Abstract
Purpose
We demonstrate clinical features, therapy and outcome of 14 patients with symptomatic spinal cavernous malformations (CM).
Methods
We retrospectively reviewed all patients who underwent microsurgical treatment of symptomatic spinal CM during the last decade in our department through an analysis of our database.
Results
We analyzed the data of 14 patients (11 females, 3 males) with symptomatic spinal CM in a range of 16–77 years (mean age 47.8 years). Seven patients (50%) experienced significant improvement of their symptoms rapidly after surgery. The remaining seven patients presented new non pre-existing complaints, which improved gradually with a favourable outcome at the last follow-up examination in six cases.
Conclusion
Microsurgical treatment under perioperative electrophysiological monitoring is justified to prevent severe neurofunctional deterioration in symptomatic spinal CM. Although some of the patients deteriorate after surgery, the symptoms are rapidly declining with a favourable outcome in majority of them.
Keywords: Cavernous malformation, Cavernous malformation spinal cord, Intramedullary cavernoma, Spinal vascular malformation
Introduction
Cavernous malformations (CMs) are angiographically occult, mulberry like dynamic vascular lesions, which account up to 15% of all the vascular malformations and occur throughout the central nervous system (CNS) [2, 11, 14, 21, 23]. An association with a venous anomaly is always given [4, 25]. A familial disposition is observed in up to 50% with an autosomal dominant inheritance with incomplete penetrance. Genetic studies of familial CMs have shown mutations at three different loci (CCM1 on 7q, CCM2 on 7p, and CCM3 on 3q). Loss of CCM1 was found to be an important factor for insufficient angiogenesis due to incomplete cell adhesion, which can lead to development of CMs. Simultaneous occurrence, affecting both cerebral and spinal cord is observed with varying prevalence (Laubage et al. 37%, Vishteh et al. 47%) [2, 5, 12, 17, 19, 23, 27].
Spinal CMs are increasingly being diagnosed by magnetic resonance imaging (MRI) in patients with varying spinal cord related symptoms or pain syndromes [2, 6, 7, 26]. Spinal CMs can occur along the neuraxis and are predominantly located intramedullary. However, exophytic growth and extradural CMs are reported as well [8, 22]. The incidence is difficult to estimate. Some of the series report an involvement of the spinal cord with 3 to 5% of all CMs of the CNS [2–4, 7, 10, 17]. Due to space occupying growth, recurrent micro-bleeding or significant haemorrhage (in respect of the available space) spinal CMs can lead to severe neurological deteriorations, such as progressive myelopathy and right up to paraplegia, unlike their intracranial counterparts. The latter typically presents with seizures, progressive focal neurological deficits and haemorrhage, whereas the risk of significant haemorrhage like other vascular malformations is considered to be low due to the low venous pressure [2, 21].
The decision between conservative management and surgical treatment of spinal CM is discussed controversially and the opinions diverge. The choice of microsurgical treatment of symptomatic spinal CMs is usually made on a case-by-case basis, considering the neurological complaints, general condition and the level of suffering of the patients as well as the surgical accessibility.
The aim of our study was to present the data of micro-surgically treated patients, harbouring symptomatic spinal CMs in our department during the last decade with the main focus on the neurofunctional outcome. The justification of micro-surgical procedure is discussed critically by an additional review of the latest published works on this topic.
Methods
We reviewed the charts of 14 micro-surgically treated patients (12 females and 2 males) suffering from symptomatic spinal CMs between 1999 and 2009 in our department. Patients with asymptomatic lesions, found incidentally, were excluded from the analysis. Simultaneous occurrence of cerebral CM was observed in three cases. On MRI, CMs typically appear as lesions with mixed signal intensity on T1-weighted images (“popcorn” appearance). Microhaemorrhages and hemosiderin deposits usually cause a low-signal-intensity rim surrounding the lesion on T1- and T2-weighted images. Gradient echo sequences demonstrate the hypointense signal from the haemorrhage [1, 2, 7]. MRI has been performed in all the cases and the findings were applicable for CMs in 11 cases (Figs. 1 and 2). The remaining three cases were initially diagnosed as neurinoma, an extradural tumour and a tumour of the vertebral body.
