Abstract
This study reviews the presentation, etiology, imaging characteristics and reasons for missed diagnosis of Andersson lesion (AL) and analyzes the surgical results of short segment fixation in the thoracolumbar region. This is a retrospective single center study. Fourteen patients (15 lesions) who were operated for AL were analyzed. The study was designed in two parts. The first part consisted of analysis of clinical and radiological features (MRI and radiographs) to highlight, whether definitive characteristics exist. The second part consisted of analysis of outcome of short segment fixation as measured by VAS, Frankel score, AsQoL index, and union, with assessment of complications. The follow-up was 42.33 ± 19.29 months (13 males and 1 female) with a mean age of 61.13 ± 19.74 years. There was predisposing trauma in five patients. There was a delay in presentation of the patients by 5.86 ± 2.50 months. There was misdiagnosis in all the cases, at primary orthopedic level (ten cases were put on anti-tuberculous treatment due to its MRI resemblance to infection) and all but one case at radiologist level. Radiographs and MRI had characteristic features in all cases, and MRI could detect posterior element affection in 14 lesions as against only 8 posterior lesions detected in radiographs. In all patients, there was a patient’s delay and/or physician’s delay to arrive at a diagnosis. Spinal fusion was seen in all the cases. Outcome measures of VAS, Frankel score, and AsQoL index showed significant improvement (P < 0.002). No major complications occurred. There is a lack of awareness of AL leading to misdiagnosis. Definite clinico-radiological features do exist in AL and short segment fixation is effective.
Keywords: Andersson lesion, Ankylosing spondylitis, Short segment, Posterior fixation
Introduction
Andersson lesion (AL) is a destructive vertebral or disco-vertebral lesion that occurs as a late non-inflammatory sequel in ankylosing spondylitis (AS). It is a state of chronic mobile non-union with an essential posterior element fracture or unfused facet joints associated with the anterior lesion [1–4]. With progression of AS, the inflammatory pain usually becomes quiescent [5]. A late onset mechanical pain is the first sign of AL but is usually neglected by physicians due to the chronicity of the AS [1, 2, 4]. Since the first description of AL by Andersson (1937), the natural history, pathophysiology, clinical features, laboratory investigations, image findings, and even the terminology of AL have been controversial. The fractures occurring in AS are acute injuries, whereas AL lesions are chronic [1].
Diagnosis of AL is difficult due to pre-existing spinal changes, osteoporosis, and radiographic resemblance to infective spondylodiscitis [1]. Surgery for unstable fractures in AS includes anterior/posterior decompression and fixation or both. Decompression, instrumentation, bone grafting, and various osteotomies for deformity correction have been reported and long segment fixation is usually preferred for fractures [1, 3, 6–8], but surgical recommendations for AL are scarce.
The aim of this study therefore is to highlight the difficulties that exist in clinic-radiological diagnosis and surgical management. We specifically set out to test two hypotheses: (1) pathophysiology, clinical and imaging features to diagnose AL, and (2) short segment fixation consistently achieves satisfactory result.
Materials and methods
Between May 2003 and May 2010, 29 patients (30 lesions) of AL underwent surgery at our institute. On excluding 8 patients with cervical and 7 patients with inadequate follow-up, 14 patients of thoracolumbar AL (15 lesions) were included in this study with a minimum follow-up of 18 months. The data was obtained from the hospital medical records and all patients were seen for final follow-up. Their age, gender, duration of spondyloarthropathy, mode of present onset and its duration, previous physician’s diagnosis, and treatment were noted. Preoperative radiographs and MRI characteristic analysis were done by (A.K.), (H.R.), and an independent radiologist.
Radiograph analysis
The radiographs were assessed for the presence of advanced features of ankylosis (syndesmophytes with bamboo spine). Anterior lesion defect, osteolysis, and widening with sclerosed irregular margins were noted along with the presence of posterior element defect.
