Abstract
Posterior lumbar interbody fusion (PLIF) is a popular procedure for treating lumbar canal stenosis with spinal instability, and several reports concerning fusion assessment methods exist. However, there are currently no definitive criteria for diagnosing a successful interbody fusion in the lumbar spine. We suggested evaluating fusion status using computed tomography (CT) in extension position to detect pseudoarthrosis more precisely. The purpose of this study was to evaluate its usefulness for determining bone union quality after PLIF. Eighty-one patients who underwent PLIF at 97 levels were retrospectively enrolled. The study population included 48 men and 33 women (mean age 58.9 years, range 21–85 years). Patients were followed up for more than 12 months after surgery. The mean follow-up period was 27.6 months (range 14–49 months). Fusion status was evaluated using three ways: flexion–extension radiographs, CT images in flexion and extension position. In the flexion–extension radiographs, mobility of more than 3°, a remaining clear zone, or an uncertain bone connection constituted an incomplete union. For CT images, a remaining clear zone, a gas pattern, or an uncertain bone connection constituted an incomplete union. Flexion–extension radiographs demonstrated a solid fusion in 90.7% of the 97 levels at 10.7 months postoperatively. When fusion was demonstrated on flexion–extension radiographs, the rate of fusion affirmed by flexion CT and extension CT was 87.6 and 69.1% of the levels assessed, respectively. The rate of pseudoarthrosis detected on extension CT images was significantly higher than that on flexion–extension radiographs (P < 0.001) and flexion CT (P < 0.01). The rate of fusion achieved on extension CT was 85.6% at 15.1 months postoperatively. Extension CT could detect pseudoarthrosis more clearly than flexion–extension radiography and flexion CT. The CT images are influenced by body position and dilating anterior disc space in extension CT contributes to detect pseudoarthrodesis. Thus, extension CT was a useful method for assessing fusion status after PLIF.
Keywords: Extension CT, Flexion–extension radiograph, Interbody fusion, Fusion rate, Radiographic assessment
Introduction
In recent years, lumbar interbody fusion has become a reliable and frequent procedure and is the treatment of choice for a number of lumbar spinal disorders. The goal of any spinal fusion is to obtain a solid arthrodesis. Determining fusion status is crucial because persistent or recurrent symptoms after surgery often correlate with pseudoarthrodesis [9, 13]. The imaging techniques currently employed to assess the status of a spinal fusion include plain radiographs, flexion–extension radiographs, and computed tomography (CT) scans, including two-dimensional or three-dimensional reconstructions. However, currently there is no universally accepted radiologic assessment method for determining fusion, and the definitive criteria for diagnosing a successful interbody fusion in the lumbar spine remain controversial [12].
Dynamic lateral flexion and extension radiographs have been most widely used for evaluating fusion status. However, there is a lack of consensus concerning the degree of detected movement that demonstrates nonunion [12]. Technical measurement errors are inevitable when assessing dynamic radiographs [6], and metal artifacts obscure the image. As a result, the concordance rate between histological evidence from bone fusion evaluations and evidence from fusion images is insufficient [6].
CT has developed into the preferred method for assessing interbody fusion [4, 13]. It is rapid and provides reformatted images in the coronal and sagittal planes. Currently, with advances in thin-section helical CT scanning it has become possible to provide high-quality osseous images. CT has become the superior diagnostic imaging method of choice to evaluate spinal fusions [5, 11]. However, CT images do not determine the extent of bone fusion present as accurately as a histological evaluation [7, 10]. One reason for this problem is that plain CT scans are subject to artifact interference through and adjacent to the fusion device [12]. Another is that CT images may change depending on the positioning of the subject.
There has been little discussion in the literature regarding the positioning of a subject during CT, but we suggested evaluating fusion status using CT in the extension position: even in cases where fusion was found to have been achieved in CT scans with the subjects in flexion or neutral positions, dilating anterior disc space in extension CT might contribute to detecting clear zones and pseudoarthrodesis. The purpose of this study was to verify the effectiveness of this method for assessing the status of lumbar interbody fusion.