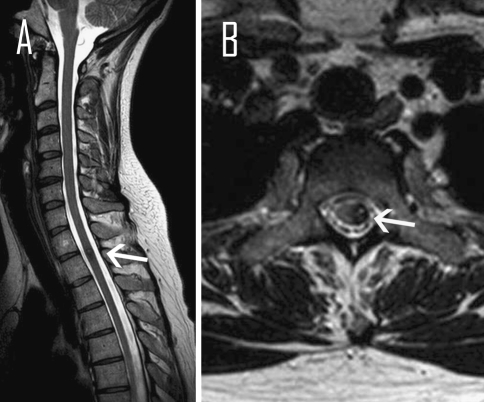
Fig. 1.
Magnetic resonance image of the spine, T2 weighted, a sagittal, b axial show cavernous malformation at the level T2 (arrows)
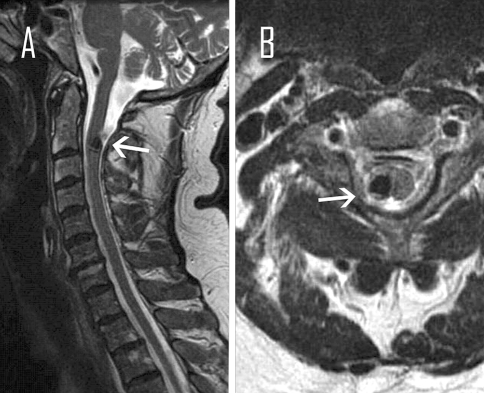
Fig. 2.
Magnetic resonance image of the cervical spine, T2 weighted, a sagittal, b axial show cavernous malformation at the C2 (arrows)
The final diagnosis was confirmed by histopathological analysis, regarding typical findings on microscopic examination, like composition of dilated, thin-walled capillaries with one layer of endothelial lining and a variable layer of fibrous adventitia. Further features were absence of elastic fibers in the walls of the vascular caverns, absence of brain tissue and usual evidence of previous haemorrhage.
It is difficult to distribute the complex neurofunctional spinal cord impairment of the patients into a classification adequately. We believe that a selective schedule of the spinal cord symptoms is more convincing than a score associated to a classification (Table 3). However, we, additionally, used the Frankel scale and the McCormick classification at the time of admission, discharge and follow up to give an overview and enable comparisons to other studies (Table 1 and 3) [9, 17, 20].
Table 3.
Neurofunctional impairments
| NO. | Admission | Discharge | Last follow-up | Duration |
|---|---|---|---|---|
| 1 | Severe ataxia, right hemihypesthesia below T10 (F:C, Mc:3) | Paresis left leg M3 with dependency on wheelchair, unchanged right hemihypesthesia, no incontinence (F:C, Mc:3) | Improvement of the paresis and hemihypesthesia. independent from wheelchair (F:D, Mc:2) | 5 months |
| 2 | Right hemihypesthesia, tendency to fall, positive pyramidal signs (F:D, Mc:2) | Significant improvement (F:E, Mc:1) | – | – |
| 3 | Back pain with radiation to the left buttock (F:E, Mc:1) | Significant pain relief (F:E, Mc:1) | No complaints (F:E, Mc:1) | 8 months |
| 4 | Right hemihypesthesia, hyperalgesia right leg (F:D, Mc:2) | Complete relief of the symptoms (F:E, Mc:1) | Stable findings (F:E, Mc:1) | 49 months |
| 5 | Dysesthesia all extremities (left > right) (F:D, Mc:2) | Slight improvement of dysesthesia, slight ataxia with closed eyes (F:C, Mc:2) | Significant improvement of dysesthesia, complete relief of ataxia (F:E, Mc:1) | 10 months |
| 6 | Progressive ataxia, dysmetria, fine motor dysfunction right hand, Back and Neck pain (F:C, Mc:3) | Left hemihypesthesia, paraparesis left > right, transient incontinence (F:C, Mc:3) | Nearly normal, significant improvement of fine motor deficits, pain and dysmetria, residual slight ataxia (F:E, Mc:1) | 36 months |
| 7 | Back pain with radiation to left leg, sensory dysfunction right leg (F:E, Mc:1) | Significant improvement (F:E, Mc:1) | No complaints (F:E, Mc:1) | 35 months |
| 8 | Back pain with radiation into both legs, neurogenic claudication (F:E, Mc:2) | Due to re-bleeding → Dysesthesia sole of the feet, proprioception impairments, gait disturbance, paraparesis, incontinence (F:B, Mc:4) | Significant improvement of dysesthesia, gait disturbance and incontinence, residual proprioception impairments (F:D, Mc:2) | 7 months |
