Abstract
Percutaneous endoscopic lumbar discectomy (PELD) can be performed under local anesthesia with intravenous analgesics. To define the incidence of piriformis syndrome (PS) after PELD via the posterolateral approach under local anesthesia compared to that of general patients presenting with low back pain with/without lower leg pain. The incidence and time of occurrence of positive FAIR test after PELD within a 3-month follow-up period were evaluated retrospectively, and compared with the prevalence of general patients who visited the pain clinic for LBP with/without lower leg pain. Factors that may increase the incidence of PS after PELD were also evaluated. There was no patient with positive FAIR test immediately after PELD in the operation room and before walking. The prevalence of PS in general patients was 317/2,320 (13.7%); however, the incidence of PS after PELD within a 3-month follow-up period was 61/151 (40.4%), peaking at 32 days. High anxiety scale scores during operation led to increased incidence of PS after PELD. PELD under local anesthesia with high level of anxiety may increase the incidence of PS after walking, peaking around the first month, compared with the results for general patients with low back pain with/without lower leg pain.
Keywords: Diskectomy, Endoscopy, Lumbar vertebrae, Percutaneous, Piriformis syndrome
Introduction
Percutaneous endoscopic lumbar discectomy (PELD) is a mainstay of minimally invasive spine surgery. It has some significant advantages over open or other minimally invasive techniques in that it can allow for better visualization of the lesion, smaller incision sizes with reduced morbidity and mortality, reduced hospital stays, and ultimately lower cost [1]. In addition, it can be performed under local anesthesia with intravenous analgesics. Therefore, it can reduce the risk of nerve damage and can result in early ambulation after operation. However, anxiety during operation may result in muscles with abnormally high tone and tension. Anxiety may increase the incidence of piriformis syndrome (PS) after PELD via the posterolateral approach under local anesthesia.
PS is a neuromuscular disorder that occurs when the sciatic nerve is compressed or otherwise irritated by the piriformis muscle, and it is characterized by pain, tingling, and numbness in the buttocks and along the path of the sciatic nerve descending down the lower thigh and into the leg. In 1947, Robinson coined the term piriformis syndrome and proposed six key features: (1) a history of trauma to the sacroiliac and gluteal region; (2) pain in the sacroiliac joint, greater sciatic notch, and the piriformis muscle, extending down the leg and causing difficulty walking; (3) acute exacerbations brought on by stooping or lifting and relieved by traction on the affected leg; (4) presence of a tender, palpable, sausage-shaped mass over the piriformis muscle; (5) a positive Lasègue’s sign; and (6) gluteal atrophy [2].
The incidence and time of occurrence of PS, after PELD via posterolateral approach under local anesthesia, were compared with the prevalence among general patients who visited a pain clinic because of low back pain (LBP) with/without lower leg pain. Factors that may increase the incidence of PS after PELD were evaluated.
Materials and methods
This study was conducted from January 2000 to August 2009 at a Pain Clinic, in Pusan National University Hospital, Korea. The protocol was approved by the Research Ethics Committee at Pusan National University Hospital.
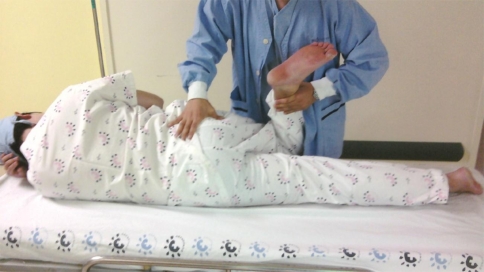
One hundred fifty-one patients who underwent performed L4-5 and/or L5-S1 PELD were selected. The indication criteria of PELD were radiculopathy on the electromyography and nerve conduction test and corresponding morphology on the magnetic resonance imaging preoperatively. A negative preoperative flexion, adduction, and internal rotation (FAIR) test was a criterion. A positive FAIR test (Pace sign) is defined as follows: the patient should be lying in the contralateral decubitus position, facing toward the examiner. Although it is somewhat painful, the physician or assistant should flex, adduct, and internally rotate the affected leg by grasping the ankle with one hand, and holding back the hip with the other to prevent the patient turning prone. One should be sure that the patient’s navel is facing above the horizontal, to avoid the patient inadvertently lying prone, and ending up in abduction instead of adduction. In flexion, the piriformis becomes an abductor; therefore, resisted abduction of the flexed hip is a provocative test (Fig. 1) [3].
