Abstract
The objective of this study was to establish the efficacy and safety of porous bioactive titanium metal for use in a spinal fusion device, based on a prospective human clinical trial. A high-strength spinal interbody fusion device was manufactured from porous titanium metal. A bioactive surface was produced by simple chemical and thermal treatment. Five patients with unstable lumbar spine disease were treated surgically using this device in a clinical trial approved by our Ethics Review Committee and the University Hospital Medical Information Network. Clinical and radiological results were reported at the minimum follow-up period of 1 year. The optimal mechanical strength and interconnected structure of the porous titanium metal were adjusted for the device. The whole surface of porous titanium metal was treated uniformly and its bioactive ability was confirmed before clinical use. Successful bony union was achieved in all cases within 6 months without the need for autologous iliac crest bone grafting. Two specific findings including an anchoring effect and gap filling were evident radiologically. All clinical parameters improved significantly after the operation and no adverse effects were encountered during the follow-up period. Although a larger and longer-term follow-up clinical study is mandatory to reach any firm conclusions, the study results show that this porous bioactive titanium metal is promising material for a spinal fusion device.
Keywords: Porous titanium metal, Spinal fusion, Biomaterial, Clinical trial
Introduction
Osteoconductive synthetic materials including sintered hydroxyapatite (Ca10(PO4)6(OH)2 or HA), Na2O-CaO-SiO2-P2O5 system (Bioglass®) and glass ceramics containing apatite and wollastonite (AW-GC) are widely used clinically as bone substitutes [7, 12, 16]. Because application for load-bearing conditions such as the spine or long bones requires high mechanical strength, solid materials are usually used. However, such materials are brittle against shearing forces and bond to the surrounding bone only at their surface. Porous materials have advantages over solid materials in terms of bone bonding, because they can demonstrate both osteoconductive bonding and mechanical interlocking through bone tissue ingrowth into the pores. Conventional porous synthetic materials such as granules of HA and AW-GC have been applied clinically as bone graft expanders for lumbar posterolateral fusion or bone void fillers after tumor excision [8]. However, because of their poor mechanical strength, porous body of such materials cannot be applied in load-bearing conditions. Thus, achieving both high bone-bonding ability and high mechanical strength is quite difficult for porous materials. To overcome this problem, we have developed porous bioactive titanium metal, which possesses both high bone-bonding ability and high mechanical strength simultaneously [24]. Titanium metal and its alloys can be changed to bioactive materials by simple chemical and thermal surface treatment [9, 18]. This can be applied to porous titanium metal as well [15]. Several experiments on animal models showed the safety and efficacy of porous bioactive titanium metal as a synthetic bone under load-bearing conditions. Our preclinical study [26] demonstrated that bioactive treatment effectively enhanced the fusion ability of the porous titanium implants in a canine model of spinal interbody fusion.
Instrumented spinal fusion with autologous iliac crest bone grafting (ICBG) is a gold-standard surgical procedure for the treatment of unstable spinal diseases. However, grafts harvested from the iliac crest are still a major source of autologous bone and the harvesting process is associated with graft site morbidities including residual pain, long operative times and significant blood loss [1].
To accelerate the fusion rate and alleviate donor site problems, several effective osteoinductive agents including recombinant human bone morphogenetic protein-2 (rhBMP-2) and osteogenic protein-1 (OP-1/BMP-7) have been introduced and are widely used clinically [3, 20]. Excellent clinical results have been documented, although some serious adverse effects (AEs) have been reported such as osteolysis around the cage implant, massive bleeding and soft tissue swelling [19, 27, 31]. Porous bioactive titanium metal is not only osteoconductive but also has osteoinductive ability without the need for additional osteogenic cells or agents [10, 25]. Although the osteoinductive ability of porous bioactive titanium metal is limited and the actual mechanism has not been clarified, the osteogenesis it induces is believed to guarantee the high osteoconductive ability of this material.
We conducted a clinical trial of porous bioactive titanium metal for lumbar interbody fusion. Here, we report our preliminary results and discuss the safety and efficacy of porous bioactive titanium metal as one of a new generation of synthetic device materials. This trial was based upon extensive experiments in animal models and clinical success in cementless total hip prosthesis using porous bioactive titanium metal [14, 26].