| 9 | Paraplegia below level L1, incontinence (F:A, Mc:4) | Declining paraplegia, able to walk short distances with assistance, intact bladder function (F:C, Mc:3) | Improvement of gait, able to walk short distances without assistance, depending on wheelchair for long distances (F:D, Mc:2) | 15 months |
| 10 | Back pain, dysesthesia left shank and foot, hyperreflexia lower limbs (F:E, Mc:1) | Improvement of dysesthesia, transient paresis of the left leg M1, persistent hyperreflexia (F:D, Mc:2) | Significant improvement of the paresis to M4, complete relief of dysesthesia and pain, persistent hyperreflexia (F:E, Mc:1) | 8 months |
| 11 | Left hemihypesthesia, fine motor dysfunction left hand (F:D, Mc:2) | Transient tetraparesis → able to walk at time of discharge, proprioception impairments, slight improvement of fine motor dysfunction (F:B, Mc:3) | Significant improvement of gait and fine motor function, residual proprioception impairments (F:D, Mc:1) | 9 months |
| 12 | Progressive ataxia → unable to walk, proprioception impairments, dysesthesia lower limbs (F:B, Mc:4) | Persistent ataxia → able to walk with assistance, persistent proprioception impairments and dysesthesia (F:C, Mc:3) | Persistent ataxia → sufficient gait with sparse assistance, improvement of dysesthesia, persistent proprioception impairments (F:D, Mc:2) | 11 months |
| 13 | Back pain, right hemihypesthesia, hyperreflexia (right > left) (F:E, Mc:1) | Transient paraparesis right > left, bilateral positive babinski’s sign (F:C, Mc:3) | Significant improvement of back pain and hemihypesthesia, almost complete relief of paraparesis (F:E, Mc:1) | 14 months |
| 14 | Back pain, dysesthesia right leg (F:E, Mc:1) | Complete relief of back pain, slight improvement of dysesthesia (F:E, Mc:1) | Constant findings (F:E, Mc:1) | 12 months |
No. patient number, Admission symptoms at time of admission, Discharge symptoms at time of discharge, Follow-up symptoms at time of last follow-up examination, Duration duration of last follow-up examination, F Frankel score, Mc McCormick classification, R right sided, L left sided
Table 1.
Frankel scale and McCormick classification
| Grade | Frankel scale | Grade | McCormick classification |
|---|---|---|---|
| A | Complete paralysis | 1 | Neurologically normal, mild focal deficit not significantly affecting function of involved limb, normal gait |
| B | Sensory function only below the injury level | 2 | Sensorimotor deficit affecting function of involved limb, mild to moderate gait difficulty, still independent walk |
| C | Incomplete motor function below injury level | 3 | More severe neurologic deficiencies, requires cane/brace for ambulation; may or may not be independent |
| D | Fair to good motor function below injury level | 4 | Severe deficit, requires wheelchair or has bilateral upper extremity impairment, usually not independent |
| E | Normal function |
The posterior surgical approach to the spine was promising and has been chosen for all patients. In detail, hemilaminectomy and laminectomy have been performed each in five, laminoplasty and interlaminar fenestration in each two cases (Table 2). Surgical procedure was done under intraoperative electrophysiological monitoring with the somatosensory evoked potentials.
