascular smooth muscle cells [7]. These findings suggest that the endoplasmic reticulum stress may translate force-induced signals into apoptotic responses.
Nitric oxide (NO), an important multifunctional biomolecule, exerts various cytotoxic effects including inhibition of mitochondrial function and activation of endoplasmic reticulum stress [14], and is known to be involved in a variety of diseases such as neurodegenerative disorders, arteriosclerosis, and diabetes mellitus [13, 20]. For instance, endoplasmic reticulum stress triggered by NO overproduction, leads to β-cell apoptosis via CHOP induction and caspase-12 activation [29]. Furthermore, cyclic stretch can induce NO overproduction [2, 33] and even cause apoptosis [34] in AF cells. These data suggest that NO may participate in force-induced signals into apoptotic responses by endoplasmic reticulum stress.
AF cells are subjected to a variety of stresses in vivo such as osmotic stress [5] and various mechanical stresses [8, 16]. Despite its obvious importance, however, endoplasmic reticulum stress response has not been documented regarding AF cell apoptosis induced by cyclic stretch so far. Therefore, we investigated the role of endoplasmic reticulum stress in the process of apoptosis induced by cyclic stretch through detecting the expression of endoplasmic reticulum stress marker GRP78, CHOP, and caspase-12. We also examined NO production, caspase-8 and 9 activity, and mitochondrial membrane potential in rat AF cells after cyclic stretch. We hypothesized that endoplasmic reticulum stress, likely mediated by NO, would be involved in the process of apoptosis induced by cyclic stretch in addition to the mitochondrial pathway.
Materials and methods
Isolation and culture of rat annular cells
All protocols were approved by the authors’ institutional Animal Care and Use Committee. The lumbar spine segment was aseptically excised from 3 months-old female Sprague–Dawley rats immediately after killed by intravenous administration of 150 mg/kg pentobarbital sodium. The annulus fibrosus of lumbar IVDs were dissected free from their upper and lower vertebrae after the NP and inner AF as well as the surrounding ligaments were discarded. AF tissues were minced into small pieces (<1 mm3) and then digested with 0.2% pronase (Roche) for 90 min followed by overnight digestion with 0.025% collagenase Type II (Sigma), 0.01% hyaluronidase Type V (Sigma) in a gyratory shaker (110 rev/min) at 37°C. Then tissue debris was passed through a 70 μm mesh to obtain a single-cell suspension.
After centrifugation for 5 min (700 rev/min) and the cellular pellet was resuspended in Dulbecco’s modified Eagle medium/Ham’s F-12 (DMEM/F-12, Gibco) containing 10% fetal bovine serum (FBS, Gibco), 1% penicillin/streptomycin (Gibco), and then plated in 60-mm tissue culture dish in a 37°C, 5% CO2 environment. The medium was changed every other day. First-passage cells maintained in a monolayer were used throughout the experiments.
Application of cyclic tension on cultured cells
For cyclic tension loading, AF cells were placed on an elastic silicone membrane coated with collagen I (BioFlex, Flexcell International, Hillsborough, NC) at a density of 2 × 105 cells/well (diameter 35 mm) for culture. After reaching 80–90% confluence, the cells were serum-starved in DMEM/F-12 containing 1% FBS for 24 h for synchronization and then stretched using a Flexercell Tension Plus system (FX-4000T, Flexcell International) at 37°C in a 5% CO2 incubator in 2.0 ml of DMEM/F-12 supplemented with 10% FBS. This device consists of a computer-controlled vacuum unit applying a precise vacuum to the silicone membrane. The deformation of the membrane is directly transmitted to the adherent cells. The experimental protocol delivered 20% stretch at a frequency of 0.5 Hz for 12, 24, 36, or 48 h. The tensile strain in this range does not result in changes in cell phenotype of AF cells [32].
Detection of apoptotic incidence by flow cytometry
Apoptotic incidence was measured with the Annexin V-FITC apoptosis detection kit I (BD Pharmingen) according to the manufacturer’s instructions. Briefly, cells were washed twice with cold PBS, and then resuspended in 500 μl of binding buffer at a concentration of 1 × 106 cells per ml. 5 μl of annexin V-FITC solution and 5 μl of PI (propidium iodide, 1 mg/ml) were added to these cells at 37°C for 30 min. The cells were analyzed by flow cytometry within 1 h. Apoptotic cells were counted and represented as a percentage of the total cell count.
Caspase-8, 9 activity assay
Caspase-8, 9 activities were determined with a caspase assay kit (Beyotime) according to the manufacturer’s protocol. After stretch, cells were lysed with lysis buffer for 15 min on ice and cell lysates were centrifuged at 16,000g for 10 min at 4°C. Caspase-8, 9 activity were quantified with a microplate spectrophotometer (Biotek) at an absorbance of 405 nm. Caspase-8, 9 activity were expressed as the fold of enzyme activity compared to that of synchronized cells.
