Abstract
Introduction
The results of platelet-rich plasma (PRP) in spinal fusion applications are limited and controversial. Both beneficial and inhibitory effects have been shown. In this prospective randomised controlled trial, our objective was to assess both the clinical and radiological effect of PRP when added to autograft iliac crest bone in posterior lumbar interbody fusion.
Methods and materials
Forty patients were recruited for the study fulfilling strict entry requirements and were randomised with a 1:1 ratio. In each group, one patient was lost to follow-up. Thirtyeight patients completed the Visual Analogue Scale (VAS), the Oswestry Disability Index (ODI), and the Short-Form 36 (SF-36) preoperatively and postoperatively at 3, 6, 12, and 24 months, respectively. CT-scans of the lumbar spine were taken at 3, 6, and 12 months. Posterior stabilisation was achieved with pedicle screws and interbody fusion was aimed at with carbon cages filled with autologous bone.
Results
Baseline demographic data (age, sex, smoking history, preoperative outcome measures) showed no relevant difference between groups. For patients who received autograft only, the mean VAS improved by 4.0 points (p < 0.01), mean ODI improved by 32.1 points (p < 0.001), and mean SF-36 showed statistically significant improvement in each of the eight domains and in the physical (p < 0.001) and mental (p < 0.001) component summary measures. For patients who received autograft with PRP, the mean VAS improved by 4.92 points (p < 0.01), mean ODI improved by 30 points (p < 0.001), and mean SF-36 showed statistically significant improvement in six of the eight domains (p < 0.02) and in the physical (p = 0.016) and mental (p < 0.001) component summary measures. The improvement of the VAS score and the physical component summary score was more pronounced in patients who received autograft with PRP. These differences were, however, not statistically significant. CT-scans showed uneventful osseous healing in all but one patient with no difference between groups.
Conclusion
In this prospective randomised controlled clinical and radiological trial, adding PRP in posterior lumbar interbody fusion did not lead to a substantial improvement or deterioration when compared with autologous bone only. No inhibitory effect of PRP was observed on CT-scans. From a clinical and radiological point of view, the use of PRP seems to be justified in posterior lumbar interbody fusion surgery. From an economical point of view, the expense of using PRP cannot be justified until statistical significance can be reached in a larger study.
Keywords: Lumbar spine, Fusion, Platelet-rich plasma, Randomised controlled trial
Introduction
Several centrifugation techniques have been described for the development of platelet concentrates for clinical use in a wide array of indications [15, 19, 21, 22, 39]. Advanced ultrafiltration techniques result in concentrated plasma of up to 10 times that of whole blood: AGF (autologous growth factors). Platelet-rich plasma (PRP) is defined as a portion of the plasma fraction of autologous blood having a platelet concentration above baseline [27]. Platelets are a key component of the initial cellular response in tissue repair, which migrate to the injury site and release a variety of growth factors. The early platelet-mediated activity induces formation of a fibrin clot as well as chemotaxis of white blood cells and stem cells. Platelet degranulation and release of platelet-derived growth factor, transforming growth factor-beta, and vascular endothelial growth factor are among the signalling substances known to be important in bone healing [17, 24].
The concept of PRP application is to enhance the healing properties of bone by stimulating osteoinduction and mitogenesis [2]. When multiple growth factors are present at the bone formation site, they may exert a synergistic effect [26].
Numerous preclinical studies have reported positive results on the use of platelet concentrates in promoting tissue healing [14, 25, 26]. Other preclinical studies have shown inhibition of osteogenic proliferation and differentiation as well as reduction of the activity of demineralized bone matrix, leading to a decrease of bone formation [3, 4, 7, 10, 11, 33].
Most clinical studies have been performed in the field of oral and maxillo-facial surgery. Several showed stimulation of bone formation [12, 26, 38] whereas others showed no effect [13, 34].
The results in spinal fusion applications are limited and controversial. Both beneficial [1, 20], and inhibitory [8, 9, 37] effects have been shown. In 2003, Hee et al. demonstrated faster fusion but no increase in fusion rates when used in transforaminal interbody fusion [20]. In that same year, Weiner and Walker reported on a detrimental effect of platelet-rich plasma on autograft in patients who had posterolateral spine fusion [37]. Manufacturers were therefore required to add a text to the warnings section of the device label, stating that the effectiveness of the device has not been established [31]. In a recent review article, platelet gel was given a grade 2B recommendation (weak recommendation; alternative approaches likely to be better) as an enhancer of the effect of autograft for both posterior lumbar fusion and anterior lumbar interbody fusion [30]. The grade of recommendation depended on the clarity of the risk/benefit and the methodological strength of supporting evidence.
