Abstract
We retrospectively evaluated 488 percutaneous pedicle screws in 110 consecutive patients that had undergone minimally invasive transforaminal lumbar interbody fusion (MITLIF) to determine the incidence of pedicle screw misplacement and its relevant risk factors. Screw placements were classified based on postoperative computed tomographic findings as “correct”, “cortical encroachment” or as “frank penetration”. Age, gender, body mass index, bone mineral density, diagnosis, operation time, estimated blood loss (EBL), level of fusion, surgeon’s position, spinal alignment, quality/quantity of multifidus muscle, and depth to screw entry point were considered to be demographic and anatomical variables capable of affecting pedicle screw placement. Pedicle dimensions, facet joint arthritis, screw location (ipsilateral or contralateral), screw length, screw diameter, and screw trajectory angle were regarded as screw-related variables. Logistic regression analysis was conducted to examine relations between these variables and the correctness of screw placement. The incidence of cortical encroachment was 12.5% (61 screws), and frank penetration was found for 54 (11.1%) screws. Two patients (0.4%) with medial penetration underwent revision for unbearable radicular pain and foot drop, respectively. The odds ratios of significant risk factors for pedicle screw misplacement were 3.373 (95% CI 1.095–10.391) for obesity, 1.141 (95% CI 1.024–1.271) for pedicle convergent angle, 1.013 (95% CI 1.006–1.065) for EBL >400 cc, and 1.003 (95% CI 1.000–1.006) for cross-sectional area of multifidus muscle. Although percutaneous insertion of pedicle screws was performed safely during MITLIF, several risk factors should be considered to improve placement accuracy.
Keywords: Risk factor, Percutaneous, Pedicle screw, Minimally invasive, TLIF
Introduction
Since the introduction of pedicle screw instrumentation, the rate of union after spinal fusion has been dramatically improved [1]. By holding the anterior and posterior columns of the spine, stable fixation and adequate correction can be provided at the instrumented segments. A meta-analysis of the published literatures has shown that the success rate of spinal fusion using pedicle screws is 94.8% [2].
The original descriptions of pedicle screw insertion, started from exposing and finding the entry point with respect to the facet joint, and screw trajectories were then determined within the pedicle cylinder based on anatomical considerations [3]. However, the accurate placement of pedicle screws in the clinical setting remains challenging due to variabilities of the pedicle structure and the insertion technique. The incidence of pedicle screw misplacement ranges from 4.9 to 55% under intraoperative fluoroscope guidance [4, 5] and up to 11% with navigation assistance [6, 7].
Percutaneous technique of pedicle screw insertion was initially introduced by Magerl [8] in 1977 as a temporary external fixation for spinal fracture and spondylodiscitis. It was also applied to test the immobilization effect for lower back pain [9, 10]. As tissue damage during spinal instrumentation and postoperative morbidity can be minimized using this technique, it became popularized as a minimally invasive alternative to open procedures [11, 12]. The evolution of percutaneous pedicle screw instrument systems and of expandable tubular retractor systems have contributed much to the popularization of minimally invasive transforaminal lumbar interbody fusion (MITLIF) [11, 12].
Because percutaneous pedicle screw insertion is performed under fluoroscopic guidance using different landmarks [11, 12], we hypothesized that some demographic and surgical factors could predict the inaccurate placement of pedicle screws. Accordingly, this study was undertaken to determine the incidence of pedicle screw misplacement during MITLIF and to identify relevant risk factors.
Materials and methods
Study participants
This study involved a retrospective analysis of 110 consecutive patients that underwent MITLIF at one institute between February 2008 and June 2010. All patients had a diagnosis of lumbar degenerative disease and had undergone a minimum of 6 months of futile conservative management. All surgeries were performed by one experienced surgeon (M.K.) using a MITLIF instrumentation system.
Patient information on age, gender, body mass index (BMI), bone mineral density (BMD), diagnosis, operation time, estimated blood loss (EBL), level of fusion, and side of surgical approach (Right, Left or bilateral) were obtained from medical records.