Materials and methods
Study population
Eighty-one patients who underwent PLIF without posterior fusion were studied retrospectively from January 2005 to December 2007. Operations were performed by one attending spine surgeon (Y.Y.). The number of fusion levels was 97: 68 single-level fusions, 10 two-level fusions, and 3 three-level fusions. There were 48 men and 33 women and their mean age was 58.9 years (range 21–85 years). The mean follow-up period was 27.6 months (range 14–49 months).
The criterion for performing the procedure was severe cauda equina and/or a radicular lesion with spinal instability, which had been unresponsive to conservative treatment. The concomitant diagnoses were degenerative spondylolisthesis in 24 patients, lumbar disc herniation in 20, lumbar spinal canal stenosis with spinal instability in 15, spondylolytic spondylolisthesis in 10, far-out syndrome in 5, degenerative scoliosis in 4, and other conditions in 3 patients. The distribution of fusion levels in this patient population group was as follows: L1/2, 5 (5.2%); L2/3, 7 (7.2%); L3/4, 22 (22.7%), L4/5, 46 (47.4%), and L5/S, 17 (17.5%).
For posterior internal fixation systems, the Texas Scottish Rite Hospital (TSRH) spinal system (Medtronic Sofamor Danek, Memphis, TN, USA) was used in 64 cases, and the Xia spinal system (Stryker, Kalamazoo, MI, USA) was used in 17 cases. The following were used for interbody reconstruction: Ogival Interbody Cage (Stryker) in 31 cases, an iliac crest graft alone in 30, a Boomerang II cage (Medtronic Sofamor Danek) in 12, and a Telamon cage (Medtronic Sofamor Danek) in eight. An autologous iliac crest bone graft was used in 69 cases (85%) and a local bone graft was used in 12 cases (15%).
All patients underwent a spinal fusion radiologic assessment using flexion–extension radiographs every 3 months postoperatively. CT was performed at 6, 12, and 24 months postoperatively, over the whole area of arthrodesis. The radiographs were assessed by two independent spine surgeons. When their opinions differed, the definition of “fusion” was determined by discussion.
The Japanese Orthopaedic Association (JOA) Score for lumbar spinal stenosis was used as measurement tool for clinical symptoms (Table 1).
Table 1.
The Japanese Orthopaedic Association (JOA) score for lumbar spinal stenosis
| Parameter | Finding | Points |
|---|---|---|
| Low-back pain | None | 3 |
| Occasional mild pain | 2 | |
| Frequent mild | 1 | |
| Occasional severe pain | 1 | |
| Frequent pain | 0 | |
| Continuous pain | 0 | |
| Leg pain and/or tingling | None | 3 |
| Occasional slight symptoms | 2 | |
| Frequent slight symptoms | 1 | |
| Occasional severe symptoms | 1 | |
| Frequent severe symptoms | 0 | |
| Continuous severe symptoms | 0 | |
| Gait | Normal | 3 |
| Able to walk >500 m although it results in pain tingling and/or muscle weakness | 2 | |
| Unable to walk >500 m owing to leg pain tingling and/or muscle weakness | 1 | |
| Unable to walk >100 m owing to leg pain tingling and/or muscle weakness | 0 | |
| Sensory disturbance | None | 2 |
| Slight disturbance (not subjective) | 1 | |
| Marked disturbance | 0 | |
| Motor disturbance | Normal (Grade 5) | 2 |
| Slight weakness (Grade 4) | 1 | |
| Marked weakness (Grades 0–3) | 0 | |
| Turn over while lying | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Standing | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Washing | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Leaning forward | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Sitting about 1 h | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Lifting or holding a heavy object | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Walking | No restriction | 2 |
| Moderate restriction | 1 | |
| Severe restriction | 0 | |
| Urinary bladder function | Normal | 0 |
| Mild dysuria | −3 | |
| Severe dysuria | −6 |
Total score = SUM (points for all parameters). Interpretation: minimum score, −6; maximum score, 29
Flexion–extension radiographs
The flexion–extension radiographs were obtained with the patient in the lateral position trying to bend or arch his or her back as far as possible. The radiographs were interpreted as showing incomplete union if there was mobility of more than 3°, a remaining clear zone, or no definite bone connection [6].
CT in flexion and extension position
To more clearly detect the changes of images influenced by body posture, images in the extension position was compared with the one in the flexion position. In the flexion position, a triangular pillow supported the upper back and shoulders and a folded towel was placed under the buttocks. In the extension position, a folded towel was placed under the lumbar region (Fig. 1a, b).