To add to the confusion, the rate of fusion depends to a great extent on the investigator’s interpretation [18, 28]. Because there is no single definition of what constitutes fusion, it is difficult if not impossible to compare the results of different studies. Moreover, it is difficult to determine radiographically whether fusion has occurred. Therefore, we tried to use the most stringent criteria for fusion and used a classification that allows to distinguish between minor changes on CT-scan.
The purpose of the current study was to assess the effect of platelet-rich plasma when added to autograft iliac crest bone in posterior lumbar interbody fusion. Both clinical and radiological outcomes were considered.
Our first hypothesis was that there would be a clinical benefit. The most important and relevant clinical difference was expected in the physical component summary (PCS) measure of the SF-36 score. Based on our experience of PRP in the period before the study, a mean difference of 5 score units was expected between the 2 groups with a variance of 30.
Our second hypothesis was that, on CT-scan, there would be no inhibitory effect of PRP on bone formation.
Methods and materials
Study design
A single-centre single-surgeon prospective randomised controlled trial was conducted. Approval from the local ethical committee and institutional research board approval was obtained for the study. The reviewers and the surgeon have and had no personal or financial interest in companies selling AGF- and PRP-technology, nor have they received any financial support from them.
Indications for surgery were both lytic and degenerative spondylolisthesis, disc degeneration not responding to conservative treatment modalities for at least 6 months, and disc herniation in patients with severe disc degeneration and persisting sciatica despite epidural steroid injections. The study was restricted to patients with single-level disc pathology. Patients with disc pathology at adjacent levels and patients undergoing multi-level surgery were excluded. Other exclusion criteria were history of spinal surgery other than discectomy, severe osteoporosis, systemic disease, malignancy, chronic use of steroids or NSAIDS, skeletally immature patients or patients over 70 years. A full informed consent was obtained from each patient. Randomisation was by sealed envelope with a 1:1 ratio opened just prior to surgery. Patients were operated on from July 2005 until December 2006.
Implants
The Monarch™ rod system (DePuy, Johnson & Johnson) was used for posterior instrumentation and 2 Saber™ cages (DePuy, Johnson & Johnson) were used for interbody fusion. The Monarch™ rod system is a titanium segmental instrumentation system. The Saber™ cages are manufactured from a carbon fibre reinforced polymer with a 70/30 PEEK (poly-ether-ether-ketone) to carbon fibre matrix ratio.
Surgical technique
Just before anaesthesia, 54 ml blood was taken from the patient through a peripheral catheter. Platelet-rich plasma was produced, using the Sympony™ Platelet Concentration System (DePuy, Johnson & Johnson). Samples of the starting material and platelet concentrates were analysed to determine the absolute concentration and yield of platelets. PRP was clotted with thrombin (1,000 U/ml in 10% CaCl2) by adding 1 part thrombin stock solution to 9 parts of plasma to yield a final thrombin concentration of 100 U/ml.
A posterior lumbar interbody fusion with posterior pedicle screw fixation was performed through a midline posterior approach. Pedicular screws were placed under fluoroscopic guidance, followed by a complete laminectomy and discectomy. The vertebral body endplates were prepared by curetting until point bleeding was seen.
Autologous cancellous bone chips were harvested unilaterally from the iliac wing, approached through the midline posterior incision. The cages of the appropriate size were filled with bone chips and were steeped in the plasma-thrombin solution until clotting occurred visually (approximately 10 min). In the control group, the cages were filled with autologous bone in the same way and were implanted after approximately 10 min, without incubation in a plasma-thrombin solution. The cages were implanted under fluoroscopic guidance, followed by placement of rods. No posterolateral grafts were used.
Three doses of intravenous antibiotics were given, one just before anaesthesia and 2 doses at 8 and 16 h postoperatively. A lumbar orthosis was worn during activity for 6 weeks.