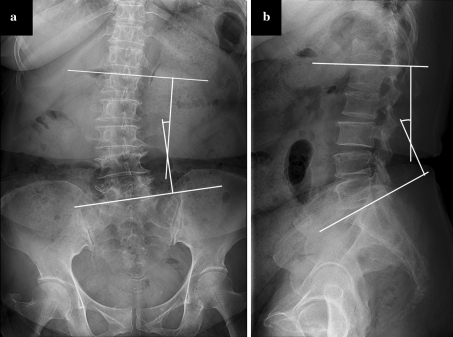
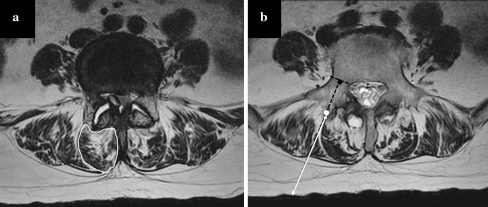
Preoperative plain radiography and magnetic resonance (MR) imaging were used to evaluate several anatomical characteristics. Anteroposterior (AP) and lateral Cobb angles of the lumbar spine were measured using standing plain radiographs (Fig. 1). Cross-sectional areas of multifidus muscle, degrees of multifidus muscle atrophy, and entry point depth were evaluated using axial T2-weighted MR images. Regions of interest outlined using a graphic cursor around the multifidus muscle on the right side were calculated for cross-sectional area at mid fusion level (Fig. 2a). Multifidus atrophy was graded as normal, mild, moderate, or severe using the same MR images according to the criteria described by Kader et al. [13]. To measure entry point depth from skin, an ideal entry point of pedicle screw insertion and a midpoint of pedicular shaft were determined at each pedicle level. A line extended from those two points was drawn. Then, the depth from skin to the ideal entry point was measured (Fig. 2b).
Fig. 1.
Measurement of the lumbar Cobb angle with AP (a) and lateral (b) radiograph
Fig. 2.
a The region of interest outlined with a graphic cursor around the multifidus muscle was calculated as a cross-sectional area. bThe black dot is a midpoint of the pedicle, and the white dot is an ideal entry point. The entry point depth is determined by measuring the length from skin to the ideal entry point at each pedicle level
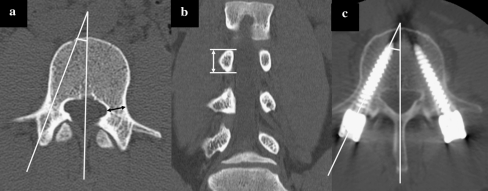
Pedicles and facet joints features were treated as the screw-related variables and assessed by preoperative computed tomography (CT). Pedicle widths, heights, and convergent angles of each level were measured in axial and coronal reformatted CT images (Fig. 3a, b).
Fig. 3.
a Measurement of the pedicle width and pedicular convergent angle. b The height of pedicle is measured with coronal reformatted CT images. c Measurement of the screw trajectory angle
Osteoarthritic grades of facet joints associated with screw entries were described as normal, mild, moderate, or severe as described by Parthria et al. [14]. Other screw-related variables were obtained from medical records and postoperative CT images (see below). All measurements were obtained using a picture archiving and communication system (Workstation; Pacsplus, Seoul, Korea).
CT was performed using an eight-detector row Lightspeed CT unit (GE Medical Systems, Milwaukee, WI, USA) in helical mode. The scanning direction was craniocaudal. Series consisted of 3.0-mm-thick CT sections (collimation, 40 mm) reconstructed at 2-mm intervals with a pitch of 0.984:1; acquisition parameters were 120 kVp and 350 mAs. Raw data were used to reconstruct transverse 3.0-mm-thick CT sections every 2 mm with a field of view adequate for visualization of the spine, as well as sagittal and coronal reformat images of the lumbar spine. MRI was performed on a 1.5 Tesla System (Siemens, Germany) with matrix size 256 × 512, field of view 260 × 260 mm, bandwidth 90 Hz/Px, and echo factor 16.4. Slice thickness was 4 mm and interslice gap was 2 mm.
All radiologic parameters were measured by three experienced spine surgeons not involved with the care of the study subjects. Individual and consensus interpretations were obtained for each parameter.