Methods
Preparation of porous implants
Porous titanium metal was manufactured from a mixture of commercially pure titanium powder <45 μm in particle size (Osaka Titanium Tech. Co. Ltd, Osaka, Japan) and ammonium hydrogen carbonate as spacer particle [32]. Sintering was carried out at 1,400°C for 2 h in Argon gas. Three types of implants, 7, 8 and 9 mm thick and 30 mm wide, were prepared for the clinical trial (Fig. 1). To improve the safety of handling during surgery, a thin outer frame was placed around the porous body and sintered. These implants were supplied by Osaka Yakin Co. (Osaka, Japan). Micro-computed tomography (CT) analysis demonstrated that more than 99% of the porous structures were interconnected and more than 80% of pores were connected through channels more than 52 μm in diameter (Fig. 2). The average porosity was 60% and the average pore size was 250 μm.
Fig. 1.

Photograph of porous bioactive titanium device for transforaminal lumbar interbody fusion
Fig. 2.

Micro-computed tomography image showing well-connected internal porous structures
Mechanical properties of the porous titanium implants
The compressive strength of the porous titanium body was measured using a universal testing machine (Model EHF-LV020K1-010, Shimadzu Corp., Kyoto, Japan) at a crosshead speed of 1 mm/min. 0.2% yield compressive strength and Young’s modulus of a typical 60% porous body were 53.0 MPa and 4.2 GPa, respectively. The stiffness was 91.5 kN/m, and this increased to 458.3 kN/m at the outer frame. The porous body combined with the outer frame proved stable against a cyclic load of 10,000 N at 4 Hz for 1,000,000 cycles.
Bioactive surface treatment
The porous implants were treated chemically and thermally to give them a bioactive surface, as described [9, 18]. Briefly, the sintered porous titanium bodies were immersed in 5 M aqueous NaOH solution at 60°C for 24 h, 0.5 mM HCl at 40°C for 24 h, ultrapure water at 40°C for 24 h and then heat-treated at 600°C for 1 h. The homogeneity of the bioactive surface was confirmed by examining the topography and the chemistry of the center and the peripheral parts of several implants using a field emission scanning electron microscope (FE-SEM; Hitachi S-4300, Ibaraki, Japan), an energy-dispersive X-ray microanalyzer (EDX) and X-ray diffractometry (XRD). In vitro apatite-forming ability was confirmed by soaking samples for 3 days in an acellular simulated body fluid (SBF) with ion concentrations (in mM) of Na+ 142.0, K+ 5.0, Mg2+ 1.5, Ca2+ 2.5, Cl− 147.8, HCO3 − 4.2, HPO4 2− 1.0 and SO4 2− 0.5: nearly equal to those of human blood plasma at 36.5°C and prerequisite conditions for generating bioactive materials [17]. The implants were sterilized by 25 kGy γ-radiology exposure before surgical implantation.
Evaluation of implants
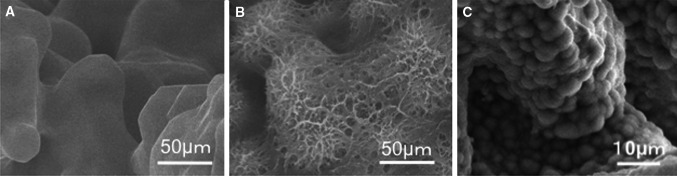
All devices with the same lot number destined for clinical use were analyzed in vitro before implantation. All parameters of mechanical strength including yield compressive strength, elastic modulus and fatigue strength were within an error of <5%. Mechanical testing found no failure of the device, nor any loss of titanium particles. FE-SEM, EDX and XRD studies confirmed the homogeneity of the bioactive surface both centrally and peripherally. After the surface treatment, the whole porous surface was uniformly changed to a bioactive thin TiO2 layer approximately 1 μm thick with sub-micron-sized pores. The walls of the porous body were completely covered with apatite within 3 days of soaking in SBF, indicating that the whole surface of the implant could be rendered bioactive by the chemical and thermal treatments (Fig. 3a–c).