Outcome measures
The visual analogue scale (VAS) of pain, the Oswestry Disability Index (ODI), and the Short-Form 36 (SF-36v2®) questionnaire were completed preoperatively and postoperatively at 3, 6, 12, and 24 months, respectively. The minimum clinically important differences for outcome measures were established, based on the literature: VAS 2 points, ODI 10 points, and SF-36 seven points in each domain [16, 32, 35]. Physical Component Summary (PCS) and Mental Component Summary (MCS) measures were scored using a three-step procedure. First, the eight health domain scales were standardised using means and standard deviations from the 1998 US population. Second, these scores were aggregated using weights from the 1990 US general population. Third, aggregate PCS and MCS scores were standardised using a linear T-score transformation [36].
Radiographs of the lumbar spine (anterior and lateral views) were taken pre-operatively and postoperatively at 3, 6, 12, and 24 months, respectively. Flexion/extension radiographs were taken postoperatively at 3, 6, 12, and 24 months, respectively. The dynamic films were performed I a sitting position.
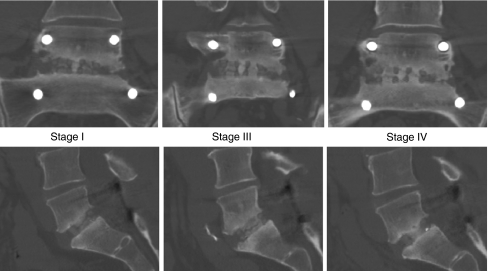
CT-scans of the fused segment were taken postoperatively at 3, 6, and 12 months, respectively. Reconstructions were made in both the coronal and the sagittal planes. We developed a classification to optimally determine the presence of bridging trabecular bone between the vertebral bodies. The status of the interbody fusion was quantified using the ‘bridging trabecular bone scale’: Visual rating from CT-reformatted images using a percentage based on the total length of the device/graft-vertebra interface superiorly and inferiorly. Rating was determined by combining both the superior and inferior edges of the device/graft-vertebra interface to yield an overall percentage of bridging bone (Table 1; Fig. 1).
Table 1.
Bridging trabecular bone scale
| 5 | 100% (complete bridging) |
| 4 | 76–99% |
| 3 | 51–75% |
| 2 | 26–50% |
| 1 | 1–25% |
| 0 | 0% (no bridging) |
Visual rating from CT-reformatted images using a percentage based on the total length of the device/graft-vertebra interface superiorly and inferiorly. Rating was determined by combining both the superior and inferior edges of the device/graft-vertebra interface to yield an overall percentage of bridging bone
Fig. 1.
Bridging trabecular bone scale. Coronal and sagittal reconstructions in one patient demonstrating stage I, III, and IV at 3, 6, and 12 months, respectively. At 6 months, trabecular bone is bridging both endplates of one of the 2 cages; at 12 months, trabecular bone is bridging both endplates of both cages
All films were read by two independent radiologists blinded to the randomization assignment. A third radiologist was consulted in case of discrepancy.
Fusion was regarded as complete when the patient had not undergone a supplemental fixation at any time during the study, if there was no evidence of pseudarthrosis on X-ray, when there was no motion on flexion/extension films, and if the bridging trabecular bone score was 3, 4 or 5.
Statistical analysis
Continuous data were tested for normality by Kolmogorov–Smirnov test. For all variables a normal distribution could be assumed. For comparisons before and after treatment, paired t tests were used; comparisons between groups were tested with independent t tests. We estimated a mean difference of 5 score units between the 2 groups with a variance of 30. With a power of 0.8 and a significance level of 0.05, a number of 20 patients was estimated to be sufficient in order to reach conclusive statistical significance.
Results
Between February 2005 and December 2006, 40 patients were recruited for the trial: 20 were randomised to the study group, receiving autograft + PRP and 20 to the control group, receiving autograft only. In each group, one patient was lost to follow-up. Patient demographics and operation details are set out in Table 2. There were no relevant differences between the groups with regard to BMI, sex, smoking history, or level of pathology (all p ≫ 0.05).
Table 2.
Patient demographics, level of surgery, operation time and blood loss
| Autograft + PRP | Autograft | p value | |
|---|---|---|---|
| Number | 19 | 19 | |
| BMI | 27.2 (5.0) [21–69] | 25.4 (3.6) [36–70] | 0.213* |
| Gender (female/male) | 7/12 | 7/12 | 1.000** |
| Smoking (yes/no) | 8/11 | 9/10 | 1.000** |
| Level of surgery | |||
| L3–L4 | 0 | 1 | |
| L4–L5 | 3 | 4 | |
| L5–S1 | 16 | 14 | |
| Operation time (min) | 193.2 | 191.1 | 0.855* |
| Blood loss (ml) | 407.1 | 402.4 | 0.926* |
Mean (SD) [range]
* t test
** Fisher’s exact test
Analysis of preoperative outcome measures showed no statistically significant difference between the two groups (Table 3).