MRI image analysis
We noted the signal intensity of AL on T1, T2-weighted and STIR images as low, iso-intense, high, or mixed, as compared with that of the nearest bone or posterior elements. Mixed signal intensity meant that the lesion was both hyperintense and hypointense (heterogeneous).
MRI image morphology
Characteristics of anterior and the posterior element affection were noted. When the signal intensity of the lesion (anterior or posterior) was as high as that of the cerebrospinal fluid, it was noted as pseudoarthrotic fluid. Loss of cortical definition of the anterior and posterior margins of the vertebral body was noted. This definition did not include endplate destruction of the vertebral body because the subchondral bone of the endplate/vertebral body is almost always involved in AL. Soft tissue mass and loss of fat planes and MR myelography block were also noted.
The senior author (B.R.D.) performed the surgeries on all the patients who were placed in the prone position under general anesthesia. The surgical indications were persistent pain, instability, and/or neurological deficit. The positioning was done with great care so as to accommodate the kyphotic deformity and the stiff neck. A midline posterior approach followed by instrumentation (monoaxial screws 6 mm with 5.5/6 mm connecting rods construct) of adjacent two vertebrae, one cephalad, and one caudal was done (four screws). In cases where it was not possible to negotiate a screw, the construct was extended to include the next segment (five screws). Laminectomy was performed in all cases with neurological deficit. Transpedicular intralesional curettage was done and material was sent for histopathological examination. Posterolateral and transpedicular bone grafting (n = 5) with locally harvested bone/tricalcium phosphate was done in all the cases. No attempt to correct the deformity was done. Physiotherapy in all cases along with ambulation (n = 6) was started in those who had adequate motor power. Patients were followed every 6 weeks until union was achieved and at final follow-up. Modified Taylor brace was given to all patients for 3 months. One patient without any evidence of posterior lesion underwent surgery with percutaneous vertebroplasty considering his age of 77 and low physical demands.
Results were analyzed at the time of presentation and at final follow-up for improvement in VAS score [9], neurological Frankel grade [10], ASQoL (Ankylosing Spondylitis Quality of Life Questionnaire scoring), [11] and radiological union. An independent radiologist read the radiographs taken at the final follow-up for determining the fusion status. The lesion was considered united if radiographs demonstrated a bilateral continuity in the fusion mass between the cephalic and caudal transverse processes with anterior defect bony bridging with no angular motion between the vertebrae noted on lateral flexion–extension radiographs. Complications or other sequelae were assessed.
Statistical analysis
Data were expressed as mean ± 2 standard deviations for demographic variables. Preoperative and postoperative differences in VAS score, Frankel neurological grade, and ASQoL score were calculated with Wilcoxon signed ranks test. All tests are two-sided and statistical significance was set at P < 0.05. All analyses were carried out using the SPSS (Statistical Package for the Social Sciences) version 17.
Results
The average follow-up was 42.33 ± 19.29 months (range 18–73 months). There was no loss of follow-up. Two patients died of other non-spinal cause. There were 13 males (14 lesions; one patient had double lesion) and 1 female with a mean age of 61.13 ± 19.74 years (range 47–83 years). The onset of spondyloarthropathy was since 30.67 ± 4.27 years (range 23–38 years). The average time from the onset of present new pain to presentation was 5.86 ± 2.50 months; five lesions had trivial traumatic history and ten had insidious onset. All the patients had consulted the general community physician following persistent mild pain for 4–6 weeks and were not diagnosed in spite of the imaging. They were diagnosed with pathology after a further delay of 5–7 weeks. The initial radiographs at onset were available with five patients but were reported to be negative, before they presented at our institute. Radiologists reported MRI as tuberculous infection (n = 12), malignancy (n = 1), pseudoarthrosis (n = 1), and AL (n = 1). Orthopedic surgeons diagnosed infection in all patients; anti-tuberculosis treatment was started in 10 and the rest were referred.
The radiological features as analyzed are tabulated (Tables 1, 2). Lesions were transdiscal (n = 9) and transvertebral (n = 6).
Table 1.
