Abstract
Patients with low back pain (LBP) suffer chronic disability. In 40% of LBP patients degenerative disc disease (DDD) seems to be the cause. This prospective case series assessed the efficacy of the interspinous device for intervertebral assisted motion (DIAM™) in patients with LBP resulting from DDD. All patients were initially assessed by physical examinations, magnetic resonance imaging, dynamic X-rays and provocative discography. Eligible patients (n = 52) had LBP for a minimum of 4 months, and received surgery with the DIAM™ system 2–4 weeks after diagnosis. Patients were evaluated pre-/post-operatively for pain severity using a visual analogue scale (VAS), and for dysfunction and disability with the Roland–Morris Disability Questionnaire (RMDQ). VAS and RMDQ score changes were assessed using the appropriate contrasts and Bonferroni-corrected P values. As a result, significant (P < 0.0001) pain score reductions were observed between baseline values, and 2 (3.7, 95% CI 3.1; 4.2) and 48 (3.1, 95% CI 2.5; 3.6) months follow-up (intent-to-treat population). Disability scores were significantly (P < 0.0001) reduced between baseline and 2 (8.6, 95% CI 7.4; 9.9) and 48 (7.5, 95% CI 6.1; 8.9) months. Disability scores were similar from months 2 to 48. At 48 months, 67.3% of patients reached the minimum clinically important difference (MCID; ≥1.5-unit improvement) in VAS score and 78.9% of patients reached the MCID (≥30% improvement) in RMDQ score. No complications were associated with surgery. In conclusion, patients with LBP treated with the interspinous DIAM™ system showed significant and clinically meaningful improvements in pain and disability for up to 4 years.
Keywords: Degenerative disc disease, DIAM, Interspinous device, Low back pain, Minimally invasive treatment, Pain reduction
Introduction
Low back pain (LBP) is a leading cause of chronic disability and psychological distress, and afflicted individuals frequently seek or require medical assistance. In Europe, estimates of the lifetime prevalence of back pain range from approximately 60 to 90% [2, 3, 8, 18].
Back pain can originate from any of the innervated structures constituting the lumbar spine and surrounding areas. Identification of the source of back pain can be challenging as different pathologies may share common symptoms. However, in 40% of reported cases the back pain is of discogenic origin [1]. Disc degeneration is considered to be the main cause of disc pain—it was proposed that nociceptors in the annulus and endplates stimulate pain, although the precise mechanism is unknown [5]. Degenerative disc disease (DDD) may give rise to segmental instability due to a reduction in the volume of the nucleus pulposus, loss of disc height and subsequent ligamentous laxity. Segmental instability, characterized by small abnormal vertebral movements outside the normal range, may be responsible for the pain. It was also proposed that LBP may originate from the facet joints, but several clinical studies have failed to verify this theory [16, 21, 22].
Provision of effective treatment for LBP associated with DDD remains challenging. Conservative physical-based treatments available include exercise therapy and manual therapy [30]. In one study, physical-based treatment was demonstrated to be effective in the short term but was not successful in the long term or as maintenance therapy [29].
Invasive treatment modalities for chronic LBP are generally applied after conservative treatments have failed to achieve the desired outcome. Fusion and total disc arthroplasty are the most common surgical options used to treat LBP resulting from DDD with or without segmental instability. However, the clinical success of fusion varies widely (16–95%) and depends mainly on the indication criteria being used [27, 28, 31]. In addition, patients who undergo spinal fusions or total disc replacement generally have more complications, longer hospital stays and higher hospital charges than patients undergoing other types of back surgery.
Percutaneous approaches were recently introduced for treatment of LBP resulting from DDD. The efficacy of these procedures has yet to be demonstrated in well-designed randomized controlled trials, but preliminary clinical studies showed beneficial effects [9, 12, 14, 15, 24, 25, 32]. Recently, a number of interspinous non-fusion devices entered clinical practice in Europe. All of these devices act on the posterior part of the functional spinal unit by distraction of the spinous processes and by avoiding extension of the treated segment. These devices appear to improve the cross-sectional area of the spinal channel and enlarge the diameter of the intervertebral foramina [13]. Several studies in patients with neurogenic claudication and back pain showed that treatment with interspinous spacers led to significant improvements in patients’ ability to walk. A collateral finding in these patients was an improvement in both claudication and LBP [4, 9, 11, 20, 23, 26].