Fig. 1.
The FAIR test. At 90° of flexion, passive adduction and internal rotation can produce symptoms
A positive FAIR test after PELD within a 3-month follow-up period, including hospital stay of 1–3 days, was retrospectively evaluated by medical chart review. The test had to change to negative immediately after the injection of a mixture of 5 mL of 1% lidocaine and 20 mg of triamcinolone into the piriformis muscle under fluoroscopic guidance. The patients had regular follow-up visits at postoperative weeks 1, 4, 8, and 12.
Seventy-two patients among 151 participants had a 10 × 14 cm lidocaine patch applied to the back for skin anesthesia of the path of the cannula 1 h before operation. An intravenous injection of 1 g of cefazolin was given after a skin test 30 min before skin incision. Basic monitoring, including electrocardiography, non-invasive blood pressure, pulse, and pulse oximetry, was performed during the operation. The patient was placed in prone position for posterolateral approach PELD. Intravenous analgesia comprising 30 mg of ketolorac and 50 μg of fentanyl was injected just before surgery.
The skin was prepared in a sterile fashion. Local anesthetic, 10 ml of 1.0% lidocaine, was injected using a 2-in.-long, 26-gauge needle, into the skin and subcutaneous tissue, but not into the psoas major and psoas minor muscles, through which the lumbar plexus passes. A 6-in.-long, 18-gauge needle was inserted at a 25- to 30-degree angle from the coronal plane, targeting the foraminal anular window.
Chromodiscography was performed to identify the targeted acidic degenerated nuclectomy, with a mixture of contrast agent and indigo carmine, such as 2 and 8 ml, respectively, while recording the patient’s experience of pain according to the volume, the pressure according to the volume, the volume injected, and the shape of spreading of the contrast agent and indigo carmine on posterolateral and lateral fluoroscopic views.
A long, thin guide wire was inserted through the 18-gauge needle channel and the nucleus. The needle was then removed. A small incision about 1 cm in length was made along the skin crease. A bluntly tapered, tissue-dilating obturator was slid over the guide wire until the tip of the obturator was firmly placed in the anular window. The working cannula was advanced over the obturator. The obturator was then removed. With use of an endoscope with irrigation, the outer anular fibers were inspected before the anulotomy to ensure that no neural structures were in the path of the cannula.
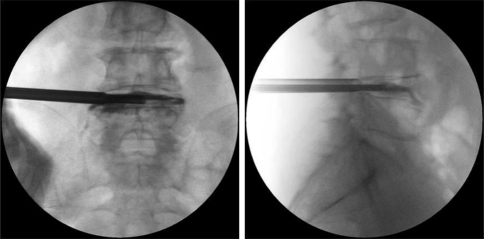
A four-quadrant anular infiltration of 0.5 ml of 1.0% lidocaine was performed with a 6-in.-long, 18-gauge needle, care being taken to avoid the spinal nerves. Anular fenestration was completed with small and large cutting trephines. The blue-stained nucleus pulposus was extracted with endoscopic rongeurs through the endoscope or with the larger straight and hinged rongeurs directly through the cannula under fluoroscopic guidance after the endoscope was removed (Fig. 2).
Fig. 2.
Percutaneous endoscopic lumbar discectomy L4–L5: a anteroposterior, b lateral fluoroscopic views
The patient was asked to cough to increase the abdominal pressure and ensure sufficient disc decompression. The flexible radiofrequency trigger-flex bipolar probe or a side-firing holmium:yttriumaluminum–garnet laser was inserted for intradiscal denervation and contraction of anular collagen at the herniation site, and the degenerated and extruded portions of the disc were removed. The endoscope was used to confirm that the discectomy was confirmed as complete at the anulotome site, neural structures, and epidural space. The endoscope and cannula were removed under direct vision.
The subcutaneous tissue was sutured with an absorbable material. The incision was dressed with an adhesive bandage, and a 2-day piroxicam patch was applied for analgesia. Postoperative computed tomography or magnetic resonance imaging was taken for checking enough decompression of the nerve root and cauda equina.