Fig. 1.
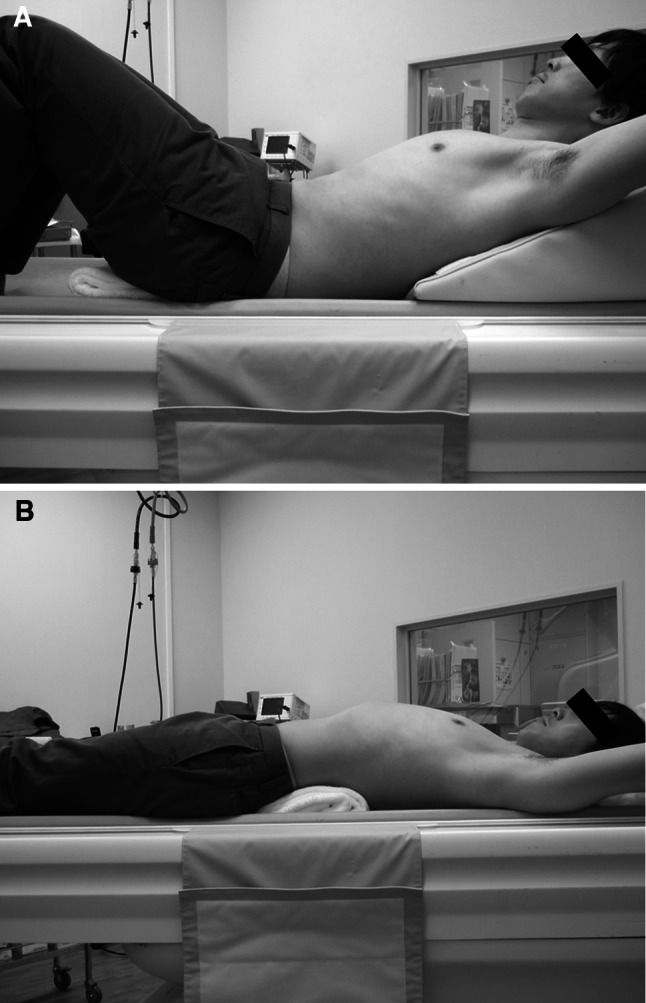
Body posture during flexion or extension computed tomography. a Flexion position: a triangular pillow supported the upper back and shoulders and a folded towel was placed under the buttocks. b Extension position: a folded towel was placed under the lumbar region
CT scans were 1-mm-thick axial helical with sagittal and coronal reconstructions. A 64-line multi-slice CT (Light Speed VCT; GE Healthcare Bio-Sciences, Piscataway, NJ, USA) was used. For dynamic CTs, a remaining clear zone, a gas pattern, or no definite bone connection was defined as incomplete union.
Statistical analysis
The StatMate (ATMS Co., Ltd., Tokyo, Japan) software package was used for data analysis. The Chi-square test was used to determine any significant differences in the apparent fusion rates between the groups. The correlation between a patient’s symptoms and the results of fusion with each evaluation method was assessed using the Student’s t test. Statistical significance was set at P < 0.05.
Results
There were no intraoperative or immediate postoperative complications related to the surgical procedures, and there were no cases of infection. The mean JOA score was 11.2 before surgery and 25.3 at the final follow-up.
Fusion assessment
The flexion–extension radiographs demonstrated a solid fusion in 90.7% (88/97 levels) of the levels assessed. The mean time by which an interbody fusion was achieved based on flexion–extension radiographs was 10.7 months postoperatively (range 6–24 months). When fusion was accomplished based on the flexion–extension radiographs, the rate of fusion affirmed by flexion CT and extension CT was 87.6% (85/97 levels) and 69.1% (67/97 levels) of the levels assessed, respectively. Statistical analysis showed that the rate of pseudoarthrosis detected on extension CT images was significantly higher than that on flexion–extension radiographs (P < 0.001) and flexion CT (P < 0.01). There were 21 levels with fusion discrepancies between the flexion–extension radiographs and extension CT scans. The characteristics of these 21 levels at which there were discrepancies regarding fusion are shown in Table 2. As in Table 2, discrepancies were detected more in cases with multi-segmental fusion and in those in which metal cages were used.