The aim of this longitudinal prospective case-series study was to assess the efficacy of the DIAM™ spinal stabilization system for the treatment of patients with LBP resulting from DDD.
Methods
This case-series report presents 4-year follow-up data in 52 patients with LBP resulting from DDD treated with the interspinous DIAM™ spinal stabilization system (Cousin Biotech; distributed by Medtronic). Surgery was performed between December 2003 and December 2004 at the Unità Funzionale di Chirurgia Spinale c.d.c. Villanova in Florence, Italy.
All treatment alternatives, the probability of success, and the risk factors associated with treatment were discussed with each patient. All patients signed an informed consent form.
Patients and eligibility criteria
Patients affected by LBP for a minimum of 4 months, with Pfirrmann type 3 or type 4 disc degeneration and with no more than Modic type 1 or type 2 vertebral body involvement, were eligible for surgery with the DIAM™ spinal stabilization system.
Exclusion criteria
Patients with more severe disc degeneration (Pfirrmann type 5), lysthesis and/or lysis, severe osteoporosis, signs of lumbar kyphosis, hypogenesis or agenesis of the spinous process, tumours, fractures, deformities or history of other diseases eventually presenting with the symptoms of LBP were not eligible. Similarly, surgery with the DIAM™ spinal stabilization system was not indicated in patients who previously were submitted to laminectomy or hemilaminectomy.
Diagnostic procedures
Diagnostic work-up included collection of patient history and physical examinations, and all patients were assessed by magnetic resonance imaging (MRI), dynamic X-rays and provocative discography.
Patient history and physical examinations
Patients were asked to describe the pain they felt and note whether the level of pain changed upon loading and unloading of the spine. A physical examination assessed whether patients experienced pain when pressure was exerted on the spinous processes. A straight leg rising test was performed and a search for signs of nerve root involvement was performed to identify any sensitivity or motor deficits.
Imaging
Magnetic resonance imaging of the lumbar spine was used to determine evidence of DDD. Each MRI examination was assessed by one radiologist.
Dynamic X-rays were reviewed for segmental instability. The spinal segment was considered unstable if the intervertebral angle in flexion was greater than 5°, or if the flexion–extension range of motion was greater than 20° at L4–L5, or greater than 19° at L3–L4 or L2–L3. Translational instability was confirmed when the translation from flexion to extension was greater than 10% of the anterior–posterior distance of the endplates.
Provocative discography was performed according to standard procedures, using a postero-lateral, extra-articular approach to the disc and an injection of 2 mL of non-ionic contrast dye inside the disc space under low to medium pressure (20–30 psi). The concordance of evoked pain and memory pain was assessed during the procedure.
Surgical procedures
All patients (with a history of LBP for a minimum of 4 months) were referred for surgery 2 weeks–1 month after diagnosis. Surgery was performed under spinal anaesthesia with patients in the knee–chest position. A C-arm control was performed to identify the correct level. A 4–8 cm midline skin incision was made and the paravertebral muscles were stripped from the spinous processes until the corresponding lamina was reached on both sides. The interspinous ligament was removed while leaving the supraspinous ligament in place (ensuring that ≥0.5-cm width of the supraspinous ligament remained to maintain its strength). The interspace between the two spinous processes was prepared and measured for the appropriately sized DIAM™.
The DIAM™ was placed from the side, under the supraspinous ligament and positioned as anteriorly as possible until it made bilateral contact with the lamina. The device was anchored by passing the laces around the superior and inferior spinous processes. No drainage was used after the procedure. The operating time for single-level surgery was 15–35 min and for double-level surgery was 35–50 min. Patients were raised from bed after 6–12 h and discharged the next day. Patients were not braced postoperatively.
Patient-reported measures
Patients reported their level of pain using a visual analogue scale (VAS), where 0 = no pain and 10 = very severe pain. The minimum clinically important difference (MCID) in pain-VAS was defined as an improvement from baseline (pre-surgery) of at least 1.2 or 1.5 units [6, 7].
Dysfunction and disability were evaluated by a self-administered Roland–Morris Disability Questionnaire (RMDQ) on a scale of 0–24 [19]. The patients completed these questionnaires prospectively at baseline and at 2, 6, 12, 24 and 48 months after surgery. The MCID for disability was defined as an improvement from baseline of at least 30% in the RMDQ score [10].