Surgical techniques
Following the induction of general endotracheal anesthesia, the patient was positioned prone on a radiolucent table, so as to maintain the physiologic lumbar lordosis. Before preparing and draping the patient, lateral and AP C-arm fluoroscopic images were obtained to ensure that pedicles and other anatomical landmarks could be adequately viewed. A 25-mm-long skin incision was placed vertically on the more affected side, two fingerbreadths off midline. After sequential dilatation, a tubular retractor system (METRx; Medtronic Sofamor Danek, Memphis, TN, USA) was docked on the facet joint. The remainder of the procedure was performed under the surgical microscope. Residual soft tissue was removed by electrocautery and pituitary rongeurs. Total facetectomy was carried out using a high-speed drill and osteotomes. The ligamentum flavum was then resected to expose the lateral margin of the ipsilateral nerve root. To achieve decompression of the central canal and the contralateral side, the tubular retractor could be angled medially and the patient tilted laterally. After adequate decompression, standard discectomy and endplate preparation were performed to construct the interbody fusion. Before inserting an interbody cage, the morselized bone graft (obtained from the removed facet and allobone graft) were packed into the contralateral and anterior disk space. A bullet-shaped single interbody cage (Capstone; Medtronic Sofamor Danek, Memphis, TN, USA) filled with autologous bone was then introduced. After the interbody fusion had been carried out, the tubular retractor was removed. In adequate fluoroscopic images, the ipsilateral pedicle was identified as a cylinder. Using a Jamshidi needle, docked against the bone at the junction of the base of the transverse process and facet joint, a PA image was obtained to localize the needle tip, and then the needle was gently tapped with a mallet to engage the tip in bone. Under intermittent fluoroscopic guidance, the needle was gently advanced to cross the pedicle cylinder and reach the cancellous bone of the vertebral body. The needle trocar was then exchanged for a flexible guidewire. The pedicle was tapped using cannulated taps, and the percutaneous cannulated pedicle screw-rod system (Sextant; Medtronic Sofamor Danek, Memphis, TN, USA) was placed. A contralateral percutaneous pedicle screw was also placed through a mirror incision under fluoroscopic guidance. Compression was applied to the construct prior to final tightening to provide compression of the bone graft and recreate lordosis. The fluoroscopic time was usually less than 30 s per one screw insertion. And, it took less than 30 s in total for the remaining procedures including cage insertion and rod connection.
Evaluation of the screw-related variables and interpretation of inserted screws
Screw location (ipsilateral or contralateral), screw length, and screw diameter were documented from medical records. Postoperative CT was performed within 2 weeks of index surgery in the same manner as preoperative CT. The screw trajectory angle was measured in axial CT images (Fig. 3c). Screw placement was verified using axial CT images as well as the coronal and sagittal reformatted images as previously described [4, 15] with modification. Briefly, screw placement was considered as “correct” if the screw was completely surrounded by pedicular cortex. An “incorrect” screw position was categorized as “cortical encroachment” if the pedicle cortex could not be visualized or as “frank penetration” when the screw was outside the pedicular boundaries. Frank penetration was subdivided as minor (≤2.0 mm), moderate (2.0–3.9 mm or <1 screw thread diameter), or severe (≥4 mm or ≥ 1 screw diameter). Depending on the direction of pedicle violation, screw misplacement was recorded as lateral, medial, inferior, superior, inferomedial, inferolateral, superomedial, or superolateral. All postoperative parameters were measured by the same three observers as the same way.
Statistical analysis
Descriptive statistics are summarized as frequencies and percentages for categorical variables, and as means and standard deviations for continuous variables. The discrepancy between the pedicle convergent angle and the screw trajectory angle between levels were compared using one-way ANOVA test. Kruskal–Wallis test was performed to compare the screw misplacement rate depending on the inserted levels. The association between the dichotomous outcome variables (correct or incorrect) and the independent variables were examined using logistic regression analysis, and the adjusted odds ratio (OR) with 95% confidence interval (CI) was calculated. Interobserver reliabilities for the interpretation of facet joint arthritis, multifidus atrophy, and pedicle screw placement were assessed using kappa values, which were interpreted as follows: moderate (0.41 ≤ κ < 0.60), substantial (0.60 ≤ κ < 0.80), and almost perfect (0.80 ≤ κ ≤ 1.00) [16]. Statistical analysis was carried out using statistical package for social sciences (SPSS) Version 12.0 software (SPSS Inc, Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
Demographics and anatomical characteristics
The demographic and anatomical characteristics of the 110 patients are summarized in Table 1. Overall mean patient age of the 41 male and 69 female patients was 59.3 ± 11.1 years (range 22–79), and mean BMI was 23.7 ± 2.9 m/kg2 (range 17.3–32.4). BMI was categorized as normal (<25 m/kg2) or obese (≥25 m/kg2). Mean value of BMD was −1.6 ± 1.5 (range −4.5–2.4). The diagnosis was degenerative lumbar instability in 47 cases, degenerative spondylolisthesis in 31 cases, isthmic spondylolisthesis in 13 cases, and recurred herniated nucleus pulposus (HNP) in 19 cases. Mean operation time was 150.7 ± 43.6 min (range 90–290). Mean EBL was 402.3 ± 147.4 cc (range 150–900). EBL was categorized as <400 cc or ≥400 cc. Single-level fusions were performed in 86 cases, and in the remaining 24 cases two levels were involved. 56 (50.9%) cases of the surgeries were performed on the left side. Mean value of AP Cobb angle of the lumbar spine was 5.2 ± 6.3° (range 0–23.4). Mean lateral Cobb angle of the lumbar spine was 30.1 ± 21.8° (range −1.0–61.2). Mean cross-sectional area of the multifidus muscle was 770.6 ± 205.6 mm2 (range 446.0–1452.5). 62 (56.4%) patients were graded as having normal or mild atrophy of multifidus muscle. The mean value of entry point depth form skin was 53.3 ± 8.8 mm (range 36.2–84.0).