Radiographic characteristic of all the lesions
| n [present (P)/absent (A)] | ||
|---|---|---|
| Radiographs | ||
| Global kyphosis | 15(P) | |
| Local kyphosis | 9(P) | 6(A) |
| Bamboo spine and syndesmophytes | 15(P) | |
| Sclerosis with irregular margins | 14(P) | 1(A) |
| Widening/osteolysis/destruction | 14(P) | 1(A) |
| Posterior defect/lesion | 7(P) | 8(A) |
| MRI | ||
| MR myelography block | 11(P) | 4(A) |
| Loss of cortical definition | 15(A) | |
| Loss of fat planes | 15(A) | |
| Soft tissue mass | 15(A) | |
Table 2.
Signal Intensity changes in MRI Sequences of all the lesions

↓, Hypointense; ↑, hyperintense;→, iso-intense; ↔, mixed number of lesion (n)
*One patient who had no posterior lesion and was treated with vertebroplasty
**Patients showed anterior pseudoarthrotic fluid intensity (iso-intense to CSF)
***Patients showed posterior pseudoarthrotic fluid intensity (iso-intense to CSF)
Increased systemic inflammatory responses, including elevation of ESR and CRP, were found in five patients. Infection was excluded with negative culture and microbiologic studies. Histopathological examinations were reported non-specific inflammation with hypo vascular fibrotic tissue (with only a few lymphocytes) in all lesions.
Neurological improvement was noted in 12 patients [Frankel B–D (n = 2), C–D (n = 1), C–E (n = 2) and D–E (n = 6), A–D (n = 1)] and 2 remained status quo (one D and one E).
VAS score improved from 9.13 ± 1.82 (range 7–10) to 3.47 ± 1.26 (range 2–4) and was statistically significant (Z = −3.457, P = 0.001). Frankel neurological grade improved from 2.53 ± 2.24 (range 1–5) to 1.33 ± 0.96 (range 1–2) which was statistically significant (Z = −3.140, P = 0.002). ASQoL score improved from 13.93 ± 7.06 (range 5–18) to 5.33 ± 9.52 (0–16) was statistically significant (Z = −3.299, P = 0.001).
There were no major intraoperative complications. Two patients had dural tears repaired with dural path graft. Instead of the four screws as planned, four patients required five screws and a segment extension. One case of superficial skin necrosis responded well to regular dressings. There were no implant failures. Following trivial trauma, a new AL developed at a different location for which one patient underwent surgery. He then died of non-spinal cause at 18 months following the second surgery. One patient expired due to ischemic heart disease and chronic alcoholism.
Discussion
The reported prevalence of vertebral fractures varies widely (10–17%) and the incidence of neurologic complications is very high (29–91%) [7, 8]. The exact prevalence of AL complicating AS in literature is unknown, but reported prevalence ranges from 1.5% to over 28% [1–8]. This large variation is because of the lack of proper diagnostic criteria and the differences in the extent of spinal survey undertaken. The eponyms ‘Andersson lesion’, ‘disco-vertebral lesion’, ‘vertebral lesion’, ‘destructive vertebral lesion’, ‘spondylodiscitis’, ‘discitis’, ‘sterile discitis’, ‘pseudarthrosis’ or ‘stress fracture’ all reflect the discrepancy in the terminology used by the specialists, both rheumatologists and orthopedic surgeons [1]. Pseudoarthrosis most commonly involves the thoracolumbar region of T11–L1 [6, 8].
Multiple possibilities for etiology
Multiple possibilities for etiology of AL have been described. These include repetitive stress on skipped segments that have not completely ossified [2], a biomechanically weak hyperkyphotic spine [2, 6, 7, 12], trauma [3–6], and progressive inflammation too [1]. It is worthwhile noting that inflammatory lesions usually affect multiple levels during the early part of AS. Nevertheless, AL often involves only a single level, indicating that additional factors could be involved [14]. An infectious origin has also been suggested because of the radiographic resemblance to spondylodiscitis [15, 16], but this has never gained much popularity in literature. Regardless of the exact etiology, a final common pathway exists, in which mechanical stresses prevent the lesion from fusion and the development of pseudarthrosis ensues [1].
