Abstract
Obesity is a risk factor for ossification of the posterior longitudinal ligament (OPLL) of the spine, which is characterized by heterotopic bone formation in the posterior longitudinal spinal ligament. Hyperleptinemia is a common feature of obese people and leptin is believed to be an important factor in the pathogenesis of OPLL. However, the association between leptin and bone metabolism and the development of OPLL is not understood fully. The objective of the present study was to determine the association between serum leptin concentration and bone metabolic markers and the extent of heterotopic ossification of the spinal ligament in patients with OPLL. The serum concentrations of leptin, insulin, fructosamine, bone-specific alkaline phosphatase, and carboxyterminal propeptide of type I procollagen, urine deoxypyridinoline levels, and the number of vertebrae with OPLL involvement were measured in 125 (68 males and 57 females) patients with OPLL. The correlation between leptin and these other factors was then examined. Serum leptin and insulin concentrations were increased significantly in OPLL females compared to non-OPLL female controls. In the females with OPLL, serum leptin concentrations corrected for body mass index correlated positively with the number of vertebrae with OPLL involvement. In females, serum leptin levels were significantly higher in patients in whom OPLL extended to the thoracic and/or lumbar spine than in patients in whom OPLL was limited to the cervical spine. Our results suggest that hyperleptinemia, in combination with hyperinsulinemia, may contribute to the development of heterotopic ossification of the spinal ligament in female patients with OPLL.
Keywords: Leptin, Ossification of the posterior longitudinal ligament (OPLL), Insulin, Gender, Bone metabolic markers
Introduction
Ossification of the posterior longitudinal ligament (OPLL) of the spine is characterized by heterotopic bone formation in the spinal canal and is considered to belong to the same pathological entity as ankylosing spinal hyperostosis. Enlarged OPLL often compresses the spinal cord and causes severe neurological disorders [33].
The Zucker fatty (fa/fa) rat, a model for hereditary obesity, exhibits hyperglycemia, hyperinsulinemia, hyperlipidemia, and heterotopic ossification of the spinal ligament [13, 20, 39]. A missense mutation (Gly269Pro) in the leptin receptor gene (Ob-R) was found in this rat where leptin-binding affinity was reduced and signal transduction was attenuated, leading to a compensatory elevation in circulating leptin levels [10, 17, 21, 28]. Since heterotopic ossification of the spinal ligament in the fa/fa rat is quite similar to that found in human OPLL, researchers in the field of spinal surgery consider the fa/fa rat as a useful animal model for studying the pathophysiology of OPLL [28, 36].
Leptin, a product of the obese (ob) gene, is secreted primarily by adipocytes and plays an important role in regulation of food intake and energy expenditure [30]. Peripheral administration of leptin increases bone growth and indices of bone formation [26, 37]. Leptin can also act directly on stromal cells to enhance their differentiation into osteoblasts and inhibit their differentiation into adipocytes [3]. On the other hand, intracerebroventricular infusion of leptin leads to rapid bone loss [32], implying that leptin regulates bone mass through alternate pathways, one involving a direct stimulatory effect on bone growth when administered peripherally and another acting indirectly via a hypothalamic relay that suppresses bone formation, when administered centrally [4]. As leptin has the potential to drive stromal cells into osteogenic differentiation, serum leptin may be associated with the development of heterotopic ossification of the spinal ligament. However, to date, the association between leptin and heterotopic ossification of the spine, particularly in OPLL, has been largely unstudied.
In this study, we hypothesized that serum leptin levels are elevated in patients with OPLL and may be associated with bone metabolic markers and the extent of OPLL development. We measured serum leptin concentrations in OPLL patients and non-OPLL controls and corrected these levels using individual body mass index (BMI). We then analyzed the association between the leptin/BMI ratio and bone metabolic markers, and the number of vertebrae with OPLL involvement. Based on these results, we discuss the possible role(s) of leptin in the development of OPLL.
Subjects and methods
The study subjects (Table 1) were 125 Japanese patients with OPLL (68 males and 57 females) and 62 non-OPLL control subjects (35 males and 27 females, the majority of who had spinal degenerative disorders other than OPLL). All patients were followed at the Department of Orthopedic Surgery of Chiba University Hospital between 1995 and 2008.
Table 1.