Table 1.
Demographics and anatomical characteristics
| Variables | N = 110 |
|---|---|
| Demographics | |
| Age (year) | 59.3 ± 11.1 |
| Gender | |
| Male | 41 (37.3%) |
| Female | 69 (62.7%) |
| Body mass index (m/kg2) | |
| Normal (< 25) | 81 (73.6%) |
| Obesity (≥ 25) | 29 (26.4%) |
| Bone mineral density | −1.6 ± 1.5 |
| Diagnosis | |
| Degenerative instability | 47 (42.7%) |
| Degenerative spondylolisthesis | 31 (27.3%) |
| Isthmic spondylolisthesis | 13 (11.8%) |
| Recurred HNP | 19 (17.3%) |
| Operation time (minute) | 150.7 ± 43.6 |
| Estimated blood loss (cc) | |
| <400 | 60 (54.5%) |
| ≥400 | 50 (45.5%) |
| Fusion level | |
| 1-level | 86 (78.2%) |
| 2-level | 24 (21.8%) |
| Side of approach | |
| Right | 48 (43.6%) |
| Left | 56 (50.9%) |
| Bilateral | 6 (5.4%) |
| Anatomical characteristics | |
| Lumbar Cobb angle (°) | |
| AP | 5.2 ± 6.3 |
| Lateral | 30.1 ± 21.8 |
| Multifidus | |
| Cross-sectional area (mm2) | 770.6 ± 205.6 |
| Atrophy | |
| Normal | 21 (19.1%) |
| Mild | 41 (37.3%) |
| Moderate | 36 (32.7%) |
| Severe | 12 (10.9%) |
| Entry point depth (mm) | 53.3 ± 8.8 |
Values are expressed as the number (%) for categorical variables, and as the mean ± standard deviation for continuous variables
Screw-related variables (Table 2)
Table 2.
Screw-related variables
| Variables | N = 488 |
|---|---|
| Pedicle | |
| Level | |
| L2 | 12 (2.5%) |
| L3 | 44 (9.0%) |
| L4 | 164 (33.6%) |
| L5 | 188 (38.5%) |
| S1 | 80 (16.4%) |
| Width (mm) | 15.17 ± 4.01 |
| Height (mm) | 12.37 ± 1.75 |
| Convergent angle (°) | 26.9 ± 5.6 |
| Facet joint osteoathritis | |
| Normal | 64 (13.1%) |
| Mild | 90 (18.4%) |
| Moderate | 208 (42.6%) |
| Severe | 126 (25.8%) |
| Screw | |
| Length | |
| 35 mm | 4 (0.8%) |
| 40 mm | 82 (16.8%) |
| 45 mm | 402 (82.4%) |
| Diameter | |
| 5.5 mm | 15 (1.4%) |
| 6.5 mm | 473 (98.5%) |
| Trajectory angle (°) | 23.9 ± 5.3 |
Values are expressed as the number (%) for categorical variables, and as the mean ± standard deviation for continuous variables
Of the 488 screw insertions, the most frequently inserted level was L5 (188 screws) followed by L4 (164 screws), and S1 (80 screws). All screws were placed bilaterally. The mean values of pedicular widths, heights, and convergent angles were 15.17 ± 4.01 mm (range 6.70–27.11), 12.37 ± 1.75 mm (range 14.0–16.82), and 26.9 ± 5.6° (range 11.2–46.6), respectively. The mean convergent angle of pedicle was 18.5 ± 2.4° (range 16.1–21.4) at L2, 20.5 ± 4.6° (range 11.2–29.2) at L3, 23.5 ± 3.8° (range 11.6–33.1) at L4, 28.3 ± 4.3° (range 18.1–38.9) at L5, and 33.4 ± 4.3° (range 26.0–46.6) at S1. One hundred and twenty-six (25.8%) facet joints were graded as having severe osteoarthritis.