Table 2.
The characteristics of the 21 levels fused when there were discrepancies about assessing fusion between the flexion–extension radiographs and extension CT images when fusion was affirmed on flexion–extension radiographs
| N | % | |
|---|---|---|
| The number of fusion levels | ||
| 1-level fusion | 15 | 22.1% of single-level fusion |
| 2-level fusion | 4 | 40.0% of two-level fusion |
| 3-level fusion | 2 | 66.7% of three-level fusion |
| The type of interbody reconstruction | ||
| Metal cage | 15 | 29.4% of metal cage used cases |
| Autologous iliac crest bone graft | 6 | 20% of iliac crest bone used cases |
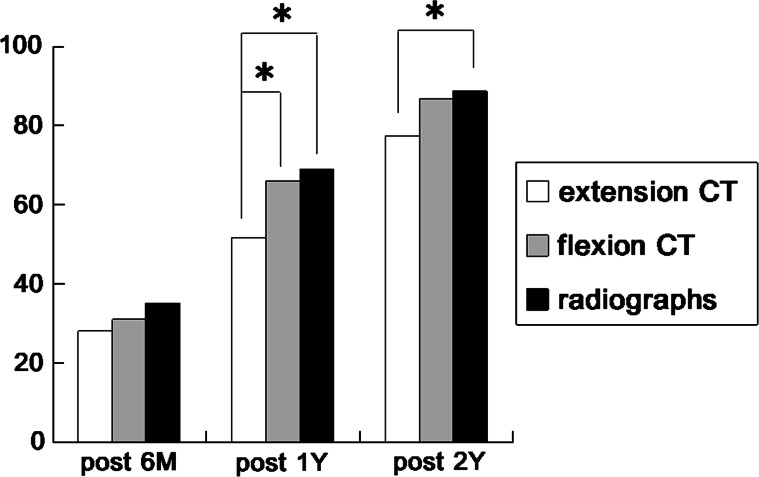
The fusion rates were confirmed on flexion–extension radiographs and CT images in flexion and extension position at 6 months, 1 year, and 2 years postoperatively (Fig. 2). The number of fusions confirmed on flexion–extension CT and flexion CT was higher than that for extension CT with statistical significance at 1 year post-surgery. The number of fusions confirmed on flexion–extension radiographs was also higher than that for extension CT with statistical significance at 2 years post-surgery. Finally, the fusion rate achieved based on extension CT was 85.6% (83/97 levels) at 15.1 months postoperatively.
Fig. 2.
The time-course changes in the rate of fusion assessment using flexion–extension radiographs (Xp), flexion and extension CT images. Asterisk indicates that the rate of fusions confirmed is different between the two groups with statistical significance (P < 0.05)
As for intraobserver variability, the degree of agreement regarding the fusion status on flexion–extension radiographs between the two independent surgeons was 84.5% (82/97 levels). On the other hand, the degree of agreement regarding the fusion status on extension CT images between the two independent surgeons was 97.9% (95/97 levels). The two levels at which there were disagreement regarding fusion had cases with two-level fusions and the use of metal cages.
The correlation between a patient’s symptoms and the results of fusion
The correlation between each patient’s symptoms at the final follow-up and the results of fusion with each evaluation method are shown in Tables 3 and 4. There was no statistically significant correlation between the symptoms and the imaging results with any of the methods. A trend toward potential correlation between the low-back pain score section of the JOA score and the results of extension CT was observed, although it was not statistically significant (Table 4).
Table 3.
The correlation between total score of the JOA score at the final follow-up and the results of the 3 evaluation methods: flexion–extension radiographs, flexion CT and extension CT
| Fusion | Pseudoarthrodesis | P | |
|---|---|---|---|
| Flexion–extension radiographs | 25.45 ± 3.12 | 24.64 ± 3.56 | 0.43 |
| Flexion CT | 25.51 ± 3.14 | 24.57 ± 3.35 | 0.32 |
| Extension CT | 25.44 ± 3.19 | 25.05 ± 3.19 | 0.64 |
Table 4.