Clinical characteristics of OPLL (ossification of the posterior longitudinal ligament) patients and non-OPLL controls
| Female OPLL versus non-OPLL | |||
|---|---|---|---|
| OPLL (n = 57) | Non-OPLL (n = 27) | p (Student’s t) | |
| Age (year) | 58.6 ± 9* | 61.7 ± 8.7 | <0.05 |
| Height (cm) | 152.9 ± 6.7 | 150.3 ± 6.7 | N.S. |
| Weight (kg) | 59 ± 9.8* | 51.9 ± 7.8 | <0.01 |
| BMI (kg/m²) | 25.2 ± 4.4* | 22.9 ± 3.1 | <0.05 |
| Serum leptin (ng/ml) | 9.67 ± 5.1* | 6.55 ± 3.67 | <0.01 |
| Leptin/BMI | 0.368 ± 0.169* | 0.275 ± 0.122 | <0.01 |
| Male OPLL versus non-OPLL | |||
|---|---|---|---|
| OPLL (n = 68) | Non-OPLL (n = 35) | p (Student’s t) | |
| Age (year) | 61.2 ± 8.1* | 56.5 ± 11.2 | <0.05 |
| Height (cm) | 163.8 ± 5.8* | 166.6 ± 5.6 | <0.05 |
| Weight (kg) | 64.6 ± 9.2 | 64.4 ± 7.8 | N.S. |
| BMI (kg/m²) | 24 ± 2.7 | 23.1 ± 2.5 | N.S. |
| Serum leptin (ng/ml) | 3.85 ± 2.2 | 3.2 ± 1.4 | N.S. |
| Leptin/BMI | 0.156 ± 0.079 | 0.136 ± 0.055 | N.S. |
N.S. not significant, BMI body mass index
* Significantly different from non-OPLL
Based on previous data that circulating leptin concentrations are significantly higher in females than in male subjects [14, 17, 22], we subdivided the OPLL and non-OPLL groups according to gender. The mean age of OPLL females, non-OPLL females, OPLL males, and non-OPLL males was 58.6 ± 9.0, 61.7 ± 8.7, 61.2 ± 8.1, and 56.5 ± 11.2 years, respectively. The mean BMI (weight in kilograms divided by the square of height in meters) of OPLL females, non-OPLL females, OPLL males, and non-OPLL males was 25.2 ± 4.4, 22.9 ± 3.1, 24.0 ± 2.7, and 23.1 ± 2.5 kg/m2, respectively. All the patients were informed that data on the blood or urine samples would be submitted for publication and the patients volunteered freely to participate in this study. This study was approved by the ethics committee of Chiba University Hospital.
A blood sample was collected from each subject between 11:00 and 13:00 h after overnight fasting and the serum immediately frozen at –80°C until analysis. For a urine analysis, the 2-h morning urine after the first void urine was tested. Serum leptin concentrations were measured using a commercially available radioimmunoassay (RIA) kit (Linco Research, Inc., St. Charles, MO). As gender and adipose tissue volume influence leptin production, the serum leptin levels were corrected for BMI, a measure of obesity, and then compared within each gender group. The minimum detection limit of serum leptin levels was 0.5 ng/ml with a 4.5% coefficient of variation. Serum insulin levels were also measured using a microparticle enzyme immunoassay (EIA) (AxSYM insulin assay kit, Dainabot Co., Ltd., Tokyo, Japan). The minimum detection limit of serum insulin levels was 0.8 μU/ml with a 5.5% coefficient of variation. The serum concentrations of bone formation markers, bone-specific alkaline phosphatase (BAP) and the carboxyterminal propeptide of type I procollagen (PICP) were measured using an EIA (Takara, Tokyo, Japan) and a RIA (Orion Diagnostica, Espoo, Finland) kit, respectively. Urine deoxypyridinoline (DPD) was measured with an EIA kit (DS Pharma Biomedical, Osaka, Japan) as a marker of bone resorption.
Radiographic evaluation of the number of vertebrae and segments with OPLL involvement in individual patients was evaluated by at least two different authors, all of whom were senior spinal surgeons. Patients with ossification of the yellow ligament of the spine, which is often seen as heterotopic ossification of the spinal ligament at the thoracic spine, were excluded from the study.
Statistical methods
Previous studies have shown that circulating leptin levels correlate positively with BMI [14, 31]. To eliminate the influence of obesity, we calculated the leptin/BMI ratio for individual patients. Comparison of age, height, body weight, BMI, serum leptin levels, and leptin/BMI ratios between OPLL patients and non-OPLL controls was performed using Student’s t test. In female OPLL patients, correlations between leptin/BMI ratios and serum BAP, PICP, insulin, and fructosamine (FRA) levels, urine DPD levels, and the number of vertebrae with OPLL involvement were analyzed using Pearson’s correction analysis.
In addition, the OPLL patients were divided into two subgroups according to the extent of OPLL development, with patients in whom OPLL was limited to the cervical spine being designated as type C-OPLL, while subjects in whom OPLL extended to the thoracic and/or lumbar spine being designated as type TL-OPLL (Fig. 1). Type C-OPLL included 63 patients (48 males and 15 females) while type TL-OPLL included 62 patients (20 males and 42 females) (Table 2). Student’s t test was then performed to analyze differences in age, height, body weight, BMI, leptin/BMI ratios, and serum insulin and FRA levels between type C-OPLL and type TL-OPLL patients. The correlation between leptin/BMI ratios and serum BAP, PICP, insulin, and FRA levels, urine DPD levels, and the number of vertebrae with OPLL involvement in both female type TL-OPLL and female type C-OPLL patients were analyzed using Pearson’s correlation analysis. All these analyses were performed with the significance level being set at p < 0.05.