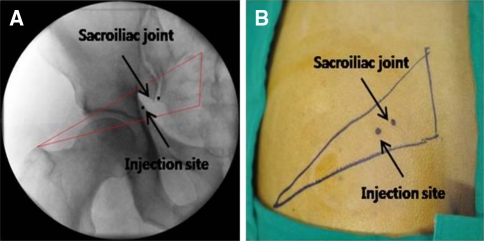
Confirmatory diagnostic and therapeutic injection into the affected piriformis muscle in the event of a positive FAIR test was performed under fluoroscopic guidance. The skin was prepared in a sterile fashion. The contour of a flat, pear-shaped piriformis muscle was drawn from the second through fourth sacral vertebrae to the greater trochanter under fluoroscopic guidance. A triangle was obtained wherein the base of the triangle, composed of the second to fourth sacral vertebrae, is the origin of the piriformis muscle, and the apex of the triangle, the medial aspect of the greater trochanter is the site of insertion into the muscle (Fig. 3) [4].
Fig. 3.
An imaginary triangle of the piriformis muscle under the fluoroscope
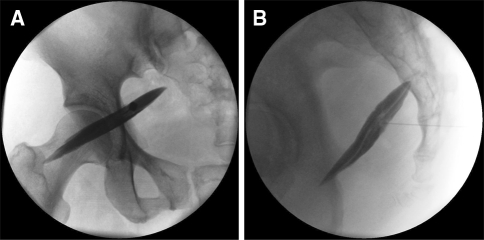
After the skin was anesthetized, a 22-gauge spinal needle was directed into the piriformis muscle belly and muscle sheath. After using loss of resistance and confirming no regurgitation of blood, contrast medium was injected until showing the shadow of the muscle under the fluoroscope anteroposterior and lateral view in succession (Fig. 4) [5]. The mixture of 5 mL of 1% lidocaine and 20 mg of triamcinolone was injected into the muscle. A changed positive into negative FAIR test immediate after piriformis muscle injection was defined as a PS.
Fig. 4.
Piriformis intramuscular injection: a anteroposterior and b lateral fluoroscopic views
Patients received co-interventions as needed: codeine-containing weak-opioid or non-opioid analgesics, muscle relaxants, and a home exercise program of stretching. Refractory PS was treated with 50 IU of botulinum toxin type A.
The incidence and time of occurrence of positive FAIR test after PELD within a 3-month follow-up period were evaluated retrospectively, and compared with the prevalence of 2,320 general patients who visited the pain clinic for LBP with/without lower leg pain.
Factors that may increase the incidence of PS after PELD, such as anxiety, age, sex, additional skin anesthetics (10 × 14 cm lidocaine patch), and performed levels of operation, were also evaluated in the group that had PELD performed. Anxiety was standardized by the State-Trait Anxiety Inventory (STAI-X) for Adults, and point 65 or over was considered anxious [6].
Statistical analysis was carried out using the SPSS ver. 12.0 for Windows software (SPSS Inc., Chicago, IL, USA). The values were calculated as the mean ± standard deviation. Demographic characteristics, including age and sex, were analyzed by a Student’s t test and chi squared test for inter-group comparison, respectively. The incidence of PS after PELD and the prevalence of PS in the general group were compared using a chi-squared test. Factors that may increase the incidence of PS after PELD were compared using intra-group comparison with a logistic regression analysis.
Results
Among 2,471 patients who suffered from LBP with/without lower leg pain, 151 patients (age 21–84 years) with no evidence of PS preoperatively received PELD. The prevalence of PS in general group (age 17–89 years) was 317/2,320 (13.7%); however, the incidence of PS after PELD within a 3-month follow-up period was 61/151 (40.4%) (Table 1).
Table 1.
Demographic characteristics and incidence and prevalence of piriformis syndrome
| P group (n = 151) | G group (n = 2,320) | |
|---|---|---|
| Age (years) | 54.5 ± 15.2 | 59.4 ± 15.1 |
| Sex ratio (M/F) | 76/75 | 1,051/1,269 |
| Number of patients with Piriformis Syndrome (M/F) | 61*(33/28) | 317*(151/166) |
P group Patients who received percutaneous endoscopic lumbar discectomy
G group General patients who visited the pain clinic for low back pain with/without lower leg pain
* P < 0.01
There was no patient with positive FAIR test results immediately after PELD in the operating room and before walking. The mean onset time of PS was 32.0 ± 30.2 days, ranging from 1 to 88 days.