Table 3.
Preoperative outcome measures
| Autograft + PRP | Autograft | p value | |
|---|---|---|---|
| ODI | 42.6 (17.4) | 49.4 (11.1) | 0.164* |
| VAS | 7.4 (1.9) | 7.4 (1.4) | 0.924* |
| SF-36 | |||
| Physical function | 16.3 (3.9) | 16.2 (4.4) | 0.907* |
| Physical role | 4.3 (0.45) | 4.5 (1.0) | 0.885** |
| Bodily pain | 4.5 (2.0) | 5.0 (1.6) | 0.415* |
| General health | 16.1 (4.3) | 16.3 (4.0) | 0.847* |
| Vitality | 16.8 (4.7) | 15.2 (4.0) | 0.272* |
| Social function | 6.1 (2.3) | 6.5 (1.8) | 0.532* |
| Emotional role | 4.2 (1.2) | 4.1 (1.2) | 0.665** |
| Mental health | 21.6 (5.5) | 20.3 (6.1) | 0.471* |
Values are expressed as mean (SD)
* t test
** Mann–Whitney U test
The mean increase in plasma concentration after centrifugation was 3.3-fold.
VAS
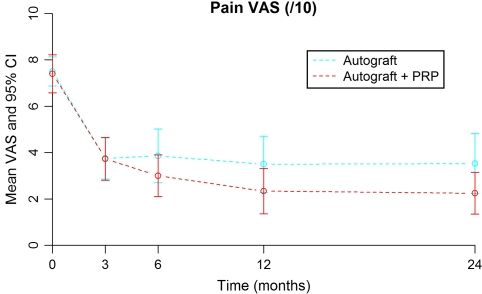
Both groups showed a significant improvement in VAS 2 years postoperatively (Fig. 2) with an improvement of 4.92 points in the study group (p < 0.001) and 4.00 points in the control group (p < 0.001). The difference in improvement was not statistically significant (p = 0.166).
Fig. 2.
Visual analogue scale preoperatively and at 3, 6, 12, and 24 months for the study (+PRP) group and control (−PRP) group
Oswestry disability index
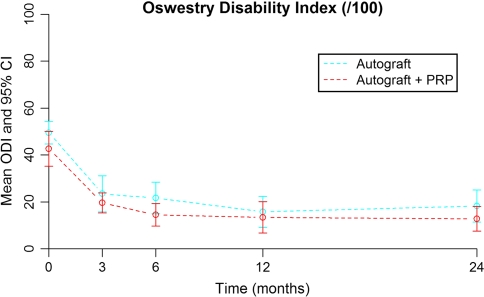
Both groups showed a significant improvement in ODI 2 years postoperatively (Fig. 3) with an improvement of 30 points in the study group (p < 0.001) and 32.1 points in the control group (p < 0.001). There was a non-significant difference in improvement between the two groups (p = 0.201). Note the preoperative difference which is more or less maintained during follow-up.
Fig. 3.
Oswestry disability index preoperatively and at 3, 6, 12, and 24 months months for the study (+PRP) group and control (−PRP) group
Short-Form-36
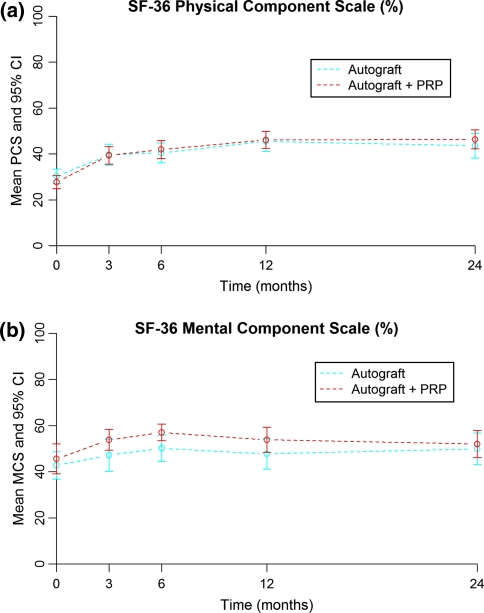
Both groups showed improvement in each domain of the SF-36. There were clinically important improvements in each domain (>7.0 points) and statistically significant improvements in all domains, except for vitality and mental health in the study group (Table 4). In these two domains, the absolute scores were markedly higher in the study group, pre-operative as well as at each post-operative interval. The improvement of the SF-36 scores was more pronounced in the study group in six domains, predominantly in physical role and social function (Table 5). Norm-based PCS and MCS measures showed improvement in both groups (Fig. 4). Mean PCS score-change was 18.38 in the study group and 14.70 in the control group. Mean MCS score-change was 8.21 in the study group and 4.19 in the control group. There was no relevant difference in improvement for both PCS (p = 0.147) and MCS (p = 0.924) between groups.