Fig. 1.
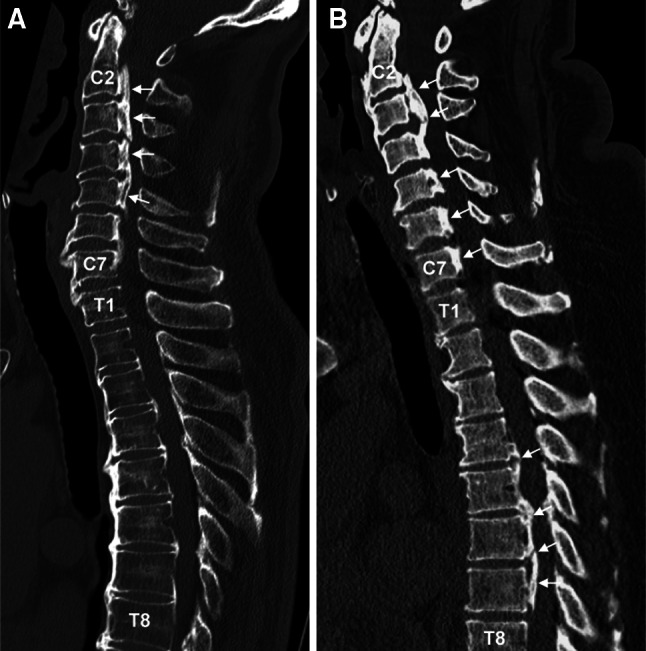
Representative, mid-sagittal reconstruction images of 3-dimensional computed tomography (CT) for type C-OPLL (a type of OPLL limited to the cervical spine) and type TL-OPLL (a type of OPLL extended to the thoracic and/or lumbar spine) patients. a A 66-year-old male patient where OPLL is limited to the cervical spine (type C-OPLL). b A 54-year-old female patient where OPLL is extended to the thoracic spine (type TL-OPLL). Arrows indicate OPLL. C2 2nd cervical vertebra, C7 7th cervical vertebra, T1 1st thoracic vertebra, T8 8th thoracic vertebra
Table 2.
Clinical characteristics of type C-OPLL (a type of OPLL limited to the cervical spine) and type TL-OPLL (a type of OPLL extended to the thoracic and/or lumbar spine) patients
| Female type C-OPLL versus type TL-OPLL | |||
|---|---|---|---|
| Type C (n = 15) | Type TL (n = 42) | p (Student’s t) | |
| Age (year) | 58.6 ± 10 | 56.1 ± 8.6 | N.S. |
| Height (cm) | 153.1 ± 6.3 | 152.8 ± 6.9 | N.S. |
| Weight (kg) | 56.6 ± 10.1 | 59.8 ± 9.6 | N.S. |
| BMI (kg/m²) | 24.2 ± 5 | 25.5 ± 4.1 | N.S. |
| Serum leptin (ng/ml) | 6.64 ± 4 | 10.7 ± 5* | <0.01 |
| Leptin/BMI | 0.261 ± 0.122 | 0.407 ± 0.168* | <0.01 |
| Serum insulin (μU/ml) | 10.1 ± 4.3 | 19.2 ± 22.2 | N.S. |
| Serum FRA (μM) | 511 ± 176 | 708 ± 418 | N.S. |
| Male type C-OPLL versus type TL-OPLL | |||
|---|---|---|---|
| Type C (n = 48) | Type TL (n = 20) | p (Student’s t) | |
| Age (year) | 60.9 ± 8.6 | 61.9 ± 6.8 | N.S. |
| Height (cm) | 164.3 ± 5.6 | 162.5 ± 6.2 | N.S. |
| Weight (kg) | 64.7 ± 9 | 64.5 ± 9.8 | N.S. |
| BMI (kg/m²) | 23.9 ± 2.6 | 24.3 ± 3.2 | N.S. |
| Serum leptin (ng/ml) | 3.62 ± 2.16 | 4.41 ± 2.33 | N.S. |
| Leptin/BMI | 0.148 ± 0.08 | 0.173 ± 0.075 | N.S. |
| Serum insulin (μU/ml) | 15 ± 16.7 | 20.1 ± 21.4 | N.S. |
| Serum FRA (μM) | 672 ± 293 | 739 ± 281 | N.S. |
N.S. not significant, BMI body mass index, FRA fructosamine
* Significantly different from type C-OPLL
Results
Serum leptin concentrations and leptin/BMI ratios in OPLL and non-OPLL patients
The characteristics of the four subgroups are presented in Table 1. Both OPLL and non-OPLL groups exhibited significantly higher serum leptin concentrations in females than in male subjects, consistent with the findings of previous studies [14, 23]. In female subjects, serum leptin concentrations in the OPLL group were 1.5-fold higher than that in the non-OPLL group (p < 0.01). However, in male subjects there was no significant difference in serum leptin concentrations between the OPLL and non-OPLL groups.