Among 61 patients, 22 patients were 65 and over in the STAI-X. Other factors examined, including age, sex, added skin anesthetics, and performed level of operation, did not affect the incidence of PS after PELD (Tables 2, 3). Fifteen patients were received 50 IU of botulinum toxin type A for refractory PS during the follow-up period.
Table 2.
Factors that may increase the incidence of piriformis syndrome (PS) after percutaneous endoscopic lumbar discectomy (PELD)
| Factors | PS (n = 61) | N (n = 90) | P value |
|---|---|---|---|
| Anxiety | 22 | 12 | 0.001* |
| Age (Y/M/O) | 15/26/20 | 23/39/28 | 0.975 |
| Sex (M/F) | 33/28 | 43/47 | 0.581 |
| Lidocaine patch (+/−) | 31/30 | 41/49 | 0.525 |
| PELD level | |||
| L4–L5 | 39 | 56 | 0.837 |
| L5–S1 | 7 | 18 | 0.157 |
| Both L4–L5 and L5–S1 | 15 | 16 | 0.309 |
PS Patients with PS after PELD
N Patients without PS after PELD
Anxiety Number of anxious patient ≥65 in STAT-X
Y Young age <45 years old
M Middle age between 45 and 64 years old
O Old age ≥65 years old
+ Lidocaine patch application 1 h before PELD
− No lidocaine patch application
The incidence of PS was increased in anxious patients (P = 0.001*)
Table 3.
Site of occurrence of piriformis syndrome (PS) after Percutaneous Endoscopic Lumbar Discectomy (PELD)
| Site of occurrence of piriformis syndrome | Number of patients | P value |
|---|---|---|
| The same side of PELD | 26 | 0.011* |
| The opposite side of PELD | 13 | 0.591 |
| Both piriformis muscles | 22 | 0.384 |
* A PS after PELD developed in the same side in which PELD was performed (P = 0.011*)
Discussion
PS is an often misdiagnosed cause of sciatica and is usually diagnosed only after excluding all other possibilities originating from the back, buttock and leg pain.
The piriformis muscle originates from the anterior surface of the S2–S4 sacral vertebrae, the gluteal surface of the ilium near the posterior surface of the iliac spine, and the capsule of the sacroiliac joint. It runs laterally through the greater sciatic foramen, becomes tendinous, and inserts into the piriformis fossa at the medial aspect of the greater trochanter of the femur. The gluteal nerves, gluteal vessels, sciatic nerve, and posterior femoral cutaneous nerve pass below the piriformis muscle. The branches of the L5, S1, and S2 spinal nerves innervate the piriformis muscle [7].
There are six possible anatomical relationships between the sciatic nerve and the piriformis muscle (1) the sciatic nerve passes below the piriformis muscle; (2) a divided nerve passes through and below the muscle; (3) a divided nerve passes through and above the muscle; (4) a divided nerve passes above and below the muscle; (5) an undivided nerve passes through the muscle; or (6) an undivided nerve passes above the muscle [7]. The most common anatomical relationship known is the sciatic nerve passing below the piriformis muscle, with the incidence between 74 and 96% [8].
The most common level of lumbosacral HNP and spinal stenosis is L4/L5 or L5/S1. The piriformis muscle is innervated from L5 to S2. This leads to confusion and need to differentiate whether the pain originates from the root or the peripheral nerve.
Physical examination of PS may reveal that the straight leg raise (SLR) test (pain on raising one leg with the knee absolutely straight) is negative, whereas a Lasègue’s sign (pain on voluntary flexion, adduction, and internal rotation of the hip) is positive. In addition, a positive FAIR test may confirm a PS suspected on physical examination. If the test turns negative after a successive diagnostic injection into the affected piriformis muscle, this may confirm the diagnosis of PS.
The other available diagnostic methods can be used, including imaging studies (such as magnetic resonance imaging, computed tomography, and ultrasonography) and electrodiagnostic studies (such as electromyography and nerve conduction test).