Real-time PCR analysis for the expression of CHOP, GRP78, Caspase-12 and iNOS
Total RNA was extracted from treated cells using 1 mL TRIzol reagent (invitrogen). cDNA was synthesized from 1 μg total RNA through reverse transcription using a TaKaRa RNA PCR Kit Ver. 2.1 (TaKaRa Bio). The sequences for primers are shown as following: CHOP: 5′-GGAAAGTGGCACAGCTTGCT-3′, TCAGGCGCTCGATTTCCT-3′, GRP78: 5′-ACGTCCAACCCGGAGAACA-3′, 5′-TTCCAAGTGCGTCCGATGA-3′, caspase-12: 5′-CGACAGCACATTCCTGGTCTT-3′, 5′-CACCCCACAGATTCCTTCCA-3′, iNOS: 5′-TGGTGAAAGCGGTGTTCTTTG-3′, 5′-ACGCGGGAAGCCATGA-3′, GAPDH: 5′-CCTGGAGAAACCTGCCAAGTAT-3′, 5′-CTCGGCCGCCTGCTT-3′. Quantitative real-time PCR was performed by using 1 μg of cDNA and SYBR Green (Bio-Bad) in 36-well plates in a LightCycler rapid thermal cycler system (Roche Diagnostics). PCR products were subjected to melting curve analysis, and the data were analyzed by the 2−ΔΔCT calculation method and standardized by GAPDH.
Effects of caspase inhibitors on the tension-induced apoptosis
Primary cells were treated as described above and synchronized for 22 h. Then, the medium was changed and cells were pre-incubated with 10 μM Ac-LEHD-CMK [18] (a caspase-9-specific inhibitor, BioVision), or 2 μM Z-ATAD-FMK (a specific inhibitor of caspase-12, thus suppressing the endoplasmic reticulum-mediated apoptosis; [18, 23, 24], BioVision) or the both for 2 h, respectively. Cells were static without any inhibitor as negative control; Cells were stretched without any inhibitor as positive control. Then, cells were stretched for 36 h and apoptotic incidence was detected as described above.
Fluorescence double labeling study
After serum starvation for synchronization, the cells were stretched in 10% FBS by Flexercell Tension Plus system for 36 h and harvested for fluorescence double labeling of CHOP and GRP78 or of CHOP and caspase-12. The cells after serum starvation were cultured for 36 h in 10% FBS without stretch as control. Briefly, Cells were fixed in 4% paraformaldehyde and permeated by 0.2% Triton X-100. Then, the cells were incubated overnight at 4°C with primary antibodies (anti-CHOP, 1:100, Santa Cruz and anti-GRP78, 1:100, Santa Cruz), or with primary antibodies (anti-CHOP, 1:100, Santa Cruz and anti-caspase-12, 1:100, Santa Cruz), then incubated with secondary antibodies (FITC-bound anti-rabbit IgG; and Cy3-bound anti-mouse IgG, 1:200, Jackson) at room temperature for 2 h. Finally, nuclear DNA was counterstained by incubating with 2 μg/ml Hoechst 33258 (Sigma) in PBS at room temperature for 5 min. The stained results were visualized under a fluorescence microscope in the same field.
Western blotting
Total protein was extracted using a Western & IP cell lysis kit after cyclic stretch. Whole cell extracts were separated by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA). After the PVDF membrane had been incubated with 10 mM TBS with 1.0% Tween 20 and 10% dehydrated skim milk to block nonspecific protein binding, the membranes were incubated at 4°C overnight with rabbit polyclonal antibody against GRP78 (Santa Cruz, sc-13968; 1:100 dilution), rabbit polyclonal antibody against caspase-12 (Santa Cruz, sc-5627; 1:75 dilution), mouse monoclonal antibody against CHOP (Santa Cruz, sc-7315; 1:100 dilution) and mouse monoclonal antibody against β-actin (Beyotime, AA128; 1:750 dilution). Then the membranes were washed with TBST and incubated with alkaline phosphatase-linked secondary antibodies (Jackson Immunoresearch, PA). After being washed with TBST, immunoreactive bands were visualized using NBT/BCIP as substrate.
Assay of mitochondrial membrane potential changes in situ
JC-1 probe was employed to measure mitochondrial depolarization in rat AF cell. After cyclic tension, the cells were incubated at 37°C for 20 min with 5 mg/l JC-1 (Beyotime), then washed twice with PBS and placed in 2 ml culture medium. A green fluorescent (JC-1 as a monomer at low membrane potentials) and a red fluorescent (JC-1 as “J-aggregates” at higher membrane potentials) were monitored under a fluorescence microscope. Mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio.
Determination of the nitric oxide in supernatant
Primary cells were seeded into 6-well plates at 2 × 105 cells per well. After cyclic tension application, the supernatants were harvested and the levels of the nitric oxide (NO) were determined with the Griess reaction [11] according to instructions provided by the manufacturer (Beyotime, China). Briefly, 100 μl of medium or standard NaNO2 was mixed with 100 μl of Griess reagent in a 96-well plate. After 15 min, optical density was read in a microplate reader at 540 nm.
Observation for the nitric oxide in situ
AF cells were stretched for 12 or 36 h, the medium was removed and 2 ml of 3-Amino,4-aminomethyl-2′,7′-difluorescein, diacetate (DAF-FM DA, 5 μmol/l) was added into the well. The cells were incubated in 5% CO2 environment at 37°C for 20 min in the dark and then washed three times with PBS (10 mM, pH 7.4). The cell staining was visualized under a fluorescence microscope.
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical analyses were performed using the SPSS 11.5 statistical software program. Statistical significance was evaluated by one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test.