Fig. 3.
Field emission scanning electron microscope (FE-SEM) images showing surface morphological changes to the porous titanium metal. a Before treatment, the surface was smooth. b After chemical and thermal treatment, a thin submicron-sized pore layer was formed on the surface. c Apatite formation on the whole surface of the porous bioactive titanium metal after soaking in simulated body fluid (SBF) for 3 days
Transforaminal lumbar interbody fusion (TLIF) [11]
All surgical procedures were performed by the two senior authors (S.F. and M.T.).
Following a midline skin incision, the lateral aspect of facet joints was exposed through a midline subperiosteal approach or Wiltse’s approach depending on the case. After bilateral pedicle screw placement, the neural foramen was exposed by excision of the ipsilateral facet joint. Disc space preparation with the removal of degenerative disc materials and cartilaginous endplate was performed carefully from the safety triangle zone between the exiting and traversing nerve roots. In the case of concomitant spinal canal stenosis, neural decompression was done using a surgical microscope. The bioactive porous titanium implant was placed into the intervertebral space through the opened safety triangle zone and small local bone chips were packed around the implant as monitoring bone material. Compressive force was applied through the pedicle screws and pre-bent rods were set on the screws bilaterally. The patients were allowed to walk while wearing a hard brace beginning on the first day after surgery.
Patients
This was a prospective clinical case series on five patients (3 men and 2 women) with degenerative unstable lumbar lesion who were eligible for surgical treatment and who were referred to our University Hospital from November 2008 to June 2009. In all cases, the patient and his or her relatives were informed about the benefits and the risks of the implant. Written informed consent was obtained from all patients and/or their relatives, in accordance with protocols approved by our Institutional Ethics Committee and in agreement with the Declaration of Helsinki.
Among the five patients enrolled there were three with degenerative spondylolisthesis and two with isthmic spondylolisthesis. Inclusion criteria for this preliminary clinical trial were symptomatic single-level unstable lumbar disc disease with or without compression of neural elements, which were refractory to adequate conservative treatments for at least 3 months preoperatively. Patients with multilevel diseases, a previously operated spine, osteoporosis, general inflammatory disease or a severe comorbidity such as cardiovascular disease or renal dysfunction were excluded. The average age of the enrolled patients at surgery was 51.6 years (range 36–61 years).
Clinical assessment
A patient self-assessed 100 mm visual analog scale (VAS) (0 mm = no pain, 100 mm = worst pain imaginable) for both low back pain (LBP) and leg pain (LP), the Japanese Orthopaedic Association (JOA) score (Table 1) and its recovery rate [recovery rate = postoperative score − preoperative score/29 (full score) − preoperative score × 100 (%)] were examined before operation and postoperatively. A self-assessed patient’s satisfaction score was examined after the surgery. For subjective assessment of the overall results of surgery, the patient was asked to select from among the options: very satisfied, satisfied, somewhat satisfied, somewhat dissatisfied or dissatisfied. The satisfaction score was recorded as a score at all time points. All patients complained of LBP preoperatively and four complained of concomitant LP. The average preoperative JOA score was 15.8 (range 11–21). The average preoperative VAS values for LBP and LP were 37.6 mm (range 10–50 mm) and 21.4 mm (range 0–60 mm), respectively. An independent expert nurse carried out the assessment of pre- and postoperative VAS and the patient’s satisfaction score. The JOA scores and VAS measures were analyzed statistically using paired t-tests and P < 0.05 was considered statistically significant.
Table 1.