The correlation between the low-back pain score section of the JOA score at the final follow-up and the results of the 3 evaluation methods: flexion–extension radiographs, flexion CT and extension CT
| Fusion | Pseudoarthrodesis | P | |
|---|---|---|---|
| Flexion–extension radiographs | 2.56 ± 0.73 | 2.45 ± 0.82 | 0.66 |
| Flexion CT | 2.57 ± 0.73 | 2.43 ± 0.76 | 0.51 |
| Extension CT | 2.63 ± 0.70 | 2.30 ± 0.80 | 0.08 |
Illustrative case
Preoperative diagnosis was L4 spondylolytic spondylolisthesis and spinal canal stenosis at the L4/5 level. PLIF at L4/5 was performed. In the flexion–extension radiographs taken 1 year post-surgery, there was mobility of less than 3° (L4/5; 1°), no apparent clear zone but definitive bone connection in the interbody space. In flexion CT, bone connection was confirmed. However, an extension CT performed at the same time revealed a clear zone. We judged that successful arthrodesis had not been achieved. (Fig. 3a–d).
Fig. 3.
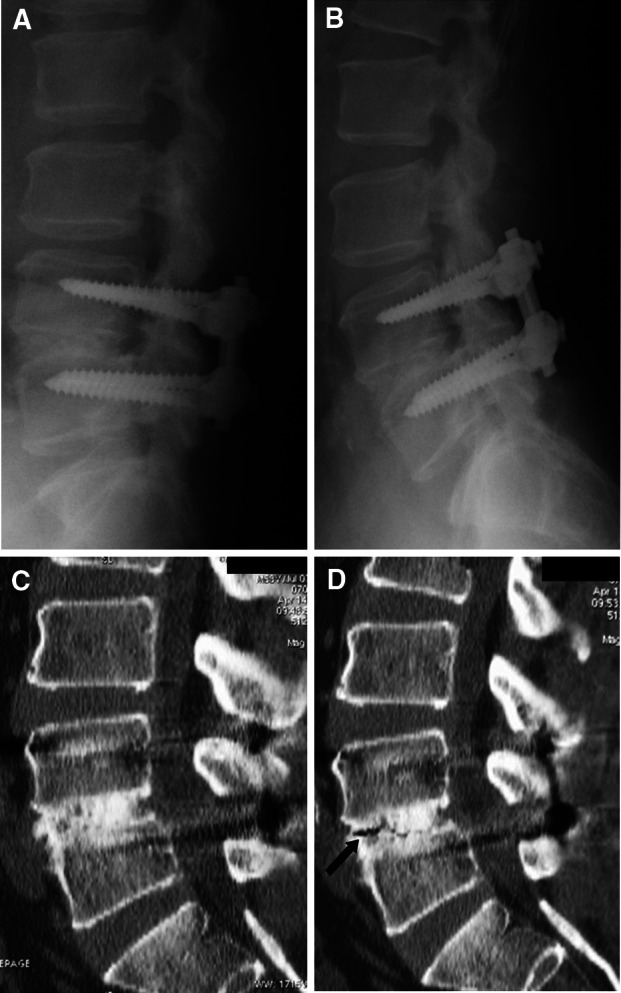
Imaging studies obtained in the illustrative case. Postoperative flexion (a) extension (b) lateral radiograph obtained 1 year after surgery. These radiographs demonstrating fusion were achieved at the L4/5 level. c and d Postoperative flexion and extension CT scan taken 1 year after surgery. Flexion CT scan (c) showing that fusion was complete at the L4/5 level. However, extension CT (d) showed a gas pattern in the interbody space. We then judged that successful arthrodesis had not been achieved
Discussion
Extension CT scan was investigated to determine whether it was a suitable method for judging fusion status after PLIF. The present study demonstrated that extension CT scan detected pseudoarthrosis more clearly than flexion–extension radiographs and flexion CT scan. When the CT scan was performed with the patient in extension position, inter-vertebral space was more likely to open up, and any clear zone or bony discontinuity was more obviously exposed. Thus, extension CT scan analysis can provide a more detailed picture of fusion status.
Accurately detecting interbody arthrodesis is crucial, especially in patients who have persistent or recurrent symptoms after surgery and thus may need further intervention [9, 13]. Several radiographic approaches have been attempted to detect pseudoarthrosis, including plain radiographs, flexion–extension radiographs, and CT scans with reconstructions. However, the definition of successful arthrodesis following lumbar fusion is controversial [12].