Results
Cyclic stretch-induced rat AF cells apoptosis
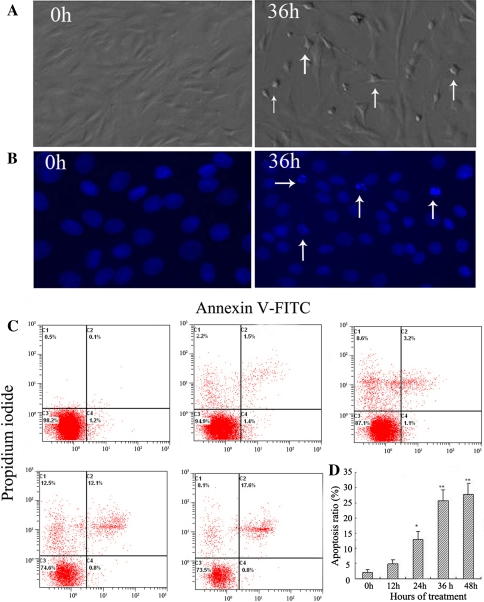
The morphologic features of stretched cells, evaluated under a phase-contrast microscope, gradually showed apoptotic morphology after 36 h of stretching, including cell rounding and shrinkage, vacuolization in the cytoplasm, shedding of smaller fragments from the cells, and detachment from the silicone membrane (Fig. 1a). As shown in Fig. 1b, the cells subjected to 20% cyclic stretch for 36 h showed an increase of chromatin condensation and nuclear fragmentation by Hoechst 33258 staining. The results indicated that the cell death occurred in an apoptotic manner.
Fig. 1.
Cyclic stretch induces cell apoptosis in AF cells. Rat AF cells were subjected to 20% stretch at a frequency of 0.5 Hz for 12, 24, 36, or 48 h, and those cells kept static were used as controls. a Phase-contrast photomicrograph of rat AF cells kept static (0 h) or subjected to cyclic stretch for 36 h. Apoptotic cells were characterized by cell shrinkage and detachment from the silicone membrane (arrows). Original magnification ×160. b Hoechst 33258 staining of AF cells: Apoptotic nuclei were featured by condensed or fragmented DNA brightly stained with Hoechst 33258 (arrows, 36 h). Original magnification ×200. c Representative graphs obtained by flow cytometry analysis for cell apoptosis after double staining with annexin V-FITC and propidium iodide. d The apoptotic incidences of rat AF cells treated for 12, 24, 36, or 48 h. Compared with static control cells (0 h, 2.0 ± 0.8%), the apoptotic rate of rat AF cells obviously increased to 12.9 ± 2.7%, 25.8 ± 3.5%, and 27.6 ± 2.8% after cyclic stretch for 24, 36, and 48 h, respectively (n = 5). *P < 0.05 vs. control, **P < 0.01 vs. control
The rate of cell apoptosis was detected by flow cytometry by double labeling with annexin V and PI. Representative graphs obtained by flow cytometry analysis of stretched cells after double staining with annexin V-FITC and PI were shown in Fig. 1c. The apoptotic rate in the static control cells was 2.0 ± 0.8%. There was a time-dependent increase in the apoptotic rate of rat AF cells exposed to the cyclic stretch. Hence, the apoptotic rates in the AF cells were increased to 4.8 ± 1.4%, 12.9 ± 2.7%, 25.8 ± 3.5%, and 27.6 ± 2.8% after cyclic stretch for 12, 24, 36, and 48 h, respectively (Fig. 1d).
Cyclic stretch enhances CHOP, GRP78, and caspase-12 expression in rat AF cells
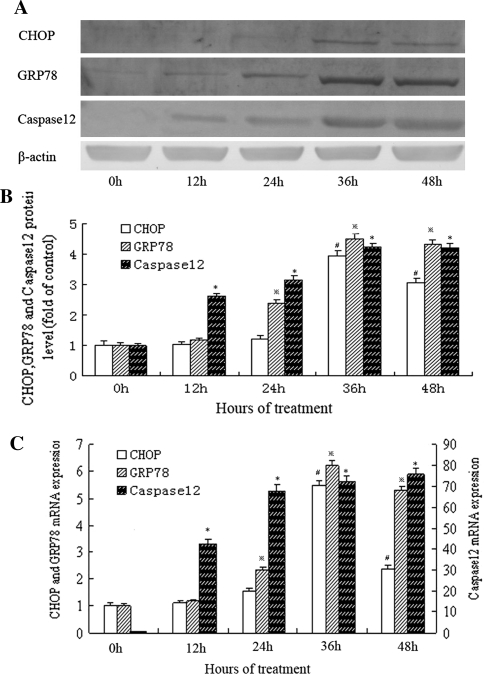
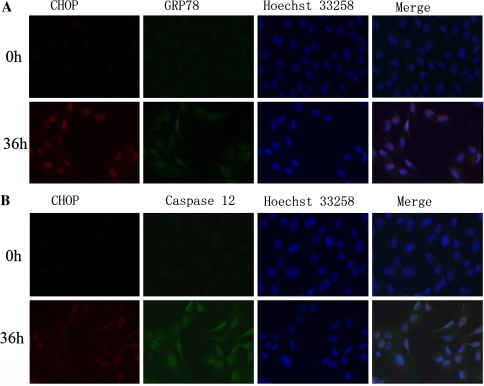
As shown in Fig. 2a, b, the level of CHOP protein began to increase as early as 24 h after stretch to 20% elongation was applied and reached its peak by 36 h. Real-time PCR analysis also showed that CHOP mRNA increased maximally after 36 h of stretch at 20% elongation (Fig. 2c). Using the immunocytological method, we further confirmed the induction of CHOP in cells subjected to cyclic stretch for 36 h but not in the static group cells (Fig. 3). According to western blot analysis, we found that the expression of GRP78 increased gradually in the cells subjected to cyclic stretch over time, reached a maximum of 4.5-fold over the static control by 36 h (Fig. 2a, b). The increase of GRP78 mRNA, as measured by real-time PCR, almost paralleled with that of GRP78 protein (Fig. 2c). This was further confirmed by an increase in the number of GRP78-positive cells after 36-h of treatment observed under the fluorescence microscope relative to the static group (Fig. 3). Our results also showed that the expression of caspase-12 at both mRNA and protein levels were markedly elevated in the cells subjected to cyclic stretch (Fig. 2a–c). The results of immunolocalization at 36 h time-point validated the findings (Fig. 3).