The presenting symptoms in cases of AL are mechanical pain, deformity, and occasionally neurological deficit [2–8]. In the context of patients with longstanding ankylosing spondylitis, the physicians tend to ignore the symptom of pain. Unfortunately, it has been shown that in a large percentage of cases, the correct diagnosis is not established until after the patient has already experienced a decline in neurologic function [17]. Unless there is a trauma with acute new back pain, spinal fracture in these patients may be easily overlooked. Because of the presence of a chronic problem, the patients and physicians might not be aware of a new pathology-pseudoarthrosis (AL) [4, 18, 19].
The extent of awareness is so low amongst the orthopedic surgeons that they even initiated anti-tuberculous treatment in ten patients, as tuberculosis is endemic in this part of the world. Thus, in all patients there was a patient’s delay (decision to seek medical attention) and physician’s delay (inability to timely recognize) to arrive at a diagnosis.
Thus, high clinical suspicion is the first diagnostic tool for the specialist surgeon in order to identify this lesion. The initial radiological study in acute trauma in AS patients may be negative (Fig. 1), necessitating the use of CT and/or MRI scanning to increase the sensitivity [1, 4, 6, 7, 19, 20]. The initial radiograph of five patients with traumatic history was reported as without any bony injury in our series. The recognition of fractures and AL in individuals with AS may be complicated by their distorted bony anatomy and progressive deformity, making it more challenging to interpret radiographs [17]. Dilhman et al. and Cawley et al. have attempted to classify inflammatory and non-inflammatory AL [14, 18]. The radiographs show destructive transdiscal/transvertebral lesion, irregular margins with sclerosis, reduction in space, local kyphosis, posterior element break and generalized changes of bamboo spine with syndesmophytes [1, 2, 19]. Most of our patients had all these features in radiographs (Table 1). Widening, osteolysis, destruction, and sclerosis were absent in only one patient who had developed a second AL after undergoing surgery for the first AL and was detected early on new MRI. Posterior lesions were detected in seven patients with radiographs.
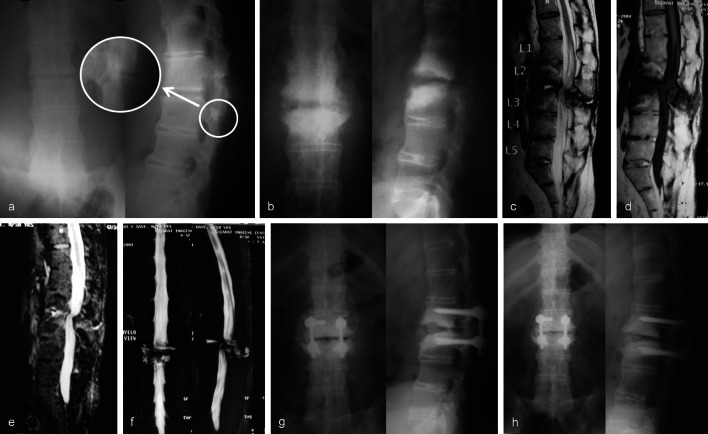
Fig. 1.
a Advanced ankylosed spine of 60 year/male with primary radiograph reported normal at first consultation. Retrospectively we noted a faint posterior fracture line (arrow-inset). b Established pseudoarthrosis at 6 months on presentation to us. Anterior lesion with marked bony destruction, irregular sclerosed margins with posterior element extension. c T2 weighted sequence: anterior and posterior lesion with pseudoarthrotic fluid (iso-intense to CSF), with vertebral margin hypointensity (suggesting sclerosis) with mixed intensity changes in adjacent vertebral bodies. d T-1 weighted sequence: Mixed intensity changes in vertebral body with hypointense lesion. e STIR image showing maintained cortical margins and no soft tissue extension. f MR myelograph showing the block with pseudoarthrotic fluid anteriorly and posteriorly. g Short segment fixation with interbody and posterolateral bone grafting with laminectomy decompression was done. h Final follow-up at 64 months with interbody/posterolateral fusion and good implant position
Flexion and extension radiographs are of value in demonstrating spinal instability [19]. CT scan has a complementary role in demonstrating bone erosion, irregularity, sclerosis and posterior element defect more accurately than conventional radiography [19].