Flexion–extension radiographs are widely used to assess fusion status mainly because of their low cost and easy availability. However, there are major concerns regarding the utility of flexion–extension radiographs and the reliability of the reported fusion rates. It has been reported that a much higher fusion rate is determined by assessment using flexion–extension radiographs than histological evaluations [1, 5, 8]. The likely reasons for this are numerous. Metal implants often obscure the trabecular remodeling and fusion image: due to the inherently strong stability provided by most posterior internal fixation systems and interbody cages, flexion–extension radiographs cannot detect the subtle motion often seen in nonunions; movement can also be masked by muscle guarding and compensation with hip flexion [12]. Moreover, measurement accuracy is largely dependent on obtaining true lateral views; hence, suboptimal radiographs are often obtained [6]. In addition, it is impossible to totally eliminate rotational movement when radiographs are obtained. Consequently, there are no definite criteria for assessing fusion after PLIF [12]. Further complicating matters is the fact that interpretation of these films has significant intra and interobserver variations because interpretation of dynamic radiographs can be confusing for spine surgeons due to the difficulty of judging fusion progression [5, 12]. Consequently, definitive objective assessment of fusion after PLIF is extremely difficult using flexion–extension radiographs.
More recently, thin-section helical CT scanning has become the most reliable method for assessing fusion [4, 13]. Reformatted coronal and sagittal CT images make it possible to more clearly evaluate osseous continuity within the graft segment. Several CT studies have shown a higher degree of specificity for detecting pseudoarthrosis than plain radiography, particularly when evaluating interbody fusion [3, 14, 15]. The CT scan was assumed to be the ideal assessment method for suspected pseudoarthrosis. However, a helical CT scan is more likely to overestimate the extent of bone fusion than histological studies. Previous studies on the accuracy of CT sagittal reconstructions reported only a 60–80% correlation with surgical exploration [2, 5, 10]. This sizeable discrepancy (20–40%) is no doubt due to some factors that adversely affect the images obtained via CT. These CT scans are subject to artifacts through and adjacent to the fusion device, a situation that can become quite serious depending on the cage material and design [12]. There is also imaging interference wherein posterior instrumentation obscures the image [14]. In addition, CT images can be affected by a patient’s body posture. Clearly, there has been a need for a more reliable method for assessing fusion.
The current study showed that extension CT has some advantages over flexion–extension radiographs and flexion CT images: extension CT is a simple evaluation method, without the cumbersome measurement of morbidity angle required with flexion–extension radiographs, and shows a higher degree of intraobserver reliability than flexion–extension radiographs. Moreover, extension CT is able to more frequently detect minor clear zones within the graft segments than flexion CT. The results of the current study showed that extension CT could detect pseudoarthrosis more clearly in cases where flexion–extension radiographs and flexion CT images seemed to indicate that fusion had been accomplished. The radiation exposure with extension CT was almost the same as with neutral CT, 10–20 vol(mGy)/1 fusion level, and is considered to be an acceptable dose, the same as that with the standard CT method.
Admittedly, it should be noted that the present study had certain potential limitations. One potential limitation was the fact that the accuracy of functional CT was not determined by comparison to a histological analysis, because of etiological problems. Another potential limitation was that a test comparing CT reconstructions with a neutral position and extension CT was not performed in this study, because of concerns about the amount of radiation exposure. However, the trend observed in this study was clear enough to show that CT in the extension position had the potential to detect pseudoarthrosis more often than in a neutral position.
In this study, there were discrepancies in the judgments regarding fusion between flexion and extension CT only after 1 year post-surgery. Because of the small number of cases with pseudoarthrodesis, many subjects will have to be examined to establish statistically significant differences in the judgments regarding fusion with various body postures. However, there is no doubt that the results of fusion assessment would change based on the body posture.
There are several reports that some patients with pseudoarthrodesis have persistent or recurrent symptoms after surgery [9, 13]. There was a trend toward potential correlation between the low-back pain score section of the JOA score and the results of extension CT in this study, although it was not statistically significant. If the study populations were increased and the number of patients with pseudoarthrosis also increased, there could be the possibility of finding some statistically significant correlation between the low-back pain and the results of pseudoarthrodesis.