Fig. 2.
Effects of cyclic stretch on the protein and mRNA levels of ER stress markers in rat AF cells. a Representative western blots for CHOP, GRP78, and caspase-12 in rat AF cells subjected to cyclic stretch by 20% elongation for 12, 24, 36, or 48 h. b Quantitative analysis of CHOP, GRP78, and caspase-12 protein levels. The values from treated cells have been normalized to values in static control cells (n = 5). #P < 0.05 vs. control;  P < 0.05 vs. control; *P < 0.05 vs. control. c The mRNA expression of CHOP, GRP78, and caspase-12 by RT-PCR in rat AF cells subjected to cyclic stretch for 12, 24, 36, or 48 h. The values from treated cells have been normalized to GAPDH measurement and then expressed as a ratio of normalized values to mRNA in control cells (n = 5). #P < 0.05 vs. control;
P < 0.05 vs. control; *P < 0.05 vs. control. c The mRNA expression of CHOP, GRP78, and caspase-12 by RT-PCR in rat AF cells subjected to cyclic stretch for 12, 24, 36, or 48 h. The values from treated cells have been normalized to GAPDH measurement and then expressed as a ratio of normalized values to mRNA in control cells (n = 5). #P < 0.05 vs. control;  P < 0.05 vs. control; **P < 0.01 vs. control
P < 0.05 vs. control; **P < 0.01 vs. control
Fig. 3.
After serum starvation for synchronization, fluorescence double labeling of CHOP and GRP78, CHOP and caspase-12 in rat AF cells subjected to cyclic stretch by 20% elongation in 10% FBS for 36 h. The cells after serum starvation were cultured for 36 h in 10% FBS without stretch (stretched for 0 h) as control. a CHOP and GRP78. b CHOP and caspase-12. In a and b, the 3 photographs in each line were taken under a same field and then were merged
Cyclic stretch enhanced NO production from rat AF cells
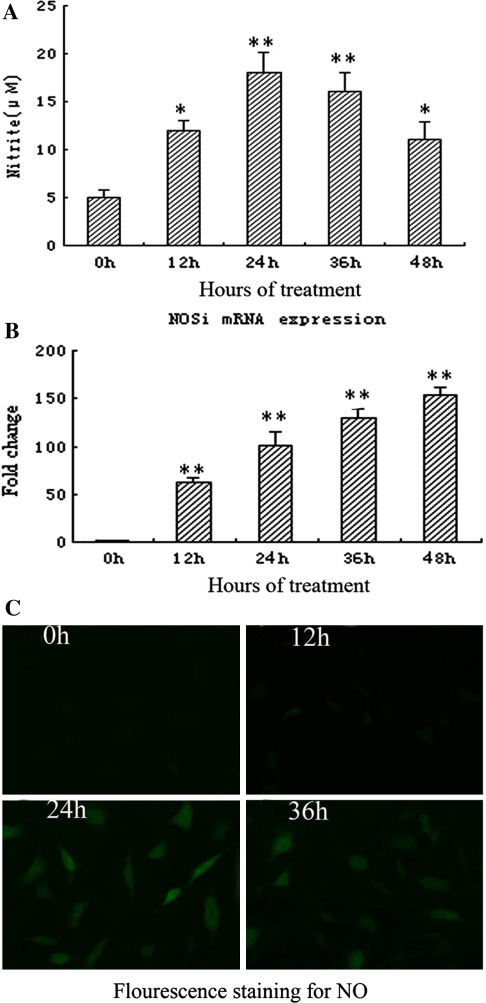
As shown in Fig. 4a, after cyclic stretch, the accumulation of NO in the supernatant was significantly increased than that in static group. Also, exposure to cyclic stretch increase the mRNA levels of iNOS in parallel with NO production. The results of fluorescence staining showed that NO-positive cells (green) significantly increased in the cells subjected to cyclic stretch for 24 and 36 h than that in static group (Fig. 4c).
Fig. 4.