MRI shows increased signal intensity in T2 in early active inflammatory AS. Vertebral lesion margins in AL show decreased intensity in T1 and T2 images as they are non-inflammatory lesions. T1 weighted contrast images shows increased uptake adjacent to the vertebral margins. Pseudo arthritic fluid if present is apparent as high signal at the center of the lesion [19, 20]. The bone and soft tissue near the fracture line could be edematous for some time after trauma and present as high signal intensity on the T2-weighted images in some cases [20]. MR imaging can additionally show occult fractures in the anterior column/spinous process/facet, cord changes, PLL/ALL tears, posterior ligament and soft tissue disruption. The imaging features characteristically correlated in all our patients (Table 2). Anterior and posterior lesions were evident in MRI of 14 lesions. Only one patient did not have the classic posterior element extension and was treated with vertebroplasty with good outcome.
The occurrence of AL is frequent enough to warrant differentiation from other infectious or tumorous conditions. The absence of soft tissue swelling, paravertebral mass or displacement and loss of fat planes are characteristic of AL which is against infection and tumor [21]. In AL the major part of the disc shows decreased signal intensity on T2 images, while generalized increased signal intensity due to inflammatory oedema and granulation tissue would be expected in established acute infective spondylitis [22]. Maintained integrity of anterior and posterior vertebral margin, absence of soft tissue mass and evident fat planes were consistently present in all our patients. This could differentiate it from infection. Contrast enhancement pattern can also help in differentiating AL from infection [20]. Both early and late bone scintigraphy can be used to identify AL complicating AS and to differentiate the lesion from infection but it lacks specificity [1, 23].
Conservative treatment with brace, rest and physiotherapy can be effective [24]. But, at the more mobile cervical and thoracolumbar junction conservative management is less efficient [1]. Surgical instrumentation and fusion is considered the principle management in symptomatic AL that fails to resolve from a conservative treatment [1, 2, 6].
Anterior decompression and bone grafting alone [2, 13, 18, 25] or with fixation [26] has been done in AL. Approaching a kyphosed spine anteriorly in presence of restrictive pulmonary function can be difficult and associated with more morbidity [7]. In everyday practice posterior stabilization is done, considering the cardiovascular and pulmonary disorders [4]. Long segmental posterior instrumentation with or without decompression followed by anterior fusion has been done for significantly displaced fractures or AL [4, 19, 27–30]. Solid fusion can be achieved in these patients, with [6] or without bone grafting [8], indicating intact bone reparative processes.
In all our cases single segment fixation and bone grafting achieved union (Figs. 1, 2). Though, single stage posterior osteotomy to correct the deformity along with the fusion for AL is done at some centers [8, 31], we only aimed to achieve fusion.
Fig. 2.
56 year old male at follow-up for operated D12–L1 Andersson lesion (arrow) presented with new lower lumbar pain following trivial trauma. Primary radiograph (a, b) were unremarkable c T2 weighted sequence was unremarkable d T1 weighted sequence shows anterior hypointense lesion through L5 body with faintly appreciated posterior extension e STIR sequence shows hyperintensity (anterior and posterior) suggesting oedema as it is fresh trauma f MR myelogram was normal g CT scan midsagittal cut showing fracture (black arrow) h Operated single segment fixation with intralesional/posterolateral bone graft and tricalcium phosphate with i fusion at follow-up (30 months first lesion and 18 months second lesion)
Surgery in elderly people is associated with higher rates of morbidity and mortality probably due to pre-existing medical conditions especially in AS [6, 7, 32–34]. Dural adhesions do occur in AL and can be recognized in MRI [19, 20]. This should be anticipated and dealt with accordingly. In our series, we had two dural tears. Five screws (instead of the planned four screws) were used in four cases due to the inability to position monoaxial screws into the slanting irregular lower vertebral body. This could have been avoided by using polyaxial screws with prior CT evaluation to anticipate the vertebral body structure and deficiency.