Statistical analysis
The intent-to-treat (ITT) population included all study patients (n = 52). Missing values were imputed using the last observation carried forward (LOCF) method for patients who were lost to follow-up. Missing values at month 48 were imputed with the patient’s baseline values for those who had a second surgery at the index level; observed values were used for patients who had a second surgery at a different level.
The per-protocol (PP) population excluded all patients who were lost to follow-up at month 48, as well as patients who had a second surgery at a level different from the index level. Patients who had a second surgery at the index level were included in the analysis; the month 48 data were imputed with the patient’s baseline values.
All statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC, USA). A generalized estimating equations (GEE) modelling approach with unstructured working correlations was used to analyze the average profile of pain-VAS and RMDQ scores. The decrease in pain-VAS and RMDQ scores after surgery was assessed using the appropriate contrasts and Bonferroni-corrected P values. P values <0.05 were considered to be statistically significant.
Results
Demographics and treatment history at baseline
A total of 52 patients presented with LBP, with 19 patients also describing slight pain irradiating to buttock and thigh. The duration of pre-operative symptoms ranged from 6 to 84 months [mean ± standard deviation (SD) 31.8 ± 20.2 months, median 24.0 months]. Ten patients had a history of discectomy without recurrence, but later developed back pain.
All patients had previously received conservative treatment (mainly physical therapy or exercise therapy) and most patients had taken non-steroidal anti-inflammatory drugs, as needed, for back pain.
Overall, the mean age of patients was 49.4 years (range 29–77 years), and 29 patients were female. Forty-four patients were employed (25 sedentary workers and 19 manual labour workers) and eight patients were retired.
Patient disposition
No complications occurred in any patients receiving the interspinous DIAM™ spinal stabilization system. Seven patients needed artificial supraspinous ligament reconstruction during surgery. Six patients underwent a second surgery—this was performed at the same index level in four patients and at a different level in two patients. Three patients needed a second surgery at the same index level (2–6 months post-surgery) because of a pull-out of the device due to inadequate securing of the laces. One other patient required a second surgery at the same index level, after a fall from a height of 3 m, which caused the device to fail. All four patients reported an immediate worsening in pain levels and MRI confirmed the aforementioned conditions. The three patients with device pull-out underwent immediate further surgery and the patient who suffered the fall underwent surgery 3 months after the trauma—all patients showed improvements in back pain. In total, three patients were lost to follow-up at 48 months.
Reductions in pain
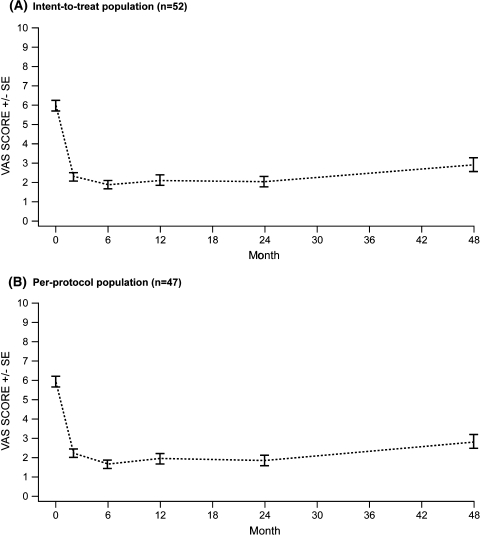
At baseline, the VAS score was 6.0 ± 1.9 (mean ± SD) for both the ITT and PP populations (Tables 1, 2). After surgery, VAS scores were lower at all follow-up visits from months 2 to 48 (range: ITT 1.9–2.9, PP 1.7–2.8) compared with baseline (Tables 1, 2; Fig. 1).
Table 1.
Summary of patient-reported pain-visual analogue scale (VAS) scores by visit (intent-to-treat population)
| Visit | Number of patients | Mean ± SD | 95% confidence intervals | Median |
|---|---|---|---|---|
| Baseline | 52 | 6.0 ± 1.9 | 5.5–6.5 | 6.0 |
| 2 months | 52 | 2.3 ± 1.5 | 1.9–2.7 | 2.0 |
| 6 months | 52 | 1.9 ± 1.4 | 1.5–2.3 | 1.5 |
| 12 months | 52 | 2.1 ± 1.9 | 1.6–2.7 | 2.0 |
| 24 months | 52 | 2.0 ± 1.8 | 1.6–2.5 | 1.5 |
| 48 months | 52 | 2.9 ± 2.5 | 2.2–3.6 | 2.0 |
Table 2.