Effects of cyclic stretch on NO production from rat AF cells. a The effect of cyclic stretch on NO production of rat AF cells for different duration in the supernatant (n = 5). *P < 0.05 vs. control, **P < 0.01 vs. control. b The effect of cyclic stretch on iNOS mRNA expression of rat AF cells for different duration (n = 5). **P < 0.01 vs. control. c Fluorescence staining for NO. NO-positive cells (green) obviously increased in the cells subjected to cyclic stretch for 24 and 36 h than that in static control (0 h)
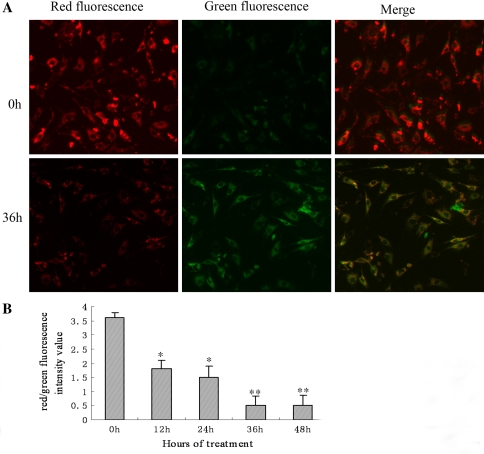
Cyclic stretch decreased mitochondrial membrane potential
Static AF cells stained with JC-1 emitted mitochondrial red fluorescence with a little green fluorescence, indicative of normal polarization state. The JC-1 aggregates were dispersed to the monomeric form (green fluorescence) in the cells subjected to cyclic stretch for 36 h (Fig. 5a). The results showed that cyclic stretch reduced the ratio of red (JC-1 aggregates) to green (JC-1 monomers), indicating that cyclic stretch caused the dissipation of mitochondrial membrane potential (Fig. 5b).
Fig. 5.
Cyclic stretch decreased mitochondrial membrane potential in rat AF cells. a Representative photographs of JC-1 staining in different groups. In a, the 2 photographs in each line were taken under a same field and then were merged. b Quantitative analysis of the shift of mitochondrial red fluorescence to green fluorescence among groups. Red/green fluorescence intensity value was calculated. All values are showed as means ± SD from 12-independent photographs shot in each group. *P < 0.05 vs. control, **P < 0.01 vs. control
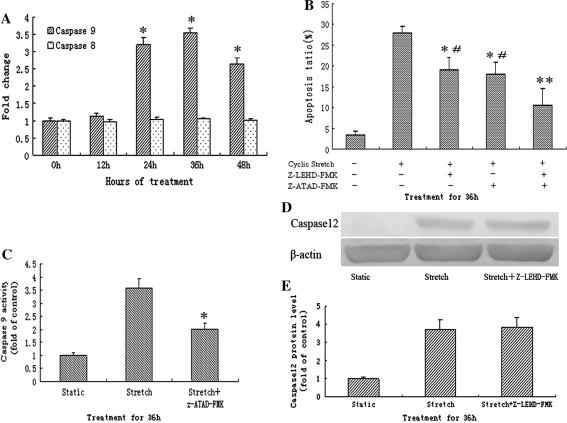
Activation of caspase-9 by cyclic stretch
As shown in Fig. 6a, compared to that in static control, caspase-9 activity in the cells subjected to cyclic stretch began to increase at 24 h and reach a maximum by 36 h with 3.2 ± 1.05 and 3.54 ± 1.07 fold, respectively. Simultaneously, we found that activity of caspase-8 was not changed in the cells subjected to cyclic stretch for different duration.
Fig. 6.
Activation of caspase-9 by cyclic stretch and the role of selective inhibitors on apoptosis. a The effect of cyclic stretch on caspase-8, 9 activities in rat AF cells. Cyclic stretch significantly increased the activity of caspase-9 after 24 h but not caspase-8 (n = 5). *P < 0.05 vs. control. b Z-LEHD-FMK and Z-ATAD-FMK suppressed of apoptosis induced by cyclic stretch for 36 h in rat AF cells (n = 5). *P < 0.05 vs. stretch alone. #P < 0.05 vs. stretch + Z-LEHD-FMK + Z-ATAD-FMK group. c Z-ATAD-FMK significantly inhibited caspase-9 activity induced by cyclic stretch for 36 h in rat AF cells (n = 5). *P < 0.05 vs. stretch alone. d Representative western blots for caspase-12 after 36 h cyclic stretch in rat AF cells pretreated with Z-LEHD-FMK or not. e Quantitative analysis of caspase-12 protein levels. The values from treated cells have been normalized to values in static control cells
Suppression of apoptosis by Z-LEHD-FMK and Z-ATAD-FMK
The effect of selective inhibitors for caspase-9 (Z-LEHD-FMK) and caspase-12 (Z-ATAD-FMK) on the rate of apoptosis at 36 h after cyclic stretch were shown in Fig. 6b. Each inhibitor, when applied alone, significantly diminished cyclic stretch-induced apoptosis in rat AF cells relative to vehicle treated cells that were exposed to the same stretch. Moreover, apoptotic incidence was further decreased in the cells that were co-incubated with Z-LEHD-FMK and Z-ATAD-FMK.
Effect of Z-ATAD-FMK on caspase-9 activity
To identify which is the upstream caspase between caspase-9 and caspase-12, we detected caspase-9 activity of the cells pretreated with the caspase-12 inhibitor (Z-ATAD-FMK), or vise versa, the expression of caspase-12 in cells pretreated with the caspase-9 inhibitor (Z-LEHD-FMK) after 36-h of cyclic stretch. As shown that in Fig. 6c, blocking caspase-12 with Z-ATAD-FMK significantly inhibited caspase-9 activity induced by cyclic stretch in rat AF cells. However, Z-LEHD-FMK had no effect on the protein expression of caspase-12 in cells applied to cyclic stretch (Fig. 6d, e).