However, simple physical examination and diagnostic injection give the patient relief from immediate pain and anxiety. If symptoms are relieved by local injection with/without steroids and further physical therapy for PS, unnecessary lumbar surgery can be avoided [9, 10]. A correct diagnosis of PS can also avoid suspicion of insufficient decompression after surgery. A FAIR test and local injection should be performed first to rule out PS in patients with sciatica.
However, this diagnostic tool, a FAIR test followed by an injection into the piriformis muscle, has a limitation in that it cannot rule out pathology of the adjacent muscles. The obturator internus muscle runs in the same direction of the piriformis—just above and below the inferior and superior gemelli, respectively. The tendon of the piriformis muscle often joins with the tendons of the superior gemellus, obturator internus, and inferior gemellus muscles prior to insertion. Although it may not be common, compression of the sacral plexus caused by dynamic motion of the obturator internus muscle should be included as a possible diagnosis for sciatic pain [11].
The incidence of PS after PELD within a 3-month follow-up period was much higher than the prevalence of PS in patients with LBP and/or lower leg pain who visited the pain clinic during our 9 year and 8 month study period. Reported prevalence of PS among patients with LBP varies widely from 5 to 36% [3, 12, 13]. In our study, the prevalence of PS among patients who visited the pain clinic for LBP with/without lower leg pain was 13.7% (317/2,320).
PS occurs most frequently during the fourth and fifth decades of life [14]. There was no difference in frequent age of PS between patients who received PELD (p group) and general patients who visited the pain clinic for LBP with/without lower leg pain (g group). However, the mean age for developing PS in this study was higher by one decade, i.e., 54.5 and 59.4 years old; in the p and g group, respectively. This age gap may result because the university hospital is usually frequented by elderly patients, patients with complications, and critically ill patients.
Piriformis syndrome is more common in women than in men, possibly because of the biomechanics associated with the wider quadriceps femoris muscle angle (“Q angle”) in the os coxae (pelvis) of women [3]. We observed no sex-related difference in the occurrence of PS, regardless of PELD.
It has known that there is a possibility to increase the incidence of PS in the hip or knee diseases. The patients who received PELD had already ruled out hip or knee originated pain and related PS preoperatively.
It was not until walking that PS after PELD showed a peak, around the first month. To consider causative factors, primary congenital anatomic variations, such as a split piriformis muscle, split sciatic nerve, or an anomalous sciatic nerve path, can be excluded. Secondary PS occurs because of a precipitating cause, including trauma, ischemic mass effect, and local ischemia [14]. Microtrauma may result from the overuse of the piriformis muscle, such as in walking or running.
Anxiety during the operation increased the incidence of PS after PELD. If this is true, the incidence should be compared to that after PELD with general anesthesia. However, local anesthesia is preferred because it has definite benefits; such as reducing the risk of nerve damage and providing early ambulation after surgery. It t is expected that patients who are administered anxiolytics such as midazolam do not cooperate easily during the operation, and this is particularly true in the case of older patients. These solutions cause more problems than they are worth.
Other factors including age, sex, lidocaine patch, and performed levels did not affect the incidence of PS after PELD. Lumbar radiculopathy, although initially believed to be a primary mechanical insult to the nerve root and dorsal root ganglion, is now thought to be at least partially due to inflammatory changes in the nerve root. Continuous irrigation for visualization by spinal endoscope may decrease chemical pain mediators [15, 16] in the nerve root. PS is a neuromuscular disorder that may occur when the sciatic nerve is irritated or the piriformis muscle is made spastic when chemical pain mediators are affected by irrigation. These irrigated chemical pain mediators may descend along the same side on which PELD is performed, and they may also cross to the opposite side of the nerve root through the epidural space. Otherwise, the pre-existing non-operated contralateral disc problem may rarely contribute in development of the contralateral PS.
It is also necessary for future study to compare the incidence of PS after PELD according to the diameter of the working channel for PELD.
Electrophysiological diagnosis for the confirming PS after PELD was made by comparing posterior tibial and peroneal H-reflexes elicited in anatomical position with those obtained in flexion, adduction, and internal rotation in five patients who were not satisfied with surgical outcomes 2–3 months after operation. All of five patients showed the prolongation of the H-reflex. In addition, they showed normal needle electromyography pattern for lumbosacral radiculopathy.