The diameters of inserted screws were 6.5 mm in 473 cases (98.5%) and 5.5 mm in 15 cases (1.4%). Screw lengths were 45 mm in 402 cases (82.4%), 40 mm in 82 cases (16.8%), and 35 mm in four cases (0.8%). The mean screw trajectory angle was 23.9 ± 5.3° (range 4.1–38.1). The discrepancy values between the pedicle convergent angle and the screw trajectory angle were −4.1 ± 1.9° (range −5.8 to −2.0) at L2, −2.6 ± 2.5° (range −9.4 to 10.1) at L3, 1.2 ± 1.9° (range −8.8 to 17.0) at L4, 4.9 ± 6.2° (range −4.8 to 23.0) at L5, and 6.8 ± 6.5° (range −14.3 to 23.9) at S1 level (P = 0.004).
Interpretation of pedicle screw misplacement
Among the 488 pedicle screws interpreted in this series, 373 (76.4%) screws were interpreted as being correctly inserted. Cortical encroachment was found for 61 (12.5%) screws, and frank penetration for 54 (11.1%). Among these frank penetrations, the lateral direction was the most frequently violated (44.4%), followed by the inferomedial (37.0%), medial (14.8%), and superomedial directions (3.7%). There was no screw violation in the superior, inferolateral, or superolateral directions (Table 3).
Table 3.
Interpretation of pedicle screw misplacement
| Severity | Direction | ||||
|---|---|---|---|---|---|
| Lateral | Inferomedial | Medial | Superomedial | Total | |
| Cortical encroachment | 52 (10.7%) | 0 | 7 (1.4%) | 2 (0.4%) | 61 (12.5%) |
| Frank penetration | |||||
| Minor (<2 mm) | 22 (4.5%) | 17 (3.5%) | 5 (1.0%) | 2 (0.4%) | 46 (9.4%) |
| Moderate (≥2, <4 mm) | 2 (0.4%) | 3 (0.6%) | 2 (0.4%) | 0 | 7 (1.4%) |
| Severe (≥ 4 mm) | 0 | 0 | 1 (0.2%) | 0 | 1 (0.2%) |
| Total | 76 (15.6%) | 20 (4.1%) | 15 (3.1%) | 4 (0.8%) | 115 (23.6%) |
Values are expressed as the number (%) of misplaced screw
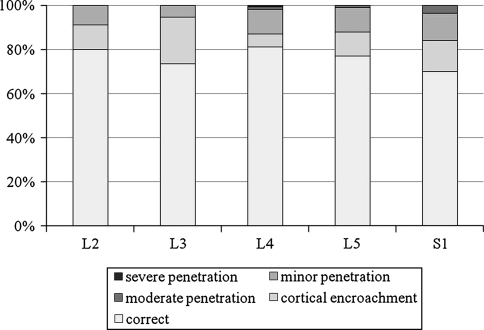
By the inserted level, S1 level was the most frequently misplaced (30%), followed by L3 (26.5%), L5 (23.1%), L2 (16.6%), and L4 (19%) (P = 0.053). One case of severe penetration occurred at L4 level. No screw was found to be misplaced by more than 2 mm at L2 and L3 levels (Fig. 4).
Fig. 4.
Screw misplacement rate for each vertebral level (P = 0.053)
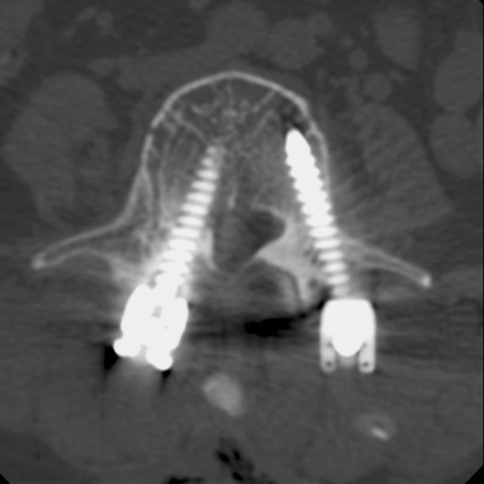
Two patients (0.2%) with medial penetration underwent revision for unbearable radicular pain and foot drop, respectively. The placement of screw in the patient with unbearable radicular pain was judged to show moderate medial misplacement; the patient recovered completely after repositioning of the screw on the following day. The other patient with neurological deficits was recognized as severe medial misplacement (Fig. 5); an immediate revision operation was done. However, the neurological deficits had not completely improved. The motor power of tibialis anterior muscle at 1-year follow-up was evaluated as fair grade.
Fig. 5.