Table 4.
Mean Short Form-36 scores
| Autograft + PRP | Autograft | |||||
|---|---|---|---|---|---|---|
| Preop | 24 months | Change (p) | Preop | 24 months | Change (p) | |
| Physical function | 31.6 | 68.4 | 36.8 (<0.001) | 30.8 | 64.2 | 33.4 (<0.001) |
| Physical role | 6.6 | 67.1 | 60.5 (<0.001) | 11.8 | 44.7 | 32.9 (=0.011) |
| Bodily pain | 25.3 | 70.0 | 44.7 (<0.001) | 30.0 | 65.3 | 35.3 (<0.001) |
| General health | 55.3 | 72.9 | 17.6 (=0.010) | 56.6 | 73.7 | 17.1 (=0.002) |
| Vitality | 63.9 | 74.5 | 10.5 (=0.263) | 56.1 | 71.8 | 15.8 (=0.005) |
| Social function | 51.3 | 82.2 | 30.9 (<0.001) | 56.6 | 74.3 | 17.8 (=0.006) |
| Emotional role | 40.4 | 77.2 | 36.8 (=0.002) | 35.1 | 64.9 | 29.8 (=0.040) |
| Mental health | 66.5 | 75.8 | 9.3 (=0.263) | 61.1 | 73.5 | 12.4 (=0.031) |
Change within each group (paired t test)
Table 5.
Comparison of changes between groups
| Increase pre-op, 24 months | p value | ||
|---|---|---|---|
| Autograft + PRP | Autograft | ||
| Physical function | 36.8 | 33.4 | 0.687 |
| Physical role | 60.5 | 32.9 | 0.080 |
| Bodily pain | 44.7 | 35.3 | 0.265 |
| General health | 17.6 | 17.1 | 0.946 |
| Vitality | 10.5 | 15.8 | 0.616 |
| Social function | 30.9 | 17.8 | 0.155 |
| Emotional role | 36.8 | 29.8 | 0.680 |
| Mental health | 9.3 | 12.4 | 0.745 |
Independent t test
Fig. 4.
a PCS score preoperatively and at 3, 6, 12, and 24 months for the study (+PRP) group and control (−PRP) group. b MCS score preoperatively and at 3, 6, 12, and 24 months for the study (+PRP) group and control (−PRP) group
Imaging
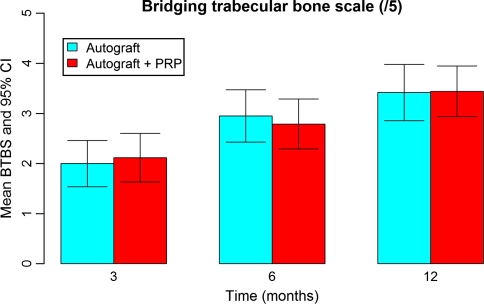
CT-graphic fusion was achieved in all but one patient from the control group (Bridging trabecular bone scale 0). He had no clinical signs or symptoms of non-union at 2 years’ follow-up. There was no intervertebral translation nor angulation on dynamic X-rays There was no difference between both groups in interbody healing on CT-scan reconstructed images at 3, 6, and 12 months (p = 0.741, p = 0.663, p = 0.951), respectively (Fig. 5).
Fig. 5.
Mean bridging trabecular bone scale at 3, 6, and 12 months for the study (+PRP) group and control (−PRP) group
Adverse events
There was one accidental dural tear, which needed repair, in the study group. There were no wound infections or vascular injuries. Transient radiculopathy occurred in one patient of the study group and in two patients of the control group. There were four cases of donor site pain in the study group and in two cases in the control group at 3 months post-operatively. At 2 years’ follow-up, no donor site pain was noted anymore. In the study group, hardware was removed in one patient after bony fusion was achieved. In the control group, instrumentation was removed in three patients after bony fusion was achieved. Revision surgery for non-union was not indicated.