In female subjects, the leptin/BMI ratio was significantly higher (1.3-fold) in the OPLL group than in the non-OPLL group (p < 0.01), whereas no significant difference was observed in the male subjects (Table 1).
Correlation of leptin/BMI ratios with biochemical markers of bone turnover, serum insulin and FRA concentrations, and the number of vertebrae with OPLL involvement in OPLL females
To determine the factors associated with the leptin/BMI ratio in OPLL females, we examined the correlation between leptin/BMI ratios and bone metabolic markers, circulating insulin and FRA concentrations, and the number of vertebrae with OPLL involvement (Fig. 2). There was only a relatively weak, non-significant positive correlation between the leptin/BMI ratio and both bone formation markers, BAP and PICP. In contrast, urine DPD levels, a bone resorption marker, showed a negative correlation with the leptin/BMI ratio (r = −0.523, p < 0.05). Serum insulin concentrations were correlated positively with the leptin/BMI ratio (r = 0.344, p < 0.01), whereas serum FRA levels showed no such significant relationship. It should be noted that there was a positive correlation between the number of vertebrae with OPLL involvement and the leptin/BMI ratio (r = 0.271, p < 0.05). We also examined all the above relationships in OPLL males and showed that there was no significant correlation between the leptin/BMI ratio and any other variable (data not shown).
Fig. 2.
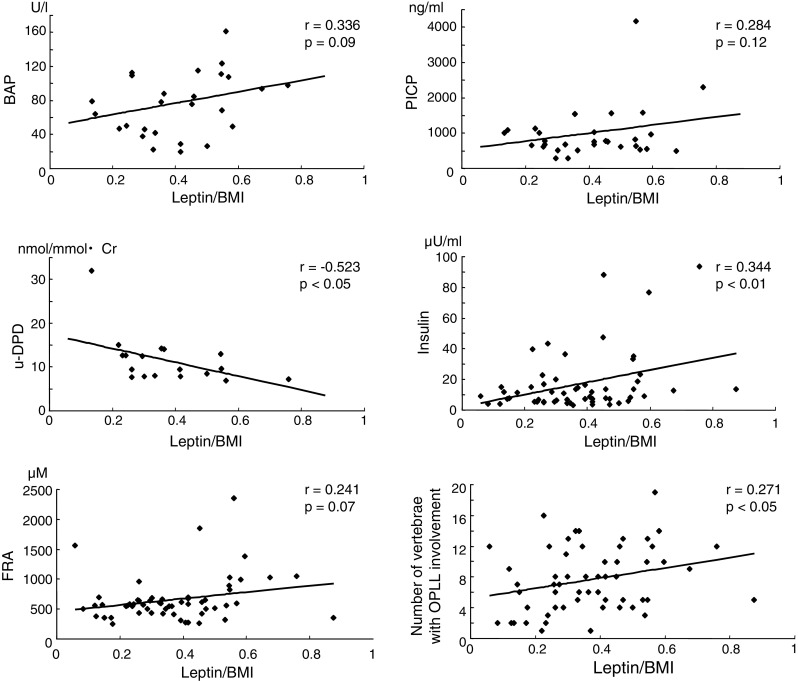
Relationship between the leptin/BMI (body mass index) ratio and bone metabolic markers including serum bone-specific alkaline phosphatase (BAP), carboxyterminal propeptide of type I procollagen (PICP), and urine deoxypyridinoline (DPD), serum insulin and fructosamine (FRA) concentrations, and the number of vertebrae with OPLL (ossification of the posterior longitudinal ligament) involvement in female OPLL patients. There was a negative, significant correlation between the leptin/BMI ratio and urine DPD levels, while a positive, significant correlation between the leptin/BMI ratio and serum insulin levels, and the number of vertebrae with OPLL involvement
Comparison of serum leptin, insulin, FRA concentrations, and leptin/BMI ratios between type C-OPLL and type TL-OPLL patients
The characteristics of the four subgroups of OPLL patients are presented in Table 2. In female subjects, there was no significant difference in age, height, weight, BMI, serum insulin and FRA concentrations between the groups except for serum leptin concentration which was 1.6-fold higher in type TL-OPLL than in type C-OPLL (p < 0.01). This difference was also found in serum leptin levels corrected by BMI, with significantly higher values in type TL-OPLL (1.6-fold, p < 0.01) than in type C-OPLL (Table 2). In male subjects, there were no significant differences in serum leptin concentrations and leptin/BMI ratios between the two subgroups (Table 2).