The potential for spinal instrumentation to subsequently act as a stress riser leading to fractures caudal or cephalad to the original level should also be kept in mind [35]. One of our cases had a second lesion caudal to the first, following trivial fall which was also managed operatively (Fig. 2).
Another important part of the management program is education of the patient and his or her caregivers about the risks of injury and its primary prevention which principally involves alerting patients to the fragility of their spine and the importance of avoiding spinal trauma. All efforts should be made to prevent patient’s delay in presentation and physician’s delay in the diagnosis [7, 36, 37].
Although, result bias has been avoided by complete assessment and analysis by the non operating authors (AK), (HR) and independent radiologist, there are limitations to the current study. This study was not a prospective study and we cannot conclude that pseudarthroses developed due to trauma in previously solid ankylosed segments or as a succession of an inflammatory lesion. Contrast MRI and CT would have given more detailed information that was not done in the current study. Union was assessed by subjective diagnostic confidence on conventional radiographs which can be unreliable. But, absence of implant failure with significant pain when taken into account is considered satisfactory. CT scan would have been ideal to assess union. Another limitation of the present study is that the long-term results are not available yet. The relatively limited number of subjects evaluated in this retrospective review does not allow us to make any definite conclusions regarding the optimal strategies for managing AL. But, there are a number of findings that are clearly of great interest to practitioners who regularly treat these patients. Although a number of case reports and small case series have been previously discussed, no studies of short segment posterior fixation are currently available to the best of our knowledge.
Conclusion
High index of suspicion is required in patients of late AS presenting with recent onset new pain. Thorough clinical and radiological assessment should be performed with MRI being a valuable tool to characterize these lesions. It is the unawareness in the orthopedic and radiology society which is actually responsible for the initial missed or wrong diagnosis. The clinical outcome of short segment fixation is favorable in AL. Registries for fractures and AL in ankylotic conditions of the spine should be maintained in order to acquire more knowledge on the natural history and prognosis of these injuries.
Conflict of interest
None.
References
- 1.Johannes LB, Mirjam KV, Marieke NS, Irene EVHB, Barend JVR. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol. 2009;28:883–892. doi: 10.1007/s10067-009-1151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang D, Leong J, Ho EKE, et al. Spinal pseudoarthrosis in ankylosing spondylitis, clinicopathological correlation and the results of anterior spinal fusion. J Bone Jt Surg Br. 1988;70:443–447. doi: 10.1302/0301-620X.70B3.3372569. [DOI] [PubMed] [Google Scholar]
- 3.Bot SD, Caspers M, Van Royen BJ, et al. Biomechanical analysis of posture in patients with spinal kyphosis due to ankylosing spondylitis: a pilot study. Rheumatology (Oxf) 1999;38:441–443. doi: 10.1093/rheumatology/38.5.441. [DOI] [PubMed] [Google Scholar]
- 4.Olerud C, Frost A, Bring J. Spinal fractures with ankylosing spondylitis. Eur Spine J. 1996;5:51–55. doi: 10.1007/BF00307827. [DOI] [PubMed] [Google Scholar]