Table 2.
Patient data
| No. | Age/sex | Duration | Spinal level | Approach |
|---|---|---|---|---|
| 1 | 23 F | 5 days | Intramedullary, T 2/3 | Laminectomy T 2-4 |
| 2 | 58 M | 18 months | Intramedullary, C 2 | Hemilaminectomy C 2 |
| 3 | 53 F | 6 weeks | Extradural, L 1 | Interlaminar fenestration L 1/2 |
| 4 | 61 M | 3 years | Intramedullary C 1/2 | Hemilaminectomy C 1 |
| 5 | 47 F | 3 weeks | Intramedullary C 5 | Laminectomy C 5 |
| 6 | 38 F | 12 weeks | Intramedullary C 5 | Laminoplasty C 4-6 |
| 7 | 44 F | 1 year | Conus | Interlaminar fenestration T 12/L 1 |
| 8 | 77 F | 2 months | Twelfth vertebral body | Laminectomy T 12 |
| 9 | 67 F | 2 days | Intramedullary T 10 | Hemilaminectomy T 10 |
| 10 | 42 F | 4 years | Intramedullary T 1/2 | Hemilaminectomy T 2 |
| 11 | 16 F | 2 weeks | Intramedullary C 1/2 | Laminoplasty C 1 |
| 12 | 63 F | 12 weeks | Extradural T 3-7 | Hemilaminectomy T 3-7 |
| 13 | 45 F | 1 week | Intramedullary, T 3 | Laminectomy T 3 |
| 14 | 17 F | 2 weeks | Conus | Interlaminar fenestration T 12/L 1 |
No. patient number, Age in years, F female, M male, Duration duration of symptoms to admission, C cervical, T thoracal, L lumbar
Postoperatively, MRI was performed in all cases, except in case number 3 (Table 2) with extradural localisation of the CM. MRI confirmed complete resection in 11 cases. In case number 10, MRI showed edema and a hemosiderin rim in the operating field in line with temporary neurofunctional deterioration. In case number 8, MRI detected re-bleeding with necessity of surgical revision. Thirteen patients were examined in our out-patient department usually 4–6 weeks after discharge and were followed-up to 45 months (mean 16.8 months) (Table 3).
Results
The clinical data are displayed in Table 2 and 3. The mean age of the patients was 47.8 years (range 16–77 years). The female to male ratio was 6:1. The CMs were located intramedullary in nine cases, affecting the cervical spine in five and the thoracic spine in four cases. Two CMs were located in the conus medullaris and additional three CMs extradurally. One extradural CM was located in the twelfth vertebral body.
The most frequent clinical symptom was sensory disturbance in ten cases, followed by ataxia, disturbances of coordination and paresis in nine cases. Neck, back and leg pain were observed in eight and positive Babinski’s sign and hyperreflexia in five cases. Twelve patients presented with slow progressive neurological deterioration over weeks and months (range 1 week to 4 years). The remaining two patients were admitted after an acute onset of sensorimotor deficits (2 and 5 days).
Seven patients (50%) experienced significant improvement of their symptoms rapidly after surgery. The remaining seven patients presented new non pre-existing complaints, which improved gradually with a favourable outcome at the last follow-up examination in six cases. As a result of postoperative re-bleeding, one patient (case no. 8, Table 2) developed severe neurological deterioration and required surgical revision. However, the impairments improved slowly and the patient reported significant relief of the symptoms at the time of last follow-up.
Discussion
Concerning the presented symptomatology of our patients, an acute and a slow progressive type can be differentiated. The acute type, with a sudden onset of neurological deterioration, is usually caused by space occupying haemorrhage in about 70% and acute decompensation due to mass effect, with previous minor complaints [15, 17, 21].