Summary of patient-reported pain-visual analogue scale (VAS) scores by visit (per-protocol population)
| Visit | Number of patients | Mean ± SD | 95% confidence intervals | Median |
|---|---|---|---|---|
| Baseline | 47 | 6.0 ± 1.9 | 5.4–6.5 | 6.0 |
| 2 months | 47 | 2.2 ± 1.5 | 1.8–2.7 | 2.0 |
| 6 months | 47 | 1.7 ± 1.2 | 1.3–2.0 | 1.5 |
| 12 months | 47 | 2.0 ± 1.8 | 1.4–2.5 | 1.7 |
| 24 months | 47 | 1.9 ± 1.7 | 1.4–2.4 | 1.5 |
| 48 months | 47 | 2.8 ± 2.6 | 2.1–3.6 | 2.0 |
Fig. 1.
Mean pain-visual analogue scale (VAS) scores in patients treated with the interspinous DIAM™ spinal stabilization system: 48-month follow-up period. SE standard error
In the ITT population, significant (P < 0.0001) VAS score reductions were observed between baseline and 2 (3.7, 95% CI 3.1; 4.2) and 48 (3.1, 95% CI 2.5; 3.6) months follow-up. VAS scores from months 2 to 24 were similar (P = 0.415). A significant (P = 0.0052) increase in VAS scores was observed from months 24 to 48 (−0.9, 95% CI −1.4; −0.4), although the 48-month VAS scores (2.9 ± 2.5) remained significantly improved compared with baseline (Table 1).
Similarly in the PP population, significant (P < 0.0001) VAS score reductions were observed between baseline and 2 (3.7, 95% CI; 3.2; 4.3) and 48 (3.1, 95% CI 2.5; 3.7) months follow-up. VAS scores were similar across months 2–24 (P = 0.144) and a significant increase was observed between month 24 and 48 (–1.0, 95% CI –1.5; –0.4) (Table 2).
A significant (P < 0.0001) proportion of patients reached the MCID (1.2 or 1.5 unit improvement) in pain-VAS score (71.2 and 67.3%, respectively) between baseline and 48 months (Table 3).
Table 3.
Proportion of patients with a change from baseline to month 48 in pain-visual analogue scale (VAS) scores reaching the minimum clinically important difference
| MCID | Patients reaching MCID (%) | 95% confidence intervals | P value |
|---|---|---|---|
| Intent-to-treat population (n = 52) | |||
| 1.2 unit improvement | 71.2 | 57.0–82.9 | <0.0001 |
| 1.5 unit improvement | 67.3 | 54.6–80.0 | <0.0001 |
| Per-protocol population (n = 47) | |||
| 1.2 unit improvement | 74.5 | 59.6–86.1 | <0.0001 |
| 1.5 unit improvement | 70.2 | 55.1–82.6 | <0.0001 |
MCID minimum clinically important difference (not minimal clinically importance difference)
Reductions in disability
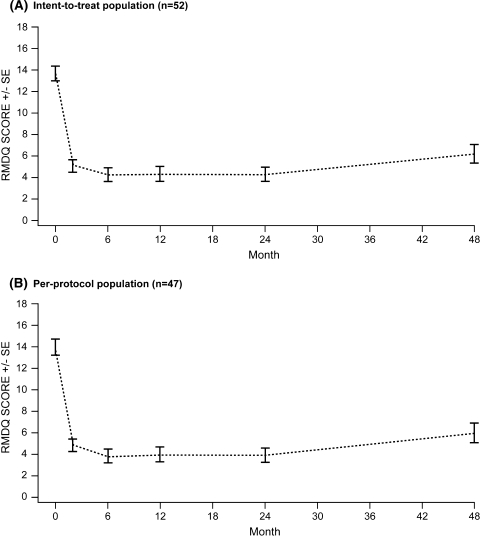
RMDQ scores at baseline were 13.7 ± 4.9 (mean ± SD) for the ITT population (Table 4) and 14.0 ± 5.0 for the PP population (Table 5). Following surgery, a reduction in disability was observed at months 2–48 (range: ITT 4.2–6.2, PP 3.9–6.0) compared with baseline (Fig. 2; Tables 4, 5).