JOA score classifications for low-back pain
| Parameter | JOA score |
|---|---|
| Subjective symptoms | 9 |
| Low-back pain | |
| None | 3 |
| Occasional mild pain | 2 |
| Frequent mild or occasional severe pain | 1 |
| Frequent or continuous severe pain | 0 |
| Leg pain and/or tingling | |
| None | 3 |
| Occasional slight symptoms | 2 |
| Frequent slight or occasional severe symptoms | 1 |
| Frequent or continuous severe symptoms | 0 |
| Gait | |
| Normal | 3 |
| Able to walk >500 m, although it causes pain, tingling, and/or muscle weakness | 2 |
| Unable to walk >500 m due to leg pain, tingling, and/or muscle weakness | 1 |
| Unable to walk >100 m due to leg pain, tingling, and/or muscle weakness | 0 |
| Clinical signs | 6 |
| Straight leg-raising test (including tight hamstrings) | |
| Normal | 2 |
| 30–70° | 1 |
| <30° | 0 |
| Sensory disturbance | |
| None | 2 |
| Slight disturbance (not subjective) | 1 |
| Marked disturbance | 0 |
| Motor disturbance | |
| Normal (Grade 5/5) | 2 |
| Slight weakness (Grade 4/5) | 1 |
| Marked weakness (Garde 0–3/5) | 0 |
| Restriction of ADL | 14 |
| ADL (restriction) | |
| Turning over while lying down | |
| Standing | |
| Washing | |
| Leaning forward | |
| Sitting (~1 h) | |
| Lifting/holding heavy objects | |
| Walking | |
| Urinary bladder function | −6 |
| Normal | 0 |
| Mild dysuria | −3 |
| Severe dysuria (incontinence, urinary retention) | −6 |
JOA Japanese Orthopaedic Association
ADL activities of daily living
For each activity of daily living category severe restriction was accorded a score of 0; moderate restriction, a score of 1; and no restriction, a score of 2
Radiological assessment
Magnetic resonance imaging (MRI), multidetector-row computed tomography (MDCT) and lateral dynamic X-rays were used to assess the neural compression and dynamic situation. Preoperative dynamic lateral X-rays showed marked segmental instability in all five patients. To assess bony union postoperatively, lateral dynamic radiographs were obtained at 3, 6 and 12 months. More than 3° motion on flexion–extension was considered to indicate nonunion. In addition, radiolucent regions around the pedicle screws and the implant were defined as showing nonunion. To evaluate the placement of implant and pedicle screws, bony union and AEs, coronal and sagittal reconstruction views using MDCT were assessed at 1 week and at 1, 3, 6 and 12 months after surgery. Bony union was defined as complete when there was osseous continuity between bony endplate and implant on both the coronal and sagittal MDCT images. Nonunion was defined as the presence of a visible gap between the vertebral endplate and implant, or radiolucency around the pedicle screws. Successful bony union was recorded when the assessments of aforementioned radiological parameters were complete. A change of 3 mm or more of implant migration into the vertebral endplate was defined as significant subsidence. MRI was performed at 1 week and at 1, 3, 6 and 12 months after surgery to assess neural decompression and dural tube extension, any AEs including inflammatory reaction around the implant such as vertebral endplate erosion, Modic change [20] and any fluid collection. Three independent experienced spinal surgeons, each with at least 10 years of experience, did all the radiological assessments. Each patient’s preoperative clinical and radiological data are summarized in Table 2.
Table 2.
Summary of preoperative patient’s demographic data
| Case | Age | Sex | Diagnosis | Level | Symptoms | Pre JOA | Pre VAS (LBP) | Pre VAS (LP) |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | DS | L4/5 | LBP + LP | 21 | 10 | 60 |
| 2 | 36 | M | IS | L5/S | LBP + LP | 12 | 80 | 50 |
| 3 | 51 | F | DS | L4/5 | LBP | 19 | 80 | 0 |
| 4 | 61 | F | DS | L4/5 | LBP + LP | 11 | 60 | 60 |
| 5 | 56 | M | IS | L5/S | LBP + LP | 16 | 50 | 20 |
DS degenerative spondylolisthesis, IS isthmic spondylolisthesis, LP leg pain, LBP low back pain, VAS visual analog scale
Ethical considerations
The study was performed in accordance with the principles of the Declaration of Helsinki and of Good Clinical Practice and was registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN000001448). Approval was obtained from the relevant competent authorities and our institutional Committee of Ethics before the trial began. As clinicians, the authors played a leading role in this new type of clinical trial, which is extremely rare in the development of new medical devices in Japan. We prepared all the protocols of this study by ourselves and were supported by a translational research center in Kyoto University. The independent clinical research coordinator of the translational research center managed all clinical data, which were extracted from each patient’s clinical research form. The endpoints of this clinical trial were achievement of good clinical results, bony union, no serious AEs and avoidance of the need for autologous ICBG.