Discussion
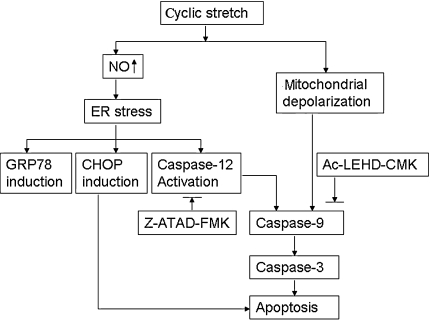
In this study, we addressed the question whether endoplasmic reticulum stress response is involved in the process of apoptosis induced by cyclic stretch in AF cells. The main findings of our study are as follows. First, we demonstrated that cyclic stretch for 20% elongation affected the morphology and induced apoptosis of rat AF cells. Second, we showed that unphysiological cyclic mechanical stretch induces NO overproduction and the expression of endoplasmic reticulum stress markers (CHOP, GRP78, and caspase-12) in rat AF cells. Further, we found that Z-ATAD-FMK, the caspase-12 inhibitor, partly suppressed apoptosis caused by cyclic stretch in rat AF cells (Fig. 7).
Fig. 7.
Proposed model of signaling pathways involved in AF cell apoptosis caused by cyclic stretch
The apoptosis of AF cells (a fibrochondrocyte) has been widely investigated for its potential involvement in the onset of IVD degeneration. AF cells are believed to experience tensile stress in vivo and response to cyclic stretch in a magnitude-dependent manner in vitro. For instance, a 15% stretch significantly increased rat AF cell apoptosis [34], but there was no change in cell viability following a 5% stretch [4] compared to unloaded cells in AF cells. In other study, the evidence showed that 20% cyclic stretch increased prostaglandin E2 synthesis and the expression of cyclooxygenase 2 mRNA in AF cells, suggesting that high level of cyclic stretch might be involved in the pathomechanism of pain induction in lumbar disc diseases [26]. Therefore, we chose 20% stretch as an unphysiological stretch to induce the apoptosis of AF cells, whereas a total of 15% area change was considered as the physiological limits of stress observed in IVD [3]. After cyclic stretch, cells gradually exhibited cell rounding and shrinkage, vacuolization and even detachment from bottom in a time-dependent manner, which were the important characteristics of cell apoptosis. Meanwhile, increased chromatin condensation and nuclear fragmentation over time were observed by Hoechst 33258 staining. Previous studies had shown that cyclic stretch (15%, 24 h) induced a significant increase in apoptosis (17 ± 11%) as compared with that in static control [34], which was further supported by our findings that the apoptosis ratio was 25.8 ± 3.5% after cyclic stretch (20%, 36 h). The variances may come from the difference with stretch in amplitude, duration and species. We conclude that unphysiological cyclic stretch to disc cells will lead to cell death through the apoptosis pathway.
In the present study, we first demonstrated that endoplasmic reticulum stress participated in the process of apoptosis induced by cyclic stretch, as coincided with the results in other studies that the apoptosis mediated by endoplasmic reticulum stress in chondrocytes contributed to the pathogenesis of several high prevalence diseases such as osteoarthritis [19] and periodontitis [41]. The endoplasmic reticulum is an important cellular organelle where secretory and membrane proteins are synthesized, folded, and assembled. ER stress can be caused by abnormality in protein glycosylation and Ca2+ homeostasis, oxidative stress and deprivation of glucose and oxygen, and is characterized by unfolded protein response (UPR), induction of numerous chaperone proteins and even apoptosis. Here, our results showed that unphysiological cyclic stretch induced the expression of CHOP, GRP78, caspase-12, and apoptosis in rat AF cells. After cyclic stretch for 24 h and beyond, we saw the expression of GRP78 and caspase-12 at both mRNA and protein levels and modest increased in apoptosis. Those phenomena were not observed at 12 h time-point. These findings indicated that ER stress occurs as early as 24-h post the application of cyclic stretch to the AF cells. Notably, the strong expression of caspase-12 and massive apoptosis was only observed at 36 h time-point, suggesting that activation of caspase-12 plays a pivotal role in the ER stress apoptotic pathway.
Previous studies have indicated that cyclic stretch leads to apoptosis mainly through the mitochondrial pathway but not Fas signaling pathway in AF cells [30, 34]. In the present study, a significant reduction of mitochondrial potentials was also observed after cyclic stretch in a time-dependent manner, as reported by other study [34]. Interestingly, depolarization of mitochondria did not synchronize with the increased apoptotic incidence, indicating there are other death pathways involved in cyclic stretch-induced apoptosis, in addition to the mitochondrial pathway.