Table 4.
Summary of Roland–Morris Disability Questionnaire (RMDQ) scores by visit (intent-to-treat population)
| Visit | Number of patients | Mean ± SD | 95% confidence intervals | Median |
|---|---|---|---|---|
| Baseline | 52 | 13.7 ± 4.9 | 12.4–15.1 | 14 |
| 2 months | 52 | 5.1 ± 3.9 | 4.0–6.2 | 4 |
| 6 months | 52 | 4.2 ± 4.2 | 3.1–5.4 | 3 |
| 12 months | 52 | 4.3 ± 4.6 | 3.0–5.6 | 3 |
| 24 months | 52 | 4.3 ± 4.3 | 3.1–5.5 | 3 |
| 48 months | 52 | 6.2 ± 5.9 | 4.6–7.8 | 3 |
Table 5.
Summary of Roland–Morris Disability Questionnaire (RMDQ) scores by visit (per-protocol population)
| Visit | Number of patients | Mean ± SD | 95% confidence intervals | Median |
|---|---|---|---|---|
| Baseline | 47 | 14.0 ± 5.0 | 12.5–15.5 | 15 |
| 2 months | 47 | 4.9 ± 3.8 | 3.8–6.0 | 4 |
| 6 months | 47 | 3.9 ± 4.1 | 2.7–5.1 | 3 |
| 12 months | 47 | 4.0 ± 4.5 | 2.7–5.3 | 3 |
| 24 months | 47 | 3.9 ± 4.2 | 2.7–5.2 | 3 |
| 48 months | 47 | 6.0 ± 6.0 | 4.2–7.8 | 3 |
Fig. 2.
Mean Roland–Morris Disability Questionnaire (RMDQ) scores in patients treated with the interspinous DIAM™ spinal stabilization system: 48-month follow-up period. SE standard error
In the ITT population, significant (P < 0.0001) reductions in disability were observed from baseline to month 2 (8.6, 95% CI 7.4; 9.9) and month 48 (7.5, 95% CI 6.1; 8.9). Mean disability scores at months 2, 6, 12, 24 and 48 were similar (P = 0.05).
In the PP population, significant (P < 0.0001) reductions in disability were observed from baseline to month 2 (9.1, 95% CI 7.8; 10.4) and month 48 (8.0, 95% CI 6.5; 9.4). Mean disability scores were similar (P < 0.1934) for months 2–24, but significantly increased from month 24 to 48 (−2.1, 95% CI −3.5; –0.7, P = 0.0216).
The proportion of patients reaching the MCID (≥30% improvement in RMDQ score) between baseline and month 48 was 78.9% for the ITT population and 80.8% for the PP population (Table 6).
Table 6.
Proportion of patients with a change from baseline to month 48 in Roland–Morris Disability Questionnaire (RMDQ) scores reaching the minimum clinically important difference
| Patient population | Proportion of patients reaching MCID (≥30% improvement) | 95% confidence intervals | P value |
|---|---|---|---|
| Intent-to-treat (n = 52) | 78.9 | 65.3–88.9 | <0.0001 |
| Per-protocol (n = 47) | 80.8 | 66.7–90.8 | <0.0001 |
MCID minimum clinically important difference
Discussion
This prospective case series demonstrated that surgery with the interspinous DIAM™ spinal stabilization system reduced pain and disability for up to 4 years post-surgery in patients with LBP.
Low back pain (LBP) is often caused by mechanical loading, which may trigger internal disc disruption and loss of water from the nucleus pulposus. The subsequent cascade of degenerative events leads to a reduction in disc height, narrowing of the intervertebral space, and malalignment of the facet joints. All of these events reduce normal physiological movements between two adjacent vertebrae and increase anomalous movements (micro-instability) arising from laxity of the ligaments and the annulus fibrosus. These events may stimulate known pain generators: mechanoreceptors in the vertebral endplates; nociceptors in the posterior part of the annulus fibrosus; the posterior longitudinal ligament; the capsule of the facet joints and the dura mater; and the sinuvertebral nerves [5]. Hence, LBP resulting from the degenerating functional spinal unit probably arises from multiple sources.