A 55-year-old female patient with degenerative spondylolisthesis underwent L4-5 MITLIF. Immediate postoperative CT showed a severe medial misplacement of L4 pedicle screw on the right side
Logistic regression analysis of screw misplacement
The OR with 95% CI for each variable is given in Table 4. Four significant risk factors of screw misplacement were identified; obesity, an EBL of >400 cc, a higher pedicular convergent angle, and a greater multifidus cross-sectional area. The ORs of these significant risk factors were 3.373 (95% CI 1.095–10.391) for obesity, 1.141 (95% CI 1.024–1.271) for pedicle convergent angle, 1.013 (95% CI 1.006–1.065) for an EBL of >400 cc, and 1.003 (95% CI 1.000–1.006) for multifidus cross-sectional area.
Table 4.
Logistic regression analysis for screw misplacement
| Variables | Odds ratio | 95% Confidence intervals | P |
|---|---|---|---|
| Demographics | |||
| Age | 1.015 | (0.969, 1.064) | 0.531 |
| Male gender | 0.481 | (0.365, 1.006) | 0.145 |
| Obesity* | 3.373 | (1.095, 10.391) | 0.034 |
| Bone mineral density | 1.214 | (0.896, 1.644) | 0.211 |
| Diagnosis | |||
| Degenerative instability | 1.000 | ||
| Degenerative spondylolisthesis | 2.468 | (0.704, 8.657) | 0.158 |
| Isthmic spondylolisthesis | 2.180 | (0.518, 9.168) | 0.288 |
| Recurred HNP | 8.045 | (0.963, 67.222) | 0.054 |
| Operation time | 1.007 | (0.995, 1.020) | 0.255 |
| EBL >400* | 1.013 | (1.006, 1.065) | 0.041 |
| 2-level fusion | 2.188 | (0.421, 10.354) | 0.367 |
| Left approach | 0.498 | (0.244, 1.014) | 0.055 |
| Anatomical characteristics | |||
| AP Cobb angle | 0.946 | (0.870, 1.029) | 0.198 |
| Lateral Cobb angle | 1.018 | (0.987, 1.049) | 0.258 |
| Pedicle level | |||
| L2 | 1.000 | ||
| L3 | 0.391 | (0.188, 0.910) | 0.126 |
| L4 | 0.499 | (0.237, 1.167) | 0.490 |
| L5 | 2.184 | (0.397, 16.164) | 0.425 |
| S1 | 6.946 | (1.671, 22.635) | 0.059 |
| Pedicle width | 0.998 | (0.983, 1.014) | 0.822 |
| Pedicle height | 1.000 | (0.976, 1.023) | 0.967 |
| Pedicle convergent angle* | 1.141 | (1.024, 1.271) | 0.017 |
| Facet joint osteoarthritis | |||
| Normal | 1.000 | ||
| Mild | 1.132 | (0.324, 2.047) | 0.940 |
| Moderate | 0.701 | (0.870, 1.029) | 0.575 |
| Severe | 0.814 | (0.987, 1.049) | 0.662 |
| Multifidus cross-sectional area* | 1.003 | (1.000, 1.006) | 0.049 |
| Multifidus atrophy | |||
| Normal | 1.000 | ||
| Mild | 1.331 | (0.206, 8.589) | 0.764 |
| Moderate | 3.930 | (0.846, 18.269) | 0.081 |
| Severe | 2.571 | (0.529, 12.504) | 0.242 |
| Entry point depth | 0.820 | (0.433, 1.551) | 0.541 |
| Screw-related variables | |||
| Ipsilateral screw | 1.525 | (0.690, 3.371) | 0.548 |
| 40 mm screw length | 0.614 | (0.189, 1.994) | 0.417 |
| 5.5 mm screw diameter | 0.845 | (0.326, 2.365) | 0.780 |
| Screw trajectory angle | 1.012 | (0.928, 1.102) | 0.792 |
* Statistically significant
Interobserver reliability assessments
The kappa values of interobserver agreement for the interpretation of facet joint arthritis, multifidus atrophy, and pedicle screw placement were 0.68, 0.78, and 0.65, respectively. All values indicated substantial agreement.
Discussion
The aim of this study was to determine the incidence of pedicle screw misplacement during MITLIF. Cannulated pedicle screws are inserted percutaneously under fluoroscopic guidance during MITLIF. Theoretically, cannulated screws are more safely and accurately inserted than conventional screws [4]. However, although wide variations exist, partly due to the lack of a standardized evaluation method, pedicle wall penetration after percutaneous pedicle screw insertion is currently reported to be around 10% [4, 17]. In the present series of 488 percutaneous pedicle screws, frank penetration was found for 54 (11.1%) screws, and 8 (1.6%) screws penetrated by more than 2 mm. Two screws (0.4%) showing medial penetration required repositioning. These results for the percutaneous insertion of pedicle screws during MITLIF concur with the published literature in terms of misplacement and neurological complication rates [4, 6, 7].