Correlation of leptin/BMI ratios with bone metabolic markers, serum insulin and FRA concentrations, and the number of vertebrae with OPLL involvement in females with type C-OPLL or type TL-OPLL
To investigate the factors associated with the leptin/BMI ratio in female patients with type TL-OPLL, we examined the correlation between leptin/BMI ratios and bone metabolic markers, serum insulin and FRA concentrations, and the number of vertebrae with OPLL involvement (Fig. 3). There was a strong, positive correlation between serum BAP levels and the leptin/BMI ratio (r = 0.518, p < 0.05), but no significant difference between PICP levels and the ratio. Urine DPD and serum FRA levels, and the number of vertebrae with OPLL involvement did not show a significant correlation with the leptin/BMI ratio. Interestingly, serum insulin concentrations correlated positively with the leptin/BMI ratio (r = 0.324, p < 0.05). We also carried out similar correlation analyses on type C-OPLL females, but found no significant relationship between the variables (data not shown).
Fig. 3.
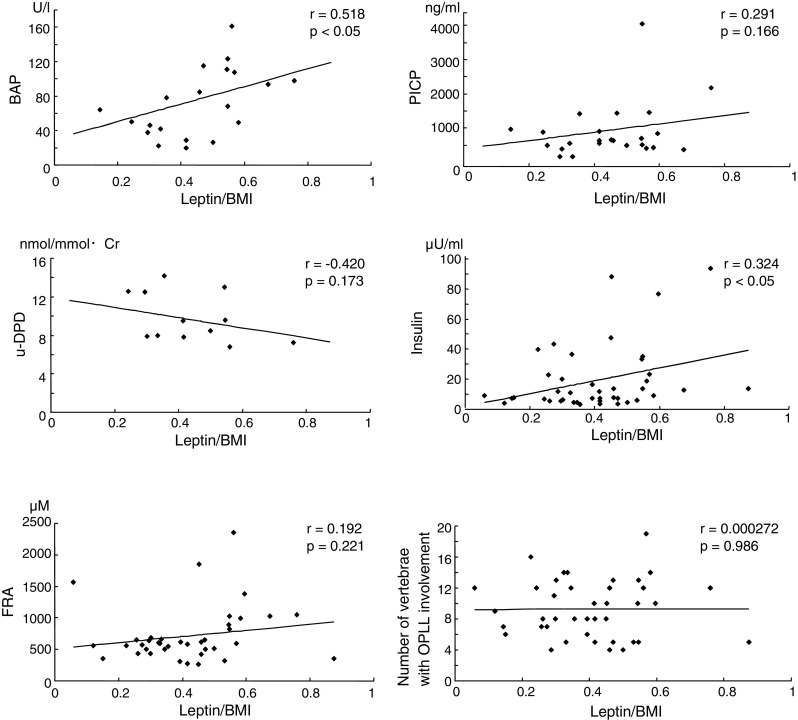
Relationship between the leptin/BMI (body mass index) ratio and bone metabolic markers including serum bone-specific alkaline phosphatase (BAP), carboxyterminal propeptide of type I procollagen (PICP), and urine deoxypyridinoline (DPD), serum insulin and fructosamine (FRA) concentrations, and the number of vertebrae with OPLL involvement in female type TL-OPLL patients. There was a positive, significant correlation between the leptin/BMI ratio and serum BAP levels, and serum insulin levels
Discussion
This study demonstrated that females with OPLL had significantly higher serum leptin levels compared with females without OPLL and that the levels correlated positively with serum insulin levels. These findings are consistent with those of a previous study by Shirakura et al. [24]. In the present study, we carried out further investigations to determine the factors associated with the leptin/BMI ratio in OPLL females and showed that urine DPD concentrations correlated negatively with the ratio. This suggests a regulatory effect of leptin on bone resorption, which is in agreement with the report of Burguera et al. [2] who demonstrated in animal experiments that leptin prevented ovariectomy-induced bone loss. Taken together, these results indicate a tendency for a positive correlation between BAP levels and the leptin/BMI ratio in OPLL females and that bone anabolism may be promoted in these patients. However, a significant correlation between urine DPD levels and the leptin/BMI ratio in OPLL females may be influenced by one sample that appears to be an outlier (leptin/BMI = 0.13, u-DPD = 32) as shown in Fig. 2 because this association lost statistical significance when that one sample was excluded (p = 0.085, data not shown). Further studies are necessary to establish association between urine DPD levels and the leptin/BMI ratio in OPLL females.