In theory, maintaining the height of the intervertebral space should help reinstate a more natural position and eliminate most of the pain. Cadaveric studies showed that interspinous devices distract the posterior part of the functional spinal unit, reposition and unload the facet joints and reduce intervertebral pressure, particularly on the posterior part of the endplates [14, 17]. Distraction of the spinous processes relieves the load from the posterior part of the intervertebral disc and diminishes the axial pressure across the endplates (i.e. some of the load is transferred from the disc to the posteriorly placed device, which releases the pressure from the endplate mechanoreceptors). Also, distraction of the posterior part of the intervertebral space may stretch the annulus fibrosus and the posterior longitudinal ligament into a more natural position, improving their capacity to resist loading stress. As a consequence, stimulation of pain receptors within these structures is likely to be minimized. Furthermore, re-positioning the facet joints relieves the axial pressure on the articular surfaces, and limits extension movements and impingement of sinuvertebral nerves. Thus, interspinous devices act on most of the known generators of pain at the lumbar level. A further advantage of interspinous devices was highlighted in biomechanical studies, which demonstrated that these devices do not impair the range of motion in adjacent vertebral levels [14, 17].
Limitations of the current study include the lack of randomization of patients or a control population receiving standard of care. Indeed, a randomized, controlled, phase III study comparing the safety and efficacy of the interspinous DIAM™ spinal stabilization system with conservative care is currently underway in patients with moderate single-level lumbar DDD (NCT00456378). Also, as the number of patients included in the study was relatively small this restricted any specific subgroup analyses (e.g. by age) being performed because the data would not be realistic. In addition, while MCID values selected for this study were associated with the minimal detectable change perceived by patients, alternative calculation methods might produce different MCID values. Finally, although vertebral disc height was not addressed in this study, a comprehensive follow-up analysis will investigate this outcome, plus MRI characteristics of disc hydration, posterior longitudinal ligament alignment and sagittal balance in the same group of patients.
In summary, these initial findings are promising and demonstrate that a substantial proportion of patients in this case series who underwent surgery with the interspinous DIAM™ spinal stabilization system experienced reductions in pain and disability for up to 4 years following surgery, with no complications.
Acknowledgments
All statistical analyses of the data were carried out by Medtronic. The authors would like to thank Karen Munro and Richard Barry (Quintiles Medical Communications) for medical writing assistance, which was funded by Medtronic.
References
- 1.Adams MA, Bogduk N, Burton K, Dolan P. The biomechanics of back pain. Edinburgh: Churchill Livingstone; 2002. [Google Scholar]
- 2.Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, Mannion AF, Reis S, Staal JB, Ursin H, Zanoli G. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15:S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton AK, Balague F, Cardon G, Eriksen HR, Henrotin Y, Lahad A, Leclerc A, Muller G, Beek AJ. European guidelines for prevention in low back pain. Eur Spine J. 2006;15:S136–S168. doi: 10.1007/s00586-006-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabraja M, Abbushi A, Woiciechowsky C, Kroppenstedt S. The short- and mid-term effect of dynamic interspinous distraction in the treatment of recurrent lumbar facet joint pain. Eur Spine J. 2009;18:1686–1694. doi: 10.1007/s00586-009-1149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson CA, Nachemson A. Neurophysiology of back pain: current knowledge. In: Nachemson A, Jonsomm E, editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 149–163. [Google Scholar]
- 6.Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 8.Hermans V, De Beeck RO (2000) Research on work-related low back disorders. Institute for Occupational Safety and Health. http://ispesl.it/profili_di_rischio%5Csitopesca/pdf/lowback.pdf. Accessed 1 November 2011
- 9.Hrabalek L, Machac J, Vaverka M. The DIAM spinal stabilisation system to treat degenerative disease of the lumbosacral spine. Acta Chir Orthop Traumatol Cech. 2009;76:417–423. [PubMed] [Google Scholar]