In comparison to this, various neurofunctional impairments develop slowly and progressively due to recurrent microhaemorrhages, mass effect, embolism and gliosis [3, 7]. The underlying cause remains often unrecognized in the early stage. Twelve of the patients in our series belong to the slowly progressive and two patients to the acute type.
Once spinal CM is diagnosed, it is to prove, whether the presented complaints are congruent to the MRI findings. Due to variance of clinical constellations and the level of suffering, the decision for microsurgical removal of spinal CMs of the slow progressive type is made individually. Possible postoperative morbidity and long-term prognosis should be considered exactly, when patients are advised and prepared for surgery. In contrast to this, surgical procedure in the cases of acute neurological deterioration is clearly indicated in the early stage.
During surgery total resection should be done to prevent recurrence and re-bleeding under preservation of the venous anomaly to avoid postoperative edema and thereby associated neurological deterioration. To reduce the iatrogenic trauma and postoperative instability, some of the authors prefer hemilaminectomy instead of laminectomy, if the posterior approach to the spine is promising and the CMs lateralizes to one side [3, 26]. This has been possible in five cases of our series, while interlaminar fenestration which seems to be the gentlest approach to accessible intradural CMs located at the dorsal surface with lateralization to one side could be used in two cases.
Intraoperative electrophysiological monitoring helps to reduce the risk of perioperative injury of the myelon and the intradural fibers, giving the surgeon additional informations. However, because of missing availability and the preference of the surgeon to operate without it, this technique is not used consistently [15]. Despite its proven effectivity, intraoperative monitoring might fail to detect neurofunctional deterioration in some of the cases with the result of false negative findings [13].
Our results show that 7 patients (50%) experienced rapid improvement of their symptoms with stable conditions or further recovery in the follow-up examinations. The remaining patients deteriorated at first in varying degrees, but improved in the continuing course and had a favourable outcome. This course is usually due to postoperative edema, residual hematoma or irritation of the spinal cord fibers during surgery. Nevertheless, the temporary deterioration with self-limiting behaviour is an important issue for the long-term prognosis, which is quite satisfying according to our results and other studies.
Bian et al. [3] reported rapid improvement of the symptoms without temporary deterioration in their series of 16 patients with symptomatic spinal CM. The 6-month follow-up results were likewise satisfying. In a multi-centre study, 37 of 40 surgically treated patients were followed and the clinical status improved in 20 patients (53%), remained unchanged in 6 (16%) and deteriorated in 11 patients (30%). Fifty percent of the patients had postoperative transient worsening, but improved in the continuing course rapidly [17]. Kivelev et al. [15] retrospectively reviewed 14 patients who underwent surgery for symptomatic spinal CM. At the last follow-up (median 3 years, range 1–10 years), 8 patients experienced improvement of their symptoms, 5 had same impairments as they had at the time of discharge and 1 patient deteriorated. In a study of 17 surgically treated patients, the clinical status improved in 12, remained unchanged in 2 and worsened in 3 patients [26]. Lu et al. treated 22 patients surgically and had a favourable outcome with improvement of the clinical status in 9, stable in 11 and worsening in 2 patients [18].
As we see that microsurgical treatment of spinal CMs becoming a safe procedure, due to assistance of high resolution imaging and intraoperative electrophysiological monitoring, it is justified to operate on patients with progressive spinal cord symptoms and MRI proven growth to avoid severe neurofunctional disabilities. The long-term outcome is satisfying and persistent severe deteriorations remain exceptional. This applies especially in the cases of CM induced pain syndromes, whereby it is discussed controversially. Some of the authors postulate that surgery might involve the risk of elevated morbidity and pain relief may be transient [6, 26]. However, we found a significant pain relief in our series without recurrence in the last follow-up examination. Our observation is supported by the results of other studies, which consider surgery as very efficient on pain, who showed that surgery lastingly improved pain in half of their patients [16].
Although observation is discussed to be an alternative to surgical procedure, different studies established the progressive behaviour of spinal CMs towards neurofunctional deterioration and signalled the need for operative treatment [14–17, 20].