The activation of caspase-8, which in turn activates downstream caspases, leads to apoptosis through death receptor pathway, whereas the activation of caspase-9, which forms the apoptosome with released Cytochrome c and Apaf-1, activates the executioner caspase-3, and induces apoptosis through mitochondrial pathway [42]. In this study, consistent with the study of Rannou et al. [34], we also found that cyclic stretch had no effect on caspase-8 activity, suggesting the death receptor caspase-8 apoptotic pathway was not involved in apoptosis induced by cyclic stretch in AF cells. Notably, we found that cyclic stretch enhanced caspase-9 activity in time-dependant manner and meanwhile induced both the activation of caspase-12 and the expression of CHOP in AF cells. Furthermore, Z-ATAD-FMK (a caspase-12-specfic inhibitor) and Z-LEHD-FMK (a caspase-9-specfic inhibitor) partly suppressed cell apoptosis induced by cyclic stretch, and a combination of the both inhibitors afforded much greater protection of the AF cells from apoptosis. The activation of caspase-12 is likely to occur prior to the activation of caspase-9 during the process of apoptosis induced by cyclic stretch, because Z-ATAD-FMK attenuated the activation of caspase-9, whereas Z-LEHD-FMK had no effect on caspase-12 activity. These results are in concordance with those reported in other studies, showing the linkage of the activated caspase-12 cleaves and activates procaspase-9 and consequently leads to activation of the caspase cascade and apoptosis [12]. Interestingly, we observed that the caspase-12 activity increased earlier than the caspase-9 activity by cyclic stretch and the phenomenon was perhaps elucidated by two reasons: One reason could be that activation of caspase-9 was dependent on caspase-12 from ER stress and the other could be that ER stress response originally occurred earlier than the depolarization of mitochondria induce by cyclic stretch.
NO plays a key role in the onset of osteoarthritis and IVD degeneration by inhibiting collagen and aggrecan synthesis, stimulating of MMP production and inducing chondrocyte apoptosis [9, 15, 21]. Excessive NO may directly disturb ER function and activate ER stress pathway [14, 17, 40]. Here, we demonstrated significant increase of NO production and iNOS mRNA in rat AF cells exposed to cyclic stretch, which was consistent with previous studies showing that NO were up-regulated by the stimulation of prolonged cyclic stretch in AF cells [2, 33]. NO overproduction appears to occur before the onset of ER stress and subsequent apoptosis, thus it is conceivable that NO production is a potential trigger of ER stress observed in rat AF cells during cyclic stretch.
In conclusion, our results demonstrated that cyclic stretch with 20% elongation at 0.5 Hz induces apoptosis in rat AF cells. Prolonged exposure of the unphysiologically cyclic stretch to cultured AF cells not only enhanced the expression of several crucial ER stress markers such as CHOP, GRP78, and caspase-12, but also induced the activation of caspase-9, the depolarization of mitochondria and NO production. Furthermore, Z-ATAD-FMK (a caspase-12-specfic inhibitor) partly suppressed cell apoptosis induced by cyclic stretch. These data suggest that ER stress apoptotic pathway, likely mediated by NO, participates in the process of apoptosis induced by cyclic stretch in addition to the mitochondrial pathway. These results could be helpful to understand the mechanism of disc cell apoptosis which contributes to the development of IVD degeneration.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (U1032001, 81000793, 81071500) and Science and Technology Commission of Shanghai Municipality (09411953000).
References
- 1.Ariga K, Yonenobu K, Nakase T, Hosono N, Okuda S, Meng W, Tamura Y, Yoshikawa H. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine. 2003;28:1528–1533. [PubMed] [Google Scholar]
- 2.Benallaoua M, Richette P, Francois M, Tsagris L, Revel M, Corvol M, Poiraudeau S, Savouret JF, Rannou F. Modulation of proteoglycan production by cyclic tensile stretch in intervertebral disc cells through a post-translational mechanism. Biorheology. 2006;43:303–310. [PubMed] [Google Scholar]
- 3.Broberg KB. On the mechanical behaviour of intervertebral discs. Spine. 1983;8:151–165. doi: 10.1097/00007632-198303000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Yan W, Setton LA. Tensile stretch alters metalloproteinase activity and gene expression in anulus fibrosus cells. Trans Orthop Res Soc. 2004;29:834. [Google Scholar]
- 5.Chen J, Baer AE, Paik PY, Yan W, Setton LA. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun. 2002;293:932–938. doi: 10.1016/S0006-291X(02)00314-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Qin J, Liu X, Han Y, Yang Z, Chang X, Ji X. Nitric oxide-mediated neuronal apoptosis in rats with recurrent febrile seizures through endoplasmic reticulum stress pathway. Neurosci Lett. 2008;443:134–139. doi: 10.1016/j.neulet.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Cheng WP, Hung HF, Wang BW, Shyu KG. The molecular regulation of GADD153 in apoptosis of cultured vascular smooth muscle cells by cyclic mechanical stretch. Cardiovasc Res. 2008;77:551–559. doi: 10.1093/cvr/cvm057. [DOI] [PubMed] [Google Scholar]
- 8.Ching CT, Chow DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech. 2003;18:182–189. doi: 10.1016/S0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 9.Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998;41:1141–1151. doi: 10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1:239–245. doi: 10.1016/S1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 11.Evans CH, Watkins SC, Stefanovic-Racic M. Nitric oxide and cartilage metabolism. Methods Enzymol. 1996;269:75–88. doi: 10.1016/S0076-6879(96)69011-9. [DOI] [PubMed] [Google Scholar]