There was no patient with PS after PELD who could not walk preoperatively due to pre-existing knee or hip problems, chronic renal failure, or a debilitated state of cancer in our experience. Overuse microtrauma from ambulation or severe sporting activity due to reduced radicular pain after PELD is one of the most contributing factors to the development of PS. The other underlying factor may be pre-existing hip or knee problems to be a burden to the piriformis muscle. Anxiety and fear during the operation may be a contributing factor to increase the muscle tension and spasm.
In conclusion, PELD under local anesthesia, with a high level of anxiety, may increase the incidence of PS after walking, showing a peak around the first month, compared with the incidence among general patients who presented with LBP with/without lower leg pain.
Conflict of interest
No funds were received in support of this study.
References
- 1.Baron EM, Levene HB, Heller JE, Jallo JI, Loftus CM, Dominique DA. Neuroendoscopy for spinal disorders: a brief review. Neurosurg Focus. 2005;19:E5. doi: 10.3171/foc.2005.19.6.6. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D. Piriformis syndrome in relation to sciatic pain. Am J Surg. 1947;73:355–358. doi: 10.1016/0002-9610(47)90345-0. [DOI] [PubMed] [Google Scholar]
- 3.Pace JB, Nagle D. Piriform syndrome. West J Med. 1976;124:435–439. [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph MC, Barry G (2001) General considerations of pain in the low back, hips, and lower extremities. In: Loeser JD (ed) Bonica’s management of pain, 3rd edn. LWW, Philadelphia, p 1494
- 5.Fishman SM, Caneris OA, Bandman TB, Audette JF, Borsook D. Injection of the piriformis muscle by fluoroscopic and electromyographic guidance. Reg Anesth Pain Med. 1998;23:554–559. doi: 10.1016/s1098-7339(98)90080-3. [DOI] [PubMed] [Google Scholar]
- 6.Spielberg CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press; 1970. [Google Scholar]
- 7.Benzon HT, Katz JA, Benzon HA, Iqbal MS. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. 2003;98:1442–1448. doi: 10.1097/00000542-200306000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Güvençer M, Iyem C, Akyer P, Tetik S, Naderi S. Variations in the high division of the sciatic nerve and relationship between the sciatic nerve and the piriformis. Turk Neurosurg. 2009;19:139–144. [PubMed] [Google Scholar]
- 9.Niu CC, Lai PL, Fu TS, Chen LH, Chen WJ. Ruling out piriformis syndrome before diagnosing lumbar radiculopathy. Chang Gung Med J. 2009;32:182–187. [PubMed] [Google Scholar]
- 10.Parziale JR, Hudgins TH, Fishman LM. The piriformis syndrome. Am J Orthop. 1996;25:819–823. [PubMed] [Google Scholar]
- 11.Murata Y, Ogata S, Ikeda Y, Yamagata M. An unusual cause of sciatic pain as a result of the dynamic motion of the obturator internus muscle. Spine J. 2009;9:e16–e18. doi: 10.1016/j.spinee.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am. 2004;35:65–71. doi: 10.1016/S0030-5898(03)00105-6. [DOI] [PubMed] [Google Scholar]
- 13.Foster MR. Piriformis syndrome. Orthopedics. 2002;25:821–825. doi: 10.3928/0147-7447-20020801-12. [DOI] [PubMed] [Google Scholar]
- 14.Boyajian-O’Neill LA, McClain RL, Coleman MK, Thomas PP. Diagnosis and management of piriformis syndrome: an osteopathic approach. J Am Osteopath Assoc. 2008;108:657–664. doi: 10.7556/jaoa.2008.108.11.657. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Moon CS, Sul D, Lee J, Bae M, Hong Y, Lee M, Choi S, Derby R, Kim BJ, Kim J, Yoon JS, Wolfer L, Kim J, Wang J, Hwang SW, Lee SH. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42:1504–1511. doi: 10.1016/j.clinbiochem.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Tolonen J, Grönblad M, Vanharanta H, Virri J, Guyer RD, Rytömaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]