- 10.Jordan K, Dunn KM, Lewis M, Croft P. A minimal clinically important difference was derived for the Roland-Morris Disability Questionnaire for low back pain. J Clin Epidemiol. 2006;59:45–52. doi: 10.1016/j.jclinepi.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Kim C, Mahar A, Perry A, Massie J, Lu L, Currier B, Yaszemski MJ. Biomechanical evaluation of an injectable radiopaque polypropylene fumarate cement for kyphoplasty in a cadaveric osteoporotic vertebral compression fracture model. J Spinal Disord Tech. 2007;20:604–609. doi: 10.1097/BSD.0b013e318040ad73. [DOI] [PubMed] [Google Scholar]
- 12.Kong DS, Kim ES, Eoh W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J Korean Med Sci. 2007;22:330–335. doi: 10.3346/jkms.2007.22.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Hida K, Seki T, Iwasaki Y, Minoru A. An interspinous process distractor (X STOP) for lumbar spinal stenosis in elderly patients: preliminary experiences in 10 consecutive cases. J Spinal Disord Tech. 2004;17:72–77. doi: 10.1097/00024720-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey DP, Swanson KE, Fuchs P, Hsu KY, Zucherman JF, Yerby SA (2003) The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine (Phila Pa 1976) 28:2192–2197 [DOI] [PubMed]
- 15.Lutz C, Lutz GE, Cooke PM. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: a prospective outcome study. Arch Phys Med Rehabil. 2003;84:23–28. doi: 10.1053/apmr.2003.50059. [DOI] [PubMed] [Google Scholar]
- 16.Marks RC, Houston T, Thulbourne T. Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain. 1992;49:325–328. doi: 10.1016/0304-3959(92)90239-8. [DOI] [PubMed] [Google Scholar]
- 17.Minns RJ, Walsh WK (1997) Preliminary design and experimental studies of a novel soft implant for correcting sagittal plane instability in the lumbar spine. Spine (Phila Pa 1976) 22:1819–1825 [DOI] [PubMed]
- 18.Palmer KT, Walsh K, Bendall H, Cooper C, Coggon D. Back pain in Britain: comparison of two prevalence surveys at an interval of 10 years. BMJ. 2000;320:1577–1578. doi: 10.1136/bmj.320.7249.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roland M, Morris R (1983) A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 8:141–144 [DOI] [PubMed]
- 20.Schiavoen AM, Pasquale G. The use of disc assisted prostheses (DIAM) in degenerative lumbar pathology: indications, techniques and results. Ital J Spinal Disord. 2003;3:213–220. [Google Scholar]
- 21.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N (1994) Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976) 19:1132–1137 [DOI] [PubMed]
- 22.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195–200. doi: 10.1016/0304-3959(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 23.Sengupta DK. Dynamic stabilization devices in the treatment of low back pain. Orthop Clin North Am. 2004;35:43–56. doi: 10.1016/S0030-5898(03)00087-7. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui M, Smith FW, Wardlaw D (2007) One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine (Phila Pa 1976) 32:1345–1348 [DOI] [PubMed]
- 25.Taylor J, Pupin P, Delajoux S, Palmer S. Device for intervertebral assisted motion: technique and initial results. Neurosurg Focus. 2007;22:E6. doi: 10.3171/foc.2007.22.1.6. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner JA, Ersek M, Herron L, Haselkorn J, Kent D, Ciol MA, Deyo R. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907–911. doi: 10.1001/jama.268.7.907. [DOI] [PubMed] [Google Scholar]
- 28.Turner JA, Herron L, Deyo RA. Meta-analysis of the results of lumbar spine fusion. Acta Orthop Scand Suppl. 1993;251:120–122. doi: 10.3109/17453679309160140. [DOI] [PubMed] [Google Scholar]
- 29.Tulder MW, Goossens M, Waddell G, Nachemson A. Conservative treatment of chronic low back pain. In: Nachemson AL, Jonssom E, editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 271–304. [Google Scholar]
- 30.Tulder MW, Waddell G. Conservative treatment of acute and subacute low back pain. In: Nachemson AL, Jonssom E, editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 241–270. [Google Scholar]
- 31.Waddell G, Gibson JNA, Grant I. Surgical treatment of lumbar disc prolapse and degenerative lumbar disc disease. In: Nachemson AL, Jonssom E, editors. Neck and back pain. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 305–325. [Google Scholar]
- 32.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, Skidmore GA, Vessa PP, Dwyer JW, Puccio S, Cauthen JC, Ozuna RM. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]