Results
Clinical results
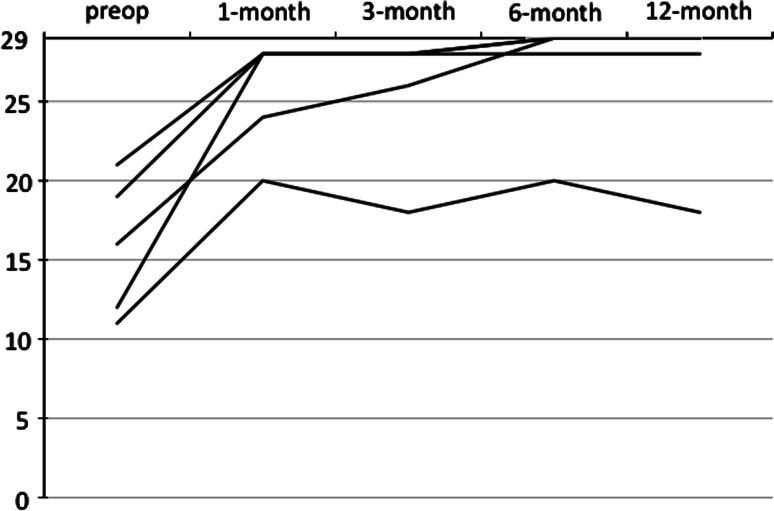
In all five patients, the preoperative LBP and radicular symptoms were resolved immediately after the operation. No surgery-related neurological deficit or wound breakdown was observed in any patient. The mean operating time was 164.6 min (range 154–179 min) and the mean estimated intraoperative blood loss was 192 mL (range 80–310 mL). No patient required transfusion or ICBG. No surgery-related complication was observed. The mean follow-up period was 15.2 months (range 12–19 months). The average postoperative JOA score was 25.6 at 1 month, 25.6 at 3 months, 27 at 6 months and 26.6 at 12 months (range 18–29). The mean recovery rate of the JOA score was 76.6% at 1 month, 77.5% at 3 months, 88.0% at 6 months and 85.8% at 12 months (range 38.9–100%). The postoperative JOA score improved significantly compared with the preoperative score at all times (P = 0.002 at 12 months). The mean VAS was 2 mm at 1 month, 2 mm at 3 months, 6 mm at 6 months and 2 mm at 12 months (range 0–30 mm) for LBP. It was 0 mm at 1 month, 0 mm at 3 months, 4 mm at 6 months and 2 mm at 12 months (range 0–20 mm) for LP. Both VAS measures were significantly improved compared with preoperative scores at all times (at 12 months; LBP P = 0.027; LP P = 0.012). All but one patient satisfied very much through the experiment periods. All clinical parameters showed rapid recovery within 1 month, which indicated a low level of invasiveness and good stabilization of the surgery (Fig. 4).
Fig. 4.
Sequential changes in the Japan Orthopaedic Association (JOA) score of the five cases. The graph indicates a rapid recovery of the patients’ clinical status within 1 month
Radiological results
Dynamic radiological examination showed a solid bony construct without abnormal segmental motion or radiolucency around the implants in all cases after 3 months. No patient exhibited significant implant subsidence during the follow-up period. Immediate postoperative MDCT demonstrated good apposition between the vertebral endplate and implant in all but one case. These findings indicated good anchoring of the porous titanium implant to the surrounding bone. Follow-up MDCT showed good bone ingrown onto the surface of the porous titanium metal without radiolucent line. It also showed remodeling not only of the monitoring bone but also of the surrounding vertebral bone. However, in Case 5, a gap was evident between porous titanium metal and surrounding vertebral endplate on the MDCT image immediately after the operation, because of a poor fit of the device surface with an irregular vertebral endplate. The gap was filled gradually and closed at the final follow-up MDCT (Fig. 5a–c). Because the radiological parameters mentioned above were complete in all cases, bony union was considered to be achieved in all cases by 6 months after the operation. Postoperative MRI scans showed no significant AE such as abnormal fluid collection or apparent change in the Modic sign. In three patients with concomitant spinal canal stenosis, successful neural tissue decompression was also confirmed. The postoperative clinical and radiological results are summarized in Table 3.