In contrast to symptomatic CMs, observation of clinical silent spinal CMs is reasonable, because the annual bleeding risk is estimated between 1.4 and 4.5% per year [17, 24]. However, patients harbouring spinal CMs should be followed closely to detect neurological deterioration. Additional to periodical neurological examination, MRI should be performed annually to rule out growth. Furthermore, it should be considered to perform cranial MRI due to reported coexistence of intracranial CMs with a high incidence. Further prospective studies are necessary to answer the open questions in the future.
Conclusion
Spinal CMs are increasingly being diagnosed early in the patients presenting with varying spinal cord symptoms and pain syndromes. Microsurgical treatment under perioperative electrophysiological monitoring is justified to prevent severe neurofunctional deterioration. Although some of the patients deteriorate after surgery, the symptoms are rapidly declining with a favourable outcome in the majority of the cases.
Conflict of interest
We declare that we have no conflict of interest.
References
- 1.Ahlhelm F, Hagen T, Schulte-Altedorneburg G, Grunwald I, Reith W, Roth C. Cavernous malformations. Radiologe. 2007;47(10):863–867. doi: 10.1007/s00117-007-1546-0. [DOI] [PubMed] [Google Scholar]
- 2.Awad I, Barrow D (1993) Cavernous malformations. Am Assoc Neurol Surg
- 3.Bian LG, Bertalanffy H, Sun QF, Shen JK. Intramedullary cavernous malformations: clinical features and surgical technique via hemilaminectomy. Clin Neurol Neurosurg. 2009;111(6):511–517. doi: 10.1016/j.clineuro.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Canavero S, Pagni CA, Duca S, Bradac GB. Spinal intramedullary cavernous angiomas: a literature meta-analysis. Surg Neurol. 1994;41(5):381–388. doi: 10.1016/0090-3019(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 5.Craig HD, Gunel M, Cepeda O, Johnson EW, Ptacek L, Steinberg GK, Ogilvy CS, Berg MJ, Crawford SC, Scott RM, Steichen-Gersdorf E, Sabroe R, Kennedy CTC, Mettler G, Beis MJ, Fryer A, Awad IA, Lifton RP. Multilocus linkage identifies two new loci for a mendelian form of stroke, cerebral cavernous malformation, at 7p15–13 and 3q25.2–27. Hum Mol Genet. 1998;7:1851–1858. doi: 10.1093/hmg/7.12.1851. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch H. Pain outcomes after surgery in patients with intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010;29(3):E15. doi: 10.3171/2010.6.FOCUS10108. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch H, Jallo GI, Faktorovich A, Epstein F. Spinal intramedullary cavernoma: clinical presentation and surgical outcome. J Neurosurg. 2000;93(1 Suppl):65–70. doi: 10.3171/spi.2000.93.1.0065. [DOI] [PubMed] [Google Scholar]
- 8.Dörner L, Buhl R, Hugo HH, Jansen O, Barth H, Mehdorn HM. Unusual locations for cavernous hemangiomas: report of two cases and review of the literature. Acta Neurochir (Wien) 2005;147(10):1091–1096. doi: 10.1007/s00701-005-0567-6. [DOI] [PubMed] [Google Scholar]
- 9.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Part I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 10.Ghogawala Z, Ogilvy CS. Intramedullary cavernous malformations of the spinal cord. Neurosurg Clin N Am. 1999;10(1):101–111. [PubMed] [Google Scholar]
- 11.Gross BA, Du R, Popp AJ, Day AL. Intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010;29(3):E14. doi: 10.3171/2010.6.FOCUS10144. [DOI] [PubMed] [Google Scholar]