Discussion
There was a significant improvement in VAS 2 years postoperatively with an improvement of 4.92 points in the study group and 4.00 points in the control group. This difference was not statistically significant.
Both groups showed a significant improvement in ODI 2 years postoperatively with overall higher scores for the study group. There was a non-significant difference in outcome between the two groups, due to a remarkable preoperative difference.
Short Form-36 analysis showed clinically important improvements in each domain and statistically significant improvements in all domains, except for vitality and mental health in the study group. In general, SF-36 scores were higher in the study group. PCS and MCS measures showed improvement in both groups. Improvement of PCS measures was more pronounced in the study group.
Imaging showed uneventful fusion in both groups, except in one patient. No stimulating or inhibitory effect of PRP was observed on CT-scan.
Instrumentation was removed in 4 out of 38 patients (10.5%) after bony fusion was achieved. We believe that this is due to the higher profile of the Monarch™ Typhoon Cap, leading to symptomatic friction of the paraspinal muscles.
The enhancement of healing by the placement of a supraphysiologic concentration of autologous platelets at the site of surgery is supported by basic science studies [5, 14, 25, 26]. Research has revealed that the role of platelets is much more involved than simply ‘plug’ formation; they are responsible for actively extruding growth factors, which initiate bone formation [5].
Beneficial effects of PRP on spinal fusion were reported by Hee et al. [20] in a prospective study comparing transforaminal lumbar interbody fusion (TLIF) with autograft + AGF to an historical cohort without AGF. They demonstrated faster fusion but no increase in fusion rates. They included both 1-level and 2-level fusions. Fusion was assessed on X-ray.
Inferior rates of arthrodesis were reported by Weiner and Walker when AGF was added to autologous bone in posterolateral spine fusion [37]. They performed a retrospective, consecutive series in 59 patients who underwent a single-level fusion. Fusion was assessed by two spine surgeons on dynamic X-rays at 1 and 2 years.
An inhibitory effect of growth factors has been suggested by Castro in spinal interbody fusion [9]. Spinal fusion was assessed in a consecutive series of 62 patients receiving autograft only, followed by a consecutive series of 22 patients receiving autograft +AGF. The arthrodesis rate appeared to decrease in the AGF group with 19%. A transforaminal lumbar interbody fusion was performed at one or two levels. Interbody cages were used from three different companies and posterior fixation instrumentation was used from five different companies. Arthrodesis was diagnosed on X-ray.
A retrospective cohort study looked at posterolateral fusion, diagnosed by X-ray, CT-scan or exploration [8]. They included 1, 2, or 3-level instrumented fusions. Only patients with persistent back pain and without a good fusion mass on X-rays were further investigated with CT-scan and eventually exploration. They found a very high non-union rate on exploration: 18% in the AGF group and 16% in the control group. They concluded that platelet gel failed to enhance fusion rate when added to autograft in patients undergoing instrumented posterolateral fusion.
The possible inhibitory effect was studied by Jenis in a prospective non-randomised study, comparing iliac crest bone graft to allograft combined with AGF [21]. Arthrodesis was diagnosed on X-ray and CT-scan. They found equivalent radiographic and clinical outcomes in 1- and 2-level interbody fusions. No inhibitory effect was noted on graft incorporation.
Most other studies looking at the combination of PRP and autograft have looked at heterogenous groups of patients, undergoing anterior, posterolateral, and posterior lumbar interbody fusion [25, 29].
The interpretation of fusion on the basis of static X-rays is subject to controversy [28]. Even on flexion–extension films, it is difficult to assess fusion for several reasons: there is a difference in range of motion of 7° to 14° in asymptomatic individuals, pain may inhibit motion, and the measurement of motion may be unreliable in the presence of pedicle-screw instrumentation [6, 28]. Therefore, we did not include X-rays in the diagnosis of fusion. We preferred to use thin-slice CT-scan with reconstruction images in the coronal and sagittal planes, allowing to determine a degree of fusion rather than distinguish between fusion and non-fusion, and to determine an eventual inhibitory effect of PRP. We therefore developed a classification for optimal determination of the presence of bridging trabecular bone between the vertebral bodies. CT-graphic fusion was determined in all but one patient in this study. Because he had no clinical signs or symptoms of non-union at 2 years follow-up, a ‘functional arthrodesis’ was diagnosed [23].