- 5.van der Horst-Bruinsma IE. Clinical aspects of ankylosing spondylitis. In: van Royen BJ, Dijkmans BAC, editors. Ankylosing spondylitis. Diagnosis and management. New York: Taylor and Francis; 2006. pp. 45–70. [Google Scholar]
- 6.Fox MW, Onofrio BM, Kilgore JE. Neurological complications of ankylosing spondylitis. J Neurosurg. 1993;78:871–878. doi: 10.3171/jns.1993.78.6.0871. [DOI] [PubMed] [Google Scholar]
- 7.Mundwiler LM, Siddique K, Dym JM, Perri B, Johnson JP, Weisman MH. Complications of the spine in ankylosing spondylitis with a focus on deformity correction. Neurosurg Focus. 2008;24(1):1–9. doi: 10.3171/FOC/2008/24/1/E6. [DOI] [PubMed] [Google Scholar]
- 8.Chang K-W, Tu M-Y, Huang H-H, Chen H-C, Chen Y-Y, Lin C-C. Posterior correction and fixation without anterior fusion for pseudoarthrosis with kyphotic deformity in ankylosing spondylitis. Spine. 2006;31(13):E408–E413. doi: 10.1097/01.brs.0000219870.31561.c2. [DOI] [PubMed] [Google Scholar]
- 9.Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15:S17–S24. doi: 10.1007/s00586-005-1044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia I. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 11.Zhao LK, Liao ZT, Li CH, Li TW, et al. Evaluation of quality of life using ASQoL questionnaire in patients with ankylosing spondylitis in a Chinese population. Rheumatol Int. 2007;27:605–611. doi: 10.1007/s00296-006-0267-4. [DOI] [PubMed] [Google Scholar]
- 12.Simmons E, Goodwin CB. Spondylodiscitis, manifestation of in ankylosing spondylitis. Orthop Trans. 1984;8:165–171. [Google Scholar]
- 13.Peh WC, Luk KD. Pseudoarthrosis in ankylosing spondylitis. Ann Rheum Dis. 1994;53:206–210. doi: 10.1136/ard.53.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dihlmann W, Delling G. Disco-vertebral destructive lesions (socalled Andersson lesions) associated with ankylosing spondylitis. Skeletal Radiol. 1978;3:10–16. doi: 10.1007/BF00365106. [DOI] [Google Scholar]
- 15.Eschelman DJ, Beers GJ, Naimark A, Yablon I. Pseudoarthrosis in ankylosing spondylitis mimicking infectious diskitis: MR appearance. Am J Neuroradiol. 1991;12(6):1113–1114. [PMC free article] [PubMed] [Google Scholar]
- 16.Kabasakal Y, Garret SL, Calin A. The epidemiology of spondylodiscitis in ankylosing spondylitis-a controlled study. Br J Rheumatol. 1996;35(7):660–663. doi: 10.1093/rheumatology/35.7.660. [DOI] [PubMed] [Google Scholar]
- 17.Whang PG, Goldberg G, Lawrence JP, et al. The management of spinal injuries in patients with ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis: a comparison of treatment methods and clinical outcomes. J Spinal Disord Tech. 2009;22:77–85. doi: 10.1097/BSD.0b013e3181679bcb. [DOI] [PubMed] [Google Scholar]
- 18.Cawley MI, Chalmers TM, Kellgren JH, et al. Destructive lesions of vertebral bodies in ankylosing spondylitis. Ann Rheum Dis. 1972;31:345. doi: 10.1136/ard.31.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y-F, Teng MM-H, Chang C-Y, Wu H-T, Wang S-T. Imaging manifestations of spinal fractures in ankylosing spondylitis. AJNR Am J Neuroradiol. 2005;26:2067–2076. [PMC free article] [PubMed] [Google Scholar]
- 20.Shih TF, Chen PQ, Li YW, Hsu CY. Spinal fractures and pseudoarthrosis complicating ankylosing spondylitis: MRI manifestation and clinical significance. J Comput Assist Tomogr. 2001;25:164–170. doi: 10.1097/00004728-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 21.De Ross A, Van Meerten EL, Bloem JL, Bluemm RG. MRI of tuberculosis spondylitis. Am J Roentgenol. 1986;146:79–82. doi: 10.2214/ajr.147.1.79. [DOI] [PubMed] [Google Scholar]