We also sought to identify the preoperative and intraoperative factors that affect pedicle screw positioning. A meta-analysis on pedicle screw placement accuracy concluded that thoracic screws were more frequently inserted inaccurately than lumbar screws [5]. Belmont et al. [18] found that coronal plane deformity is a significant risk factor of pedicle screw penetrations. Facet osteoarthritis may distort the contour of the superior articular process, making this structure difficult to use as an anatomical landmark [3]. The insertion of S1 screws is frequently interrupted by the iliac crest [4, 12].
Based on our experiences of difficult cases, we hypothesized that several demographic and surgical factors could be an important predictor of an inaccurate placement of pedicle screw. Statistical analysis revealed that obesity, higher pedicle convergent angle, EBL more than 400 cc, and greater multifidus cross-sectional area affect the accurate placement of pedicle screw.
Although it is unclear if obesity adversely affects the rate of intraoperative complication associated with surgical procedures, obese patients present more technical challenges during spinal surgery [19, 20]. Positioning of obese patients without abdominal compression was very difficult. Intraoperative fluoroscopic or radiographic identification of anatomical landmarks are frequently blurred. Moreover, surgical procedures were performed in deeper place with poor visualization and illumination. Obesity also brings more bleeding [21, 22].
Minimally invasive techniques have a virtue of treating obese patients [22, 23]. Rosen et al. [22] reported that obesity per se is not a risk factor of complications during MITLIF. However, accurate insertion of pedicle screw is affected by obesity in our series. In addition, the quantity of the multifidus muscle was also found to be a significant risk factor of pedicle screw misplacement. These results infer that fluoroscopic images are frequently blurred in bulky patient and procedures in deep space are more difficult to perform. Therefore, we conclude that surgeons should pay more attention to inserting pedicle screws in bulky patients.
EBL more than 400 cc was also an associated factor for pedicle screw misplacement in the presenting study. Excessive EBL implies hemodynamic problem, longer operation time, and blurred surgical fields. While a direct correlation of EBL to technical complication has not been defined, excessive bleeding during spinal surgery may influence the surgeon’s surgical performance [24, 25].
Another relevant factor for screw misplacement in our series was pedicle convergent angle. Except for L2 and L3 pedicles in which the sample size was too small, more discrepancy between the pedicle convergent angle and the screw trajectory angle was found to the lower levels, i.e., more convergent angle should be given to the lower levels. We assumed that it was due to the surgeon’s tendency. However, the impingement effect of iliac crest could explain the high misplacement rate of S1 screw insertion.
We thought about other demographic and anatomical variables. However, these variables were not found to be associated with the pedicle screw misplacement in the present study.
This study had several limitations. The retrospective design involving possible data associated with the use of medical records, miscoding, and a lack of clinical information may cause uncertainty in the results. Another limitation was that the diameter and length of inserted screws were mostly 6.5 and 45 mm, (98.5 and 82.4%, respectively). Last, this study does not include other radiologic outcome including fusion rates or screw failure.
Conclusion
In the presenting study, the accuracy of percutaneous pedicle screw insertion during MITLIF and associated neurological complication rates are comparable to other publications. Logistic regression for the correctness of pedicle screw placement revealed that accurate placement is affected by obesity, pedicle convergent angle, EBL, and quantity of multifidus muscle.
Conflict of interest
None.