- 12.Guzeloglu-Kayisli O, Amankulor NM, Voorhees J, Luleci G, Lifton RP, Gunel M. KRIT1/cerebral cavernous malformation 1 protein localizes to vascular endothelium, astrocytes, and pyramidal cells of the adult human cerebral cortex. Neurosurgery. 2004;54(4):943–949. doi: 10.1227/01.NEU.0000114512.59624.A5. [DOI] [PubMed] [Google Scholar]
- 13.Jones SJ, Buonamassa S, Crockard HA. Two cases of quadriparesis following anterior cervical discectomy, with normal perioperative somatosensory evokedpotentials. J Neurol Neurosurg Psychiatry. 2003;74:273–276. doi: 10.1136/jnnp.74.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharkar S, Shuck J, Conway J, Rigamonti D. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007;60(5):865–872. doi: 10.1227/01.NEU.0000255437.36742.15. [DOI] [PubMed] [Google Scholar]
- 15.Kivelev J, Niemelä M, Hernesniemi J. Outcome after microsurgery in 14 patients with spinal cavernomas and review of the literature. J Neurosurg Spine. 2010;13(4):524–534. doi: 10.3171/2010.4.SPINE09986. [DOI] [PubMed] [Google Scholar]
- 16.Kim LJ, Klopfenstein JD, Zabramski JM, Sonntag VK, Sptezler RF. Analysis of pain resolution after surgical resection of intramedullary spinal cord cavernous malformations. Neurosurgery. 2006;1:106–111. doi: 10.1227/01.NEU.0000192161.95893.D7. [DOI] [PubMed] [Google Scholar]
- 17.Labauge P, Bouly S, Parker F, Gallas S, Emery E, Loiseau H, Lejeune JP, Lonjon M, Proust F, Boetto S, Coulbois S, Auque J, Boulliat J, French Study Group C of Spinal Cord avernomas Outcome in 53 patients with spinal cord cavernomas. Surg Neurol. 2008;70(2):176–181. doi: 10.1016/j.surneu.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Lu DC, Lawton MT. Clinical presentation and surgical management of intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010;29(3):E12. doi: 10.3171/2010.6.FOCUS10139. [DOI] [PubMed] [Google Scholar]
- 19.Li DY, Whitehead KJ. Evaluating strategies for the treatment of cerebral cavernous malformations. Stroke. 2010;41(10 Suppl):S92–S94. doi: 10.1161/STROKEAHA.110.594929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick PC, Michelsen WJ, Post KD. Cavernous malformations of the spinal cord. Neurosurgery. 1988;23:459–463. doi: 10.1227/00006123-198810000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Mehdorn HM, Stolke D. Cervical intramedullary cavernous angioma with MRI-proven haemorrhages. J Neurol. 1991;238(8):420–426. doi: 10.1007/BF00314647. [DOI] [PubMed] [Google Scholar]
- 22.Minh NH. Cervicothoracic spinal epidural cavernous hemangioma: case report and review of the literature. Surg Neurol. 2005;64(1):83–85. doi: 10.1016/j.surneu.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT, Spetzler RF. Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med. 1988;319(6):343–347. doi: 10.1056/NEJM198808113190605. [DOI] [PubMed] [Google Scholar]
- 24.Sandalcioglu IE, Wiedemayer H, Gasser T, Asgari S, Engelhorn T, Stolke D. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253–256. doi: 10.1007/s10143-003-0260-2. [DOI] [PubMed] [Google Scholar]
- 25.Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2 Suppl):145–156. doi: 10.3171/spi.2002.96.2.0145. [DOI] [PubMed] [Google Scholar]
- 26.Steiger HJ, Turowski B, Hänggi D. Prognostic factors for the outcome of surgical and conservative treatment of symptomatic spinal cord cavernous malformations: a review of a series of 20 patients. Neurosurg Focus. 2010;29(3):E13. doi: 10.3171/2010.6.FOCUS10123. [DOI] [PubMed] [Google Scholar]
- 27.Vishteh AG, Zabramski JM, Spetzler RF. Patients with spinal cord cavernous malformations are at an increased risk for multiple neuraxis cavernous malformations. Neurosurgery. 1999;45(1):30–32. doi: 10.1097/00006123-199907000-00008. [DOI] [PubMed] [Google Scholar]