Fig. 5.
Sagittal multidetector-row computed tomography (MDCT) images taken immediately postoperatively and at 3 and 12 months for Case 5. The immediate postoperative image (left) shows an apparent gap between the porous titanium metal and vertebral bone. The 3-month image (center) demonstrates bone ingrowth cranial to the porous titanium metal. The 12-month image (right) demonstrates complete gap filling and direct bone bonding to the porous titanium metal
Table 3.
Summary of postoperative patient’s demographic data
| Case | Op. time (min.) | Blood loss (mL) | Post JOA score | JOA score recovery rate (%) | Post VAS (LBP) | Post VAS (LP) | Satisfaction score | ICBG | AEs | Bony union (month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 173 | 140 | 29 | 100 | 0 | 0 | 1 | – | – | 3 |
| 2 | 179 | 310 | 29 | 100 | 0 | 0 | 1 | – | – | 3 |
| 3 | 160 | 80 | 28 | 90 | 0 | 0 | 1 | – | – | 3 |
| 4 | 154 | 228 | 18 | 38.9 | 10 | 10 | 4 | – | – | 6 |
| 5 | 157 | 192 | 29 | 100 | 0 | 0 | 1 | – | – | 3 |
ICBG Iliac crest bone graft, AEs adverse effects
Satisfaction score 1, very satisfied 2, satisfied 3, somewhat satisfied 4, somewhat dissatisfied 5, dissatisfied
JOA score, VAS, and satisfaction score are obtained at 12 months after the surgery
Illustrative case (Case 1)
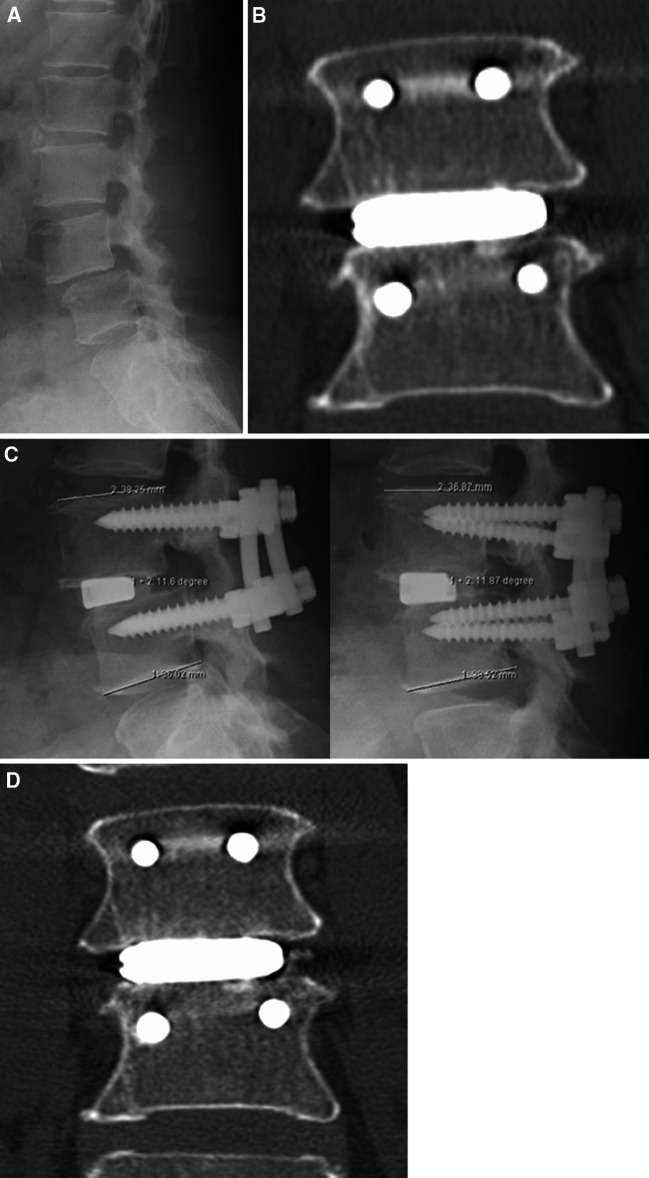
This 54-year-old woman had complained of LBP and intractable bilateral LP for 3 years before surgery. These were refractory to adequate conservative treatment. She also complained of an inability to walk for longer than 10 min, with intermittent claudication. A physical examination demonstrated bilateral dysesthesia on the L5 sensory dermatome. Her preoperative JOA score was 21 points and her self-reported VAS was 10 mm for LBP and 60 mm for LP. X-ray images showed degenerative spondylolisthesis at the L4–5 level with instability (Fig. 6a). Preoperative MR imaging demonstrated severe spinal canal stenosis at the L4–5 level. Transforaminal lumbar interbody fusion and spinal canal decompression using our bioactive titanium was performed. The operating time was 173 min and the estimated intraoperative blood loss was 140 mL. Immediate postoperative coronal imaging using MDCT demonstrated a press fit at the interface between the porous titanium metal and the vertebral endplate (Fig. 6b). Three months after the operation, dynamic X-ray imaging demonstrated no abnormal movement (Fig. 6c). Sagittal imaging using MDCT showed a stable interface without a radiolucent line or any clear zones around the pedicle screws, indicating a successful bony union (Fig. 6d). Postoperative MR imaging showed good neural decompression without any AEs. Her JOA score recovered to 29 points and the VAS score was 0 mm for LBP and 0 mm for LP at the final follow-up.
Fig. 6.