The first hypothesis, that there would be a clinical benefit in the study group, could not be confirmed. In the present study, the variance was higher than expected and the observed differences in PCS were lower than expected. As a result of this, the study turned out to be insufficiently powered, and as a consequence the differences in PCS and VAS did not reach statistical significance.
The second hypothesis, that there would be no inhibitory effect of PRP on graft incorporation on CT-scan at 3, 6, and 12 months postoperatively, was confirmed. Although we distinguished four stages of fusion, it was unlikely to find a remarkable difference in the speed of bone formation, only by adding PRP. Also, it was unlikely to find a difference in the rate of non-union because the incidence of non-union in a well-performed fusion + PLIF in a healthy person is extremely low.
This prospective randomised controlled clinical and radiological trial shows no substantial improvement or deterioration in clinical and radiographical outcome when using autologous bone with PRP in posterior lumbar interbody fusion when compared with the use of autologous bone only. Therefore, the expense of using PRP cannot be justified until statistical significance can be reached in larger studies.
Conclusion
Using PRP provided no substantial improvement or deterioration in clinical and radiographical outcomes in posterior lumbar interbody fusion.
No significant benefit on the clinical course after spinal lumbar interbody fusion could be observed in this study. Accordingly, from an economical viewpoint, the cost/benefit ratio remains high and the use of PRP cannot be recommended on a routine basis until statistical significance can be reached in a larger study.
On CT-scan, no inhibitory effect on graft incorporation was seen.
Local ethical committee and institutional research board approval were obtained for the study.
Conflict of interest
The reviewers and the surgeon have and had no personal or financial interest in companies selling AGF- or PRP-technology, nor have they received any financial support from them.
References
- 1.Archundia TR, Soriano JC, Corona JN (2007) Utility of platelet-rich plasma and growth factors in bone defects. Act Orthop Mexicana [PubMed]
- 2.Arm DM, Lowery GL, Hood AG Characterization of an autologous platelet gel containing multiple growth factors. Presented at the 45th Orthopaedic Research Society meeting, Anaheim
- 3.Arpornmaeklong P, Kochel M, Depprich R, Kubler NR, Wurzler KK. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 1999;33:60–70. doi: 10.1054/ijom.2003.0492. [DOI] [PubMed] [Google Scholar]
- 4.Aspenberg P, Jeppsson C, Wang JS, Bostrom M. Transforming growth factor beta and bone morphogenetic protein 2 for bone ingrowth: a comparison using bone chambers in rats. Bone. 1996;19:499–503. doi: 10.1016/S8756-3282(96)90257-4. [DOI] [PubMed] [Google Scholar]
- 5.Bose B, Balzarini MA. Bone graft gel: autologous growth factors in lumbar spine fusions. Adv Ther. 2002;19:170–175. doi: 10.1007/BF02848692. [DOI] [PubMed] [Google Scholar]
- 6.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM (1996) Lumbar interbody fusion using the Brantigan I/F cage for PLIF and the VSP pedicle screw system: two-year results of a Food and Drug Administration IDE clinical trial. In: Hudson, LeHuec JC (eds) Intersomatique du Rachis Lumbaire, Montpellier, France, Sauramps Medical
- 7.Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63:370–376. doi: 10.1016/j.joms.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Carreon LY, Glassman SD, Anekstein Y, Puno RM. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30:E243–E247. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- 9.Castro FP., Jr Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17:380–384. doi: 10.1097/01.bsd.0000110342.54707.19. [DOI] [PubMed] [Google Scholar]
- 10.Cenni E, Ciapetti G, Pagani S, Perut F, Giunti A, Baldini N. Effects of activated platelet concentrates on human primary cultures of fibroblasts and osteoblasts. J Periodontol. 2005;76:323–328. doi: 10.1902/jop.2005.76.3.323. [DOI] [PubMed] [Google Scholar]
- 11.Choi BH, Im CJ, Huh JY, Suh JJ, Lee SH. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33:56–59. doi: 10.1054/ijom.2003.0466. [DOI] [PubMed] [Google Scholar]