References
- 1.Gaines RW., Jr The use of pedicle-screw internal fixation for the operative treatment of spinal disorders. J Bone Joint Surg Am. 2000;82-A:1458–1476. doi: 10.2106/00004623-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Yahiro MA (1994) Comprehensive literature review. Pedicle screw fixation devices. Spine (Phila Pa 1976) 19:2274S–2278S [DOI] [PubMed]
- 3.Su BW, Kim PD, Cha TD, Lee J, April EW, Weidenbaum M, Vaccaro AR (2009) An anatomical study of the mid-lateral pars relative to the pedicle footprint in the lower lumbar spine. Spine (Phila Pa 1976) 34:1355–1362. doi:10.1097/BRS.0b013e3181 [DOI] [PubMed]
- 4.Schizas C, Michel J, Kosmopoulos V, Theumann N. Computer tomography assessment of pedicle screw insertion in percutaneous posterior transpedicular stabilization. Eur Spine J. 2007;16:613–617. doi: 10.1007/s00586-006-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosmopoulos V, Schizas C (2007) Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976) 32:E111–E120. doi:10.1097/01.brs.0000254048.79024 [DOI] [PubMed]
- 6.Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J. 2000;9:235–240. doi: 10.1007/s005860000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amiot LP, Lang K, Putzier M, Zippel H, Labelle H (2000) Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976) 25:606–614 [DOI] [PubMed]
- 8.Magerl FP (1984) Stabilization of the lower thoracic and lumbar spine with external skeletal fixation. Clin Orthop Relat Res:125–141 [PubMed]
- 9.Olerud S, Sjostrom L, Karlstrom G, Hamberg M. Spontaneous effect of increased stability of the lower lumbar spine in cases of severe chronic back pain. The answer of an external transpeduncular fixation test. Clin Orthop Relat Res. 1986;203:67–74. [PubMed] [Google Scholar]
- 10.Soini J, Slatis P, Kannisto M, Sandelin J. External transpedicular fixation test of the lumbar spine correlates with the outcome of subsequent lumbar fusion. Clin Orthop Relat Res. 1993;293:89–96. [PubMed] [Google Scholar]
- 11.Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97:7–12. doi: 10.3171/spi.2002.97.1.0007. [DOI] [PubMed] [Google Scholar]
- 12.Harris EB, Massey P, Lawrence J, Rihn J, Vaccaro A, Anderson DG. Percutaneous techniques for minimally invasive posterior lumbar fusion. Neurosurg Focus. 2008;25:E12. doi: 10.3171/FOC/2008/25/8/E12. [DOI] [PubMed] [Google Scholar]
- 13.Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–149. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- 14.Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164:227–230. doi: 10.1148/radiology.164.1.3588910. [DOI] [PubMed] [Google Scholar]
- 15.Learch TJ, Massie JB, Pathria MN, Ahlgren BA, Garfin SR. Assessment of pedicle screw placement utilizing conventional radiography and computed tomography: a proposed systematic approach to improve accuracy of interpretation. Spine(Phila Pa 1976) 2004;29:767–773. doi: 10.1097/01.BRS.0000112071.69448.A1. [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 17.Wiesner L, Kothe R, Ruther W. Anatomic evaluation of two different techniques for the percutaneous insertion of pedicle screws in the lumbar spine. Spine(Phila Pa 1976) 1999;24:1599–1603. doi: 10.1097/00007632-199908010-00015. [DOI] [PubMed] [Google Scholar]
- 18.Belmont PJ, Jr, Klemme WR, Robinson M, Polly DW., Jr Accuracy of thoracic pedicle screws in patients with and without coronal plane spinal deformities. Spine(Phila Pa 1976) 2002;27:1558–1566. doi: 10.1097/00007632-200207150-00015. [DOI] [PubMed] [Google Scholar]
- 19.Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg. 2002;97:20–24. doi: 10.3171/spi.2002.97.1.0020. [DOI] [PubMed] [Google Scholar]
- 20.Patel N, Bagan B, Vadera S, Maltenfort MG, Deutsch H, Vaccaro AR, Harrop J, Sharan A, Ratliff JK. Obesity and spine surgery: relation to perioperative complications. J Neurosurg Spine. 2007;6:291–297. doi: 10.3171/spi.2007.6.4.1. [DOI] [PubMed] [Google Scholar]
- 21.Zheng F, Cammisa FP, Jr, Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine(Phila Pa 1976) 2002;27:818–824. doi: 10.1097/00007632-200204150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Rosen DS, Ferguson SD, Ogden AT, Huo D, Fessler RG. Obesity and self-reported outcome after minimally invasive lumbar spinal fusion surgery. Neurosurgery. 2008;63:956–960. doi: 10.1227/01.NEU.0000313626.23194.3F. [DOI] [PubMed] [Google Scholar]
- 23.Park P, Upadhyaya C, Garton HJ, Foley KT. The impact of minimally invasive spine surgery on perioperative complications in overweight or obese patients. Neurosurgery. 2008;62:693–699. doi: 10.1227/01.neu.0000317318.33365.f1. [DOI] [PubMed] [Google Scholar]
- 24.Szpalski M, Gunzburg R, Sztern B. An overview of blood-sparing techniques used in spine surgery during the perioperative period. Eur Spine J. 2004;13(Suppl 1):S18–27. doi: 10.1007/s00586-004-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carl A, Kaufman E, Lawrence J. Complications in spinal deformity surgery: issues unrelated directly to intraoperative technical skills. Spine(Phila Pa 1976) 2010;35:2215–2223. doi: 10.1097/BRS.0b013e3181fd591f. [DOI] [PubMed] [Google Scholar]