- 12.Fribley A, Zhang K, Kaufman RJ. Regulation of apoptosis by the unfolded protein response. Methods Mol Biol. 2009;559:191–204. doi: 10.1007/978-1-60327-017-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friederich M, Hansell P, Palm F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr Diabetes Rev. 2009;5:120–144. doi: 10.2174/157339909788166800. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Ballou LR, Hasty KA. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene. 2007;404:101–109. doi: 10.1016/j.gene.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Hutton WC, Yoon ST, Elmer WA, Li J, Murakami H, Minamide A, Akamaru T. Effect of tail suspension (or simulated weightlessness) on the lumbar intervertebral disc: study of proteoglycans and collagen. Spine. 2002;27:1286–1290. doi: 10.1097/00007632-200206150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kawahara K, Oyadomari S, Gotoh T, Kohsaka S, Nakayama H, Mori M. Induction of CHOP and apoptosis by nitric oxide in p53-deficient microglial cells. FEBS Lett. 2001;506:135–139. doi: 10.1016/S0014-5793(01)02898-8. [DOI] [PubMed] [Google Scholar]
- 18.Kosuge Y, Imai T, Kawaguchi M, Kihara T, Ishige K, Ito Y. Subregion-specific vulnerability to endoplasmic reticulum stress-induced neurotoxicity in rat hippocampal neurons. Neurochem Int. 2008;52:1204–1211. doi: 10.1016/j.neuint.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn K, D’Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartil. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Lee JR, Kim JK, Lee SJ, Kim KP. Role of protein tyrosine nitration in neurodegenerative diseases and atherosclerosis. Arch Pharm Res. 2009;32:1109–1118. doi: 10.1007/s12272-009-1802-0. [DOI] [PubMed] [Google Scholar]
- 21.Lotz M. The role of nitric oxide in articular cartilage damage. Rheum Dis Clin North Am. 1999;25:269–282. doi: 10.1016/S0889-857X(05)70067-3. [DOI] [PubMed] [Google Scholar]
- 22.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Luthra S, Dong J, Gramajo AL, Chwa M, Kim DW, Neekhra A, Kuppermann BD, Kenney MC. 7-Ketocholesterol activates caspases-3/7, -8, and -12 in human microvascular endothelial cells in vitro. Microvasc Res. 2008;75:343–350. doi: 10.1016/j.mvr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Mao W, Iwai C, Keng PC, Vulapalli R, Liang CS. Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: inhibition of phosphatidylinositol 3-kinase survival pathway. Am J Physiol Cell Physiol. 2006;290:C1373–C1384. doi: 10.1152/ajpcell.00369.2005. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Kawakami M, Kuribayashi K, Takenaka T, Tamaki T. Cyclic mechanical stretch stress increases the growth rate and collagen synthesis of nucleus pulposus cells in vitro. Spine. 1999;24:315–319. doi: 10.1097/00007632-199902150-00002. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto H, Doita M, Nishida K, Yamamoto T, Sumi M, Kurosaka M. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine. 2006;31:4–9. doi: 10.1097/01.brs.0000192682.87267.2a. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 29.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 30.Park JB, Lee JK, Park SJ, Kim KW, Riew KD. Mitochondrial involvement in fas-mediated apoptosis of human lumbar disc cells. J Bone Joint Surg Am. 2005;87:1338–1342. doi: 10.2106/JBJS.D.02527. [DOI] [PubMed] [Google Scholar]
- 31.Park JB, Park IC, Park SJ, Jin HO, Lee JK, Riew KD. Anti-apoptotic effects of caspase inhibitors on rat intervertebral disc cells. J Bone Joint Surg Am. 2006;88:771–779. doi: 10.2106/JBJS.E.00762. [DOI] [PubMed] [Google Scholar]
- 32.Rannou F, Poiraudeau S, Foltz V, Boiteux M, Corvol M, Revel M. Monolayer anulus fibrosus cell cultures in a mechanically active environment: local culture condition adaptations and cell phenotype study. J Lab Clin Med. 2000;136:412–421. doi: 10.1067/mlc.2000.109755. [DOI] [PubMed] [Google Scholar]
- 33.Rannou F, Richette P, Benallaoua M, Francois M, Genries V, Korwin-Zmijowska C, Revel M, Corvol M, Poiraudeau S. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90:148–157. doi: 10.1002/jcb.10608. [DOI] [PubMed] [Google Scholar]
- 34.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 36.Rodella LF, Ricci F, Borsani E, Stacchiotti A, Foglio E, Favero G, Rezzani R, Mariani C, Bianchi R. Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histol Histopathol. 2008;23:433–439. doi: 10.14670/HH-23.433. [DOI] [PubMed] [Google Scholar]
- 37.Sowa G, Agarwal S. Cyclic tensile stress exerts a protective effect on intervertebral disc cells. Am J Phys Med Rehabil. 2008;87:537–544. doi: 10.1097/PHM.0b013e31816197ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxid Redox Signal. 2007;9:553–561. doi: 10.1089/ars.2006.1524. [DOI] [PubMed] [Google Scholar]
- 39.Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Liu L, Charles IG, Moncada S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat Cell Biol. 2004;6:1129–1134. doi: 10.1038/ncb1188. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi M, Kasai K. Inflammation in periodontal tissues in response to mechanical forces. Arch Immunol Ther Exp. 2005;53:388–398. [PubMed] [Google Scholar]
- 42.Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079–2088. doi: 10.1007/s10495-006-0290-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]