Preoperative and postoperative radiological studies obtained from a 54-year-old woman with degenerative spondylolisthesis at L4–5 (Case 1). a Plain lateral X-ray demonstrating L4 listhesis. b Immediate postoperative MDCT image demonstrating a press fit of the porous titanium metal implant to the vertebral endplate. c Dynamic lateral radiographs at 3 months showing a solid construct without abnormal segmental motion (left flexion; right extension). d Coronal MDCT image demonstrating solid bony union without device subsidence or any radiolucency at 3 months after surgery
Discussion
Here, we report the safety and efficacy of porous bioactive titanium metal for the treatment of unstable lumbar disc disease. All cases showed early bony union by 6 months without autologous ICBG and rapid recovery after the surgery. The patients’ satisfaction and clinical recovery rates were both acceptable.
The use of an interbody fusion cage with autologous bone grafting is a standard procedure for lumbar spinal fusion. However, nonunion, cage subsidence, implant failure and donor site morbidity are still of concern [1]. Porous materials with adequate pore structure and appropriate mechanical properties might represent an alternative to traditional cage implants. Interconnected pores permit tissue ingrowth and thus anchor the prosthesis to the surrounding bone, preventing loosening. This concept also allows a larger support area because no graft space is required and it might be effective for the prevention of implant subsidence. In the current study, significant implant subsidence has not occurred throughout the follow-up periods. Furthermore, if bone bridging can be achieved across the whole implant through the interconnected pores from one vertebra to the other it reduces the risk of implant failure and ensures long-term stability.
The kind of material and its pore characteristics influence the mechanical strength of porous biomaterials. Porous HA implants have good biocompatibility and osteoconductivity, but their mechanical properties are not adequate for load-bearing conditions. The clinical application of such conventional porous materials is limited to non-load-bearing conditions. Therefore, the use of metal to produce porous implants with higher mechanical strength is required. By using titanium metal as a starting material, our device with high porosity and large pore size acquired a high mechanical strength that is adequate for load-bearing conditions. In a previous study, we investigated the relationship between pore structure and bone ingrowth in vivo using several types of porous titanium implants. We concluded that not only high porosity and large pore size but also high interconnectivity of the pores is effective for bone ingrowth and tissue differentiation [23]. Based on this previous study, an optimally structured porous titanium metal was developed and used for this clinical trial. It has 60% porosity, 250 μm average pore size and more than 99% pore interconnectivity.