It is interesting that the number of vertebrae with OPLL involvement correlated positively with the leptin/BMI ratio in female patients with OPLL. To the best of our knowledge, there have been no other reports demonstrating an association between serum leptin levels and the extent of OPLL development in humans. We showed previously that the A861G variant in the leptin receptor gene (Ob-R) was associated with more extensive OPLL [29]. Although the effects of the variant in Ob-R on leptin signaling are unknown, this finding suggests that altered leptin signaling in spinal ligament cells may be a factor that regulates the extent of OPLL development.
To date, a few in vitro studies have revealed the mechanisms by which leptin induces osteogenic differentiation in spinal ligament cells [6, 24]. Fan et al. [6] demonstrated that leptin caused significant increases in mRNA expression of alkaline phosphatase (ALP) and osteocalcin in thoracic ossification of ligament flavum (TOLF) cells, but not in non-TOLF cells, and that the effect was both dose- and time-dependent. These findings suggest that TOLF cells are considerably more sensitive to leptin stimulation and that leptin has the potential to promote osteogenic differentiation in ligament flavum cells. However, this leptin-induced osteogenic differentiation was observed only in response to pharmacological doses, which are not equivalent to physiological concentrations in humans. This implies that ligand stimulation with cytokines, other than leptin, is required to induce osteogenic differentiation in spinal ligament cells.
Insulin has been implicated in the development of heterotopic ossification of spinal ligaments [8, 12, 15]. Spinal ligament cells express insulin receptor substrate (IRS)-1, a common major substrate for both insulin and insulin-like growth factor (IGF)-I receptor tyrosine kinase [12]. We have reported previously that IGF-I stimulates proliferation and collagen type I synthesis in the majority of spinal ligament cells [8]. Li et al. [15] showed that both insulin and IGF-I increased proliferation and ALP activity in human spinal ligament cells. It has been reported that leptin acts through some of the components of the insulin signaling cascade by recruiting several IRSs [1, 7, 9, 19]. This implies that there is cross-talk between leptin signaling and insulin-induced pathways. Our finding that the leptin/BMI ratio correlated positively with serum insulin levels in OPLL females suggests that increased levels of both leptin and insulin may act synergistically to strengthen downstream signaling, thereby contributing to the development of OPLL.
As the number of vertebrae with OPLL involvement correlated positively with serum leptin levels in OPLL females, we hypothesized that female patients with extended OPLL may have higher serum levels of leptin than those with limited OPLL. To verify this hypothesis, we divided the OPLL females into two further subgroups; type C and type TL groups. We then compared BMI, serum leptin levels, leptin/BMI ratios, and serum insulin and FRA concentrations in these two groups. The results showed that relative to type C-OPLL, serum leptin levels and leptin/BMI ratios were increased significantly in type TL-OPLL by 1.6- and 1.9-fold, respectively. Although there were no statistical differences in serum insulin and FRA concentrations between the groups, higher levels of insulin and FRA were detected in type TL-OPLL, which supports the concept that both hyperleptinemia and hyperinsulinemia contribute to extension of heterotopic ossification of the spinal ligament in OPLL females.
It is also of interest that there was a positive, significant correlation between the leptin/BMI ratio and BAP levels in type TL-OPLL females whose serum leptin concentrations were significantly higher than type C-OPLL, whereas no significant correlation was detected in all OPLL females. These results indicate that bone anabolism is elevated substantially in female type TL-OPLL patients compared to female type C-OPLL patients. However, this is inconsistent with a previous report that serum leptin levels are negative regulators of bone mass [5]. We speculate that in OPLL females there may be a decrease in leptin sensitivity in the hypothalamus, which in turn, increases circulating leptin levels, resulting in the development of OPLL through the direct and anabolic effects of leptin on bone and spinal ligament cells.
OPLL occurs frequently at the cervical spine [33]. However, it has not been determined why OPLL is sometimes limited to the cervical spine or alternatively extends to the thoracic and/or lumbar spine. As shown in our study, higher serum leptin levels, detected in female patients with OPLL extending to the thoracic and/or lumbar spine, may be a causative factor determining the extension of OPLL. However, even if serum leptin levels or other systemic factors including serum insulin or FRA levels affect the extension of OPLL, there still remains the question as to why the limited type of OPLL is seen frequently at the cervical spine. We speculate that mechanical stress, which is supposedly a local factor, could be a prime candidate to induce heterotopic ossification in the cervical spine but is unlikely to occur in the thoracic spine. Iwasawa et al. [11] demonstrated that exposure to mechanical stress such as uniaxial stretching upregulated various genes related to bone metabolism including endothelin-1 (ET-1) and prostaglandin I2 (PGI2) in OPLL cells. This shows clearly the importance of mechanical stress in heterotopic ossification of the spinal ligament. Several biomechanical studies have revealed that the range of motion (extension and flexion) of the cervical spine is much larger than that of the thoracic spine [18, 27, 35]. Taken together, it is conceivable that moderately elevated levels of leptin, in combination with mechanical stress to the ligament cells, may contribute to the development of cervical OPLL, while highly elevated levels of leptin may have a role in the extension of OPLL to the thoracic and/or lumbar spine.