- 12.Cieslick-Bielecka A, Bielecki T, Gazdzik TS, Cieslik T, Szczepanski T. Improved treatment of mandibular odontogenic cysts with platelet-rich gel. Oral Surg. 2008;105(4):423–429. doi: 10.1016/j.tripleo.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Dori F, Nikolidakis D, Huszar T, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an enamel matrix protein derivative and a natural bone mineral. J Clin Periodont. 2008;35(1):44–50. doi: 10.1111/j.1600-051X.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 14.Dugrillon A, Eichler H, Kern S, Kluter H. Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002;31:615–619. doi: 10.1054/ijom.2002.0322. [DOI] [PubMed] [Google Scholar]
- 15.Eppley B, Woosell J, Higgins J. Platelet quantification and growth factor analysis from platelet–rich plasma (PRP): implications in wound healing. Plastic Reconstruct Surgery. 2004;114:1502–1508. doi: 10.1097/01.PRS.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 16.Fairbank J, Pynsent B. The Oswestry disability index. Spine. 2002;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Houck K, Jakeman L, Leung D. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 18.Fraser RD. Interbody, posterior, and combined lumbar fusions. Spine. 1995;90(24S):167S–177S. doi: 10.1097/00007632-199512151-00016. [DOI] [PubMed] [Google Scholar]
- 19.Hannon T, Polston G, Petarske W, et al. Determination of platelet yields from platelet rich plasma for five autotransfusion machines. Anaesth Analg. 1996;88:104–109. [Google Scholar]
- 20.Hee HT, Majd ME, Holt RT, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12:400–407. doi: 10.1007/s00586-003-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenis LG, Banco RJ, Kwon B. A prospective study of autologous growth factors in lumbar interbody fusion. Spine J. 2006;6:14–20. doi: 10.1016/j.spinee.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Kevy S, Jacobson M. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corp Technol. 2004;36:28–35. [PubMed] [Google Scholar]
- 23.Kumar A, Kozak JA, Doherty BJ, Dickson JH. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine. 1993;18:2393–2400. doi: 10.1097/00007632-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Lind M, Deleuran B, Thestrup-Pedersen K, et al. Chemotaxis of human osteoblasts. Effect of osteotropic growth factors. APMIS. 1995;103:140–146. doi: 10.1111/j.1699-0463.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 25.Lowery GL, Kulkarni S, Pennisi AE. Use of autologous growth factors in lumbar spinal fusion. Bone. 1999;25(2 Suppl):47S–50S. doi: 10.1016/S8756-3282(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 26.Marx RE. Platelat-rich Plasma: what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 28.Mc Afee PC. Current concepts review: interbody fusion cages in reconstructive operations of the spine. J Bone Joint Surg Am. 1999;81:859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. JOT. 2008;22(6):432–438. doi: 10.1097/BOT.0b013e31817e793f. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Tsumura H, Wang JC, Alanay A. An update on bone substitutes for spinal fusion. Eur Spine J. 2009;6:783–799. doi: 10.1007/s00586-009-0924-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obremskey WT, Marotta JS, Yaszemski MJ, Churchill LR, Boden SD, Dirschl DR. The introduction of biologics in orthopaedics: issues of cost, commercialism, and ethics. J Bone Joint Surg Am 89:1641–1649 [DOI] [PubMed]
- 32.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Ranly DM, McMillan J, Keller T, Lohmann CH, Meunch T, Cochran DL, Schwartz Z, Boyan BD. Platelet-derived growth factor inhibits demineralized bone matrix-induced intramuscular cartilage and bone formation. A study of immunocompromised mice. J Bone Joint Surg Am. 2005;287:2052–2064. doi: 10.2106/JBJS.D.02752. [DOI] [PubMed] [Google Scholar]
- 34.Schaaf H, Strekbein P, Lendeckel S, Heidinger K, Gortz B, Bein G, Boedecker RH, Schlegel KA, Howalt HP. Topical use of platelet-rich plasma to influence bone volume in maxillary augmentation: a prospective randomized trial. Vox Sang. 2008;94(1):64–69. doi: 10.1111/j.1423-0410.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 35.Ware J, Sherbourne C. The MOS 36 Item Short Form Health Survey (SF36). Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User’s Manual fot the SF-36v2® Health Survey. 2. Lincoln: QualityMetric Incorporated; 2007. [Google Scholar]
- 37.Weiner BK, Walker M. Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine. 2003;28:1968–1971. doi: 10.1097/01.BRS.0000083141.02027.48. [DOI] [PubMed] [Google Scholar]
- 38.Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–1299. doi: 10.1016/S0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman R, Jacubietz R, Jacubiets M, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2002;41:1217–1224. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]