- 22.Modic MT, Feiglin DH, Piraindo DW, et al. Vertebral osteomyelitis: assessment by MR. Radiology. 1985;157:157–166. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 23.Park WM, Spencer DG, McCall IW, Ward J, Buchanan WW, Stephens WH. The detection of spinal pseudarthrosis in ankylosing spondylitis. Br J Radiol. 1981;54(642):467–472. doi: 10.1259/0007-1285-54-642-467. [DOI] [PubMed] [Google Scholar]
- 24.Rasker JJ, Prevo RL, Lanting PJ. Spondylodiscitis in ankylosing spondylitis, inflammation or trauma? A description of six cases. Scand J Rheumatol. 1996;25(1):52–57. doi: 10.3109/03009749609082669. [DOI] [PubMed] [Google Scholar]
- 25.Ha KY, Cho HL. Andersson lesion in ankylosing spondylitis: a case report. Korean Soc Spine Surg. 1998;5(1):148–153. [Google Scholar]
- 26.Escosa-Bage M, Garcia-Navarrete E, Pascual-Garvi meetings JM, Sola RG. Surgical treatment of spondylodiscitis in ankylosing spondylitis Report of two cases. Rev neurol. 2001;33:964–966. [PubMed] [Google Scholar]
- 27.Jayaswal A (2003) Pseudarthrosis in ankylosing spondylitis. Abstract from the SRS Annual Meeting
- 28.Krishnan A, Karunagaran, Hegde S (2009) Surgical outcome in ankylosing spondylitis patients with Andersson’s lesion and neurological deficit. J Bone Jt Surg(Br) 91-B(Supp_III):483
- 29.Serin E, Karakurt L, Yilmaz E, Belhan O, Varol T. Effects of two-levels, four-levels, and four-levels plus offset-hook posterior fixation techniques on protecting the surgical correction of unstable thoracolumbar vertebral fractures: a clinical study. Eur J Orthop Surg Traumatol. 2004;14:1–6. doi: 10.1007/s00590-003-0110-5. [DOI] [Google Scholar]
- 30.Tezeren G, Kuru I. Posterior fixation of thoracolumbar burst fracture: short-segment pedicle fixation versus long-segment instrumentation. J Spinal Disord Tech. 2005;18:485–488. doi: 10.1097/01.bsd.0000149874.61397.38. [DOI] [PubMed] [Google Scholar]
- 31.Kim KT, Lee SH, Suk KS, Lee JH, Im YJ (2007) Spinal pseudarthrosis in advanced ankylosing spondylitis with sagittal plane deformity. Clinical characteristics and outcome analysis. Spine 32 15(1):641–1647 [DOI] [PubMed]
- 32.Hornick TR. Surgical innovations: impact on the quality of life of the older patient. Clin Geriatr Med. 2006;22:499–513. doi: 10.1016/j.cger.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Murray A, Persellin RH. Cervical fracture complicating ankylosing spondylitis: a report of eight cases and review of the literature. Am J Med. 1981;70:1033–1041. doi: 10.1016/0002-9343(81)90860-3. [DOI] [PubMed] [Google Scholar]
- 34.Gold RH, Bassett LW, Seeger LL. The other arthritides. Roentgenologic fractures osteoarthritis, erosive osteoarthritis, ankylosing spondylitis, psoriatic arthritis, Reiter’s disease, multi-centric reticulohistiocytosis, and progressive systemic sclerosis. Radiol Clin North Am. 1988;26:1195–1212. [PubMed] [Google Scholar]
- 35.Samartzis D, Anderson DG, Shen FH. Multiple and simultaneous spine fractures in Ankylosing spondylitis: case report. Spine. 2005;30:E711–E715. doi: 10.1097/01.brs.0000188272.19229.74. [DOI] [PubMed] [Google Scholar]
- 36.Wade W, Saltzstein R, Maiman D. Spinal fractures complicating ankylosing spondylitis. Arch Phys Med Rehabil. 1989;70:398–401. [PubMed] [Google Scholar]
- 37.Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment neurological status and complications. Eur Spine J. 2009;18:145–156. doi: 10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]