With the results of extension CTs in mind, we can carefully decide the duration of lumbar corset use and the timing of resuming sports. In addition, in cases with multi-segmental fusion and in those in which metal cages were used, i.e., where the fusion assessment was difficult in this study, extension CT can be a measure of fusion. And, above all, extension CT can be effectively used to investigate the cause of postoperative recurrent or persistent symptoms after PLIF in cases where fusion was indicated both in flexion–extension X-rays and standard neutral CTs. Overall, extension CT is a simple and easy assessment method, and can be a strong support tool for assessing fusion after PLIF.
Conclusions
Extension CT could detect pseudoarthrosis after PLIF more clearly than flexion–extension radiography and flexion CT. CT images are influenced by body position, and dilating anterior disc space in extension CT contributes to detecting pseudoarthrodesis. We recommend extension CT especially in patients with recurrent or persistent symptoms after PLIF in spite of fusion having been indicated both in flexion–extension X-rays and standard neutral CTs.
Conflict of interest
None.
References
- 1.Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine. 1993;18:1186–1189. doi: 10.1097/00007632-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky AE, Evan SK, Momtaz AK. Correlation of radiographic assessment of lumbar spine fusions with surgical exploration. Spine. 1991;16:S261–S265. doi: 10.1097/00007632-199106001-00017. [DOI] [PubMed] [Google Scholar]
- 3.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Chafetz N, Cann CE, Morris JM, Steinbach LS, Goldberg HI, Ax L. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1987;162:803–805. doi: 10.1148/radiology.162.3.3809497. [DOI] [PubMed] [Google Scholar]
- 5.Cook SD, Patron LP, Christakis PM, Bailey KJ, Banta C, Glazer PA. Comparison of methods for determining the presence and extent of anterior lumbar interbody fusion. Spine. 2004;29:118–123. doi: 10.1097/00007632-200405150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer JW, Matteri RE, Hanley EN, Kuhlmann D, Howe J. Failed lumbar disc surgery requiring second operation: a long-term follow-up study. Spine. 1978;3:7–11. doi: 10.1097/00007632-197803000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Herzog RJ, Marcotte PJ. Imaging corner assessment of spinal fusion. Critical evaluation of imaging techniques. Spine. 1996;21:1114–1118. doi: 10.1097/00007632-199605010-00027. [DOI] [PubMed] [Google Scholar]
- 8.Kant AP, Daum WJ, Dean SM, Uchida T. Evaluation of lumbar spine fusion. Plain radiographs versus direct surgical exploration and observation. Spine. 1995;20:2313–2327. doi: 10.1097/00007632-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Korovessis P, Repantis T, Papazisis Z, Iliopoulos P. Effect of sagittal spinal balance, levels of posterior instrumentation, and length of follow-up on low back pain in patients undergoing posterior decompression and instrumented fusion for degenerative lumbar spine disease: a multifactorial analysis. Spine. 2010;15:898–905. doi: 10.1097/BRS.0b013e3181d51e84. [DOI] [PubMed] [Google Scholar]
- 10.Laasonen EM, Soini J. Low back pain after lumbar fusion: surgical and computed tomographic analysis. Spine. 1989;14:210–213. doi: 10.1097/00007632-198902000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Lang P, Genant HK, Chafetz N, Steiger P, Morris JM. Three-dimensional computed tomography and multiplanar reformations in the assessment of pseudarthrosis in posterior lumbar fusion patients. Spine. 1988;13:69–75. doi: 10.1097/00007632-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 12.McAfee PC, Boden SD, Brantigan JW, Fraser RD, Kuslich SD, Oxland TR, Panjabi MM, Ray CD, Zdeblick TA. Symposium: a critical discrepancy: a criteria of successful arthrodesis following interbody spinal fusions. Spine. 2001;26:324–334. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 13.Rothman SLG, Glenn WV., Jr CT evaluation of interbody fusion. Clin Orthop Rel Res. 1985;193:47–56. [PubMed] [Google Scholar]
- 14.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiographic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/01.BRS.0000061988.93175.74. [DOI] [PubMed] [Google Scholar]
- 15.Shah RR, Mohammed S, Saifuddin A, Taylor BA. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12:378–385. doi: 10.1007/s00586-002-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]