The important issue that is not clarified by the present study is the significant gender difference between serum leptin levels and the development of OPLL. It is likely that some gender-specific factors such as estrogen may have an important role in the development of OPLL. Recent studies on human periodontal ligament cells have shown that estrogen is capable of inducing osteogenic differentiation in ligament cells [16, 25, 38]. It has also been shown previously in the Japanese population that serum estrogen levels are elevated significantly in OPLL patients, and that the levels are related to the extent of heterotopic ligament ossification [34]. These observations suggest a pivotal role for estrogen in heterotopic ossification of the spinal ligament. We therefore speculate that serum leptin do not regulate solely the development of OPLL, but may contribute to the development of OPLL in combination with some gender-specific factors such as estrogen.
In summary, we showed that serum leptin concentrations are elevated in females with OPLL and are associated with extension of OPLL, i.e. an increased number of vertebrae with OPLL involvement. In addition, serum leptin levels in patients with extensive OPLL (thoracic and/or lumbar type) were higher than in patients with limited OPLL (cervical type). These observations indicate that hyperleptinemia may contribute to the development of heterotopic ossification of the spinal ligament in females with OPLL. However, it is also clear that this scenario does not completely explain the entire mechanism of spinal ligament ossification in OPLL patients as other complicated pathological factors associated with OPLL such as hyperinsulinemia, hyperlipidemia, and high glucose levels coexist with hyperleptinemia. The present observations are, nevertheless, important for future work and may provide information on the mechanisms underlying the development of OPLL, and ultimately may lead to potential drug therapies for management of this disease.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, and by a grant for Intractable Diseases from the Public Health Bureau, the Ministry of Health and Welfare of Japan (Investigation Committee on Ossification of the Spinal Ligaments).
Footnotes
Y. Ikeda and A. Nakajima contributed equally to this work.
References
- 1.Benomar Y, Roy AF, Aubourg A, Djiane J, Taouis M. Cross down-regulation of leptin and insulin receptor expression and signaling in a human neuronal cell line. Biochem J. 2005;388:929–939. doi: 10.1042/BJ20041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/en.142.8.3546. [DOI] [PubMed] [Google Scholar]
- 3.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–412. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 4.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/S0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 5.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Nat Acad Sci. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan D, Chen Z, Chen Y, Shang Y. Mechanistic roles of leptin in osteogenic stimulation in thoracic ligament flavum cells. J Biol Chem. 2007;282:29958–29966. doi: 10.1074/jbc.M611779200. [DOI] [PubMed] [Google Scholar]
- 7.Fruhbeck G, Salvador J. Relation between leptin and the regulation of glucose metabolism. Diabetologia. 2000;43:3–12. doi: 10.1007/s001250050002. [DOI] [PubMed] [Google Scholar]
- 8.Goto K, Yamazaki M, Tagawa M, Goto S, Kon T, Moriya H, Fujimura S. Involvement of insulin growth factor I in development of ossification of the posterior longitudinal ligament of the spine. Calcif Tissue Int. 1998;62:158–165. doi: 10.1007/s002239900410. [DOI] [PubMed] [Google Scholar]
- 9.Hegyi K, Fulop K, Kavacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine-proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 11.Iwasawa T, Iwasaki K, Sawada T, Okada A, Ueyama K, Motomura S, Harata S, Toh S, Furukawa KI. Pathophysiological role of endothelin in ectopic ossification of human spinal ligaments induced by mechanical stress. Calcif Tissue Int. 2006;79:422–430. doi: 10.1007/s00223-006-0147-7. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki T, Tobe K, Honda-Yamamoto R, Tamemoto H, Kaburagi Y, Momomura K, Ueki K, Takahashi Y, Yamauchi T, Akanuma Y, Yazaki Y. Signal transduction mechanism of insulin and insulin-like growth factor-1. Endocr J. 1996;43(suppl):S33–S41. doi: 10.1507/endocrj.43.Suppl_S33. [DOI] [PubMed] [Google Scholar]
- 13.Kawai K. Ossification of the insertion of the spinal ligament (Enthesis) in Zucker fatty rats and the effects of ethane-1-hydroxy-1, 1-diphosphonate (EHDP) on its rat. J Tokyo Med Coll. 1989;47:558–567. [Google Scholar]
- 14.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jc.82.4.1293. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Liu D, Zhao CQ, Jiang LS, Dai LY. Insulin potentiates the proliferation and bone morphogenetic protein-2-induced osteogenic differentiation of rat spinal ligament cells via extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Spine. 2008;33:2349–2402. doi: 10.1097/BRS.0b013e3181838fe5. [DOI] [PubMed] [Google Scholar]
- 16.Liang L, Yu JF, Wang Y, Wang G, Ding Y. Effect of estrogen beta on the osteoblastic differentiation function of human periodontal ligament cells. Arch Oral Biol. 2008;53:553–557. doi: 10.1016/j.archoralbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H. A radiographic study of the progression of ossification of the cervical posterior longitudinal ligament and that of the anterior longitudinal ligament. Nippon Seikeigeka Gakkai Zasshi. 1994;68:725–730. [PubMed] [Google Scholar]
- 19.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/S0091-3022(02)00105-X. [DOI] [PubMed] [Google Scholar]
- 20.Okano T, Ishidou Y, Kato M, Imamura T, Yonemori K, Origuchi N, Matsunaga S, Yoshida H, ten Dijke P, Sakou T. Orthotopic ossification of the spinal ligaments of Zucker fatty rats: a possible animal model for ossification of the human posterior longitudinal ligament. J Orthop Res. 1997;15:820–829. doi: 10.1002/jor.1100150606. [DOI] [PubMed] [Google Scholar]
- 21.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nature Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 22.Reseland J, Gordeladze J. Role of leptin in bone growth: central player or peripheral supporter? FEBS Lett. 2002;528:40–42. doi: 10.1016/S0014-5793(02)03161-7. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocriol Metab. 1996;81:3424–3427. doi: 10.1210/jc.81.9.3424. [DOI] [PubMed] [Google Scholar]
- 24.Shirakura Y, Sugiyama T, Tanaka H, Taguchi T, Kawai S. Hyperleptinemia in female patients with ossification of spinal ligaments. Biochem Biophys Res Commun. 2000;267:752–755. doi: 10.1006/bbrc.1999.2027. [DOI] [PubMed] [Google Scholar]
- 25.Shu L, Guan SM, Fu SM, Guo T, Cao M, Ding Y. Estrogen modulates cytokine expression in human periodontal ligament cells. J Dent Res. 2008;87:142–147. doi: 10.1177/154405910808700214. [DOI] [PubMed] [Google Scholar]
- 26.Steppan CM, Crawford DT, Chidsey Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/S0167-0115(00)00152-X. [DOI] [PubMed] [Google Scholar]
- 27.Takatsu T, Ishida Y, Suzuki K, Inoue H. Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1999;12:271–273. [PubMed] [Google Scholar]
- 28.Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, Mori K, Tamura N, Hosoda K, Nakao K. Molecular cloning of rat leptin receptor isoform complementary DNAs: identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun. 1996;225:75–83. doi: 10.1006/bbrc.1996.1133. [DOI] [PubMed] [Google Scholar]
- 29.Tahara M, Aiba A, Yamazaki M, Ikeda Y, Goto S, Moriya H, Okawa A. The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphate gene and leptin receptor gene polymorphisms. Spine. 2005;30:877–880. doi: 10.1097/01.brs.0000160686.18321.ad. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S. Ossification of the spinal ligaments in Zucker fatty rat. J Tokyo Med Coll. 1994;52:19–32. [Google Scholar]
- 31.Tasaka Y, Yanagisawa Y, Iwamoto Y. Human plasma leptin in obese subjects and diabetics. Endocr J. 1997;44:671–676. doi: 10.1507/endocrj.44.671. [DOI] [PubMed] [Google Scholar]
- 32.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/en.140.4.1630. [DOI] [PubMed] [Google Scholar]
- 33.Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;184:71–84. [PubMed] [Google Scholar]
- 34.Wada A. Affinity of estrogen binding in the cultured spinal ligament cells: an in vitro study using cells from spinal ligament ossification patients. Nippon Seikeigeka Gakkai Zasshi. 1995;69:440–449. [PubMed] [Google Scholar]
- 35.White AA, Panjabi MM. Clinical biomechanics of the spine. 2. Philladelphia: Lippincott-Raven; 1990. [Google Scholar]
- 36.Yamashita T, Murakami T, Iida M, Kuwajima M, Shima K. Leptin receptor of Zucker fatty rat performs reduced signal transduction. Diabetes. 1997;46:1077–1080. doi: 10.2337/diabetes.46.6.1077. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Fu Y, Li JP, Qi LY. The role of estrogen in osteogenic cytokine expression in human periodontal ligament cells. Int J Periodontics Restorative Dent. 2009;29:507–513. [PubMed] [Google Scholar]
- 39.Zucker LM, Antoniades HN. Insulin and obesity in the Zucker genetically obese rat “fatty”. Endocrinology. 1972;90:1320–1330. doi: 10.1210/endo-90-5-1320. [DOI] [PubMed] [Google Scholar]