Another important issue associated with porous metal implants is the difficulty in producing bioactive properties on the inner surfaces of implants using conventional methods such as applying a plasma-sprayed HA coating. In the absence of a bioactive surface, the osteoconductivity of these implants and their capacity to promote fusion is limited. The thickness of a conventional HA coating layer is about 40–50 μm, which cannot be applied to critical supporting structures without changes to surface morphology [5]. Moreover, the conventional HA coating for titanium metal implants has the potential for degradation, absorption and third body wear during long-term implantation, which might be related to its poor clinical results [2, 21]. Our chemical and thermal treatments ensured that bioactive properties were applied to the whole surface of the porous titanium implants without reducing the pore space available for bone ingrowth [15]. Adequate stability of the thin treated layer was confirmed both in vitro and in vivo and might assure the long-term apposition with surrounding bone [9]. This material also offers sufficient resistance to shearing forces during the implantation of treated cementless hip prostheses.
Although titanium metal and its alloys are ‘gold standard’ materials in orthopedics, one of the potential disadvantages of a metal device is a high elastic modulus. The elastic modulus of solid titanium metal is more than 100 GPa and this will lead to stress shielding around the metal device during long-term implantation. To reduce such a mechanical mismatch between the implant and host bone, several types of soft material including polyetheretherketone and carbon have been introduced clinically [6, 28]. Although there are no long-term results as yet, porous titanium metal will reduce stress shielding, because the elastic modulus of this material with 60% porosity is 4.2 GPa, close to the value of human cortical bone and much less than solid metal materials. According to Nachemson’s study, loads to the human lumbar spine are between 1,000 and 3,000 N during most everyday activities, and increases in different body positions give possible values in excess of 3,000 N during significant lifting [22]. Based upon these data, Brantigan suggested that a lumbar interbody fusion construct must bear an immediate postoperative load at the bone-implant interface of at least 2,400 N during activities of daily living [4]. On the other hand, breakage of the carbon cage and dissemination of free carbon particles occurred in one case of implant nonunion [29]. A biomechanical study revealed that the carbon cage fractures at around 5,800–8,800 N and concluded that at least 5,000 N was required for an interbody fusion cage [13]. The fatigue strength of porous titanium metal combined with an outer frame is more than 10,000 N under a repetitive compressive load, so it can be used as a spinal interbody fusion device safely.
Another potential disadvantage of a pure metal device is the difficulty of confirming fusion status radiologically because of its high radiodensity. Usually, bony union is confirmed when the following radiological parameters are complete: visible continuous grafted bone trabeculation, no abnormal movement on dynamic study and no radiolucency around the implants. In the case of metal devices such as a titanium cage, bone trabeculation through the cage is difficult to identify on plain X-ray images. However, definitive diagnoses of bony union have become easier with the introduction of MDCT, especially in the case of porous titanium metal, because the metal content is less than with the solid form, recognition of fusion status such as bone-implant interface and bony trabeculation around the implant is not so difficult on MDCT images. In the current study, two specific radiological findings were evident. The first of these was the anchoring effect between the porous bioactive titanium implant and the surrounding vertebral endplate seen on the images taken immediately after surgery. This could be attributed to the optimally rough surface of the porous bioactive device. The second finding was the gap-filling effect. The radiological evidence of gap filling as shown in Fig. 6 resembles the results of alkali- and heat-treated total hip prostheses. Radiological gaps between alkali- and heat-treated metal shells and the acetabulum were filled within 1 year, which indicated the high osteoconductive ability of bioactive titanium metal [14]. The best feature of porous bioactive titanium metal is that it permits bone ingrowth through the inner pore structure. Although we reported good bone ingrowth to the pores in several studies using animal models, we could not confirm this evidence using noninvasive radiological examinations in the present study.
There are some limitations to this study, including its small sample size, short follow-up period and preliminary nature. Our chemical and thermal treatment has been applied clinically for cementless total hip prostheses after a strict clinical trial, which was approved by the Ministry of Health, Labor and Welfare in Japan. Excellent mid-term (4.8 years) clinical results and early bone apposition were reported [14]. These results are encouraging for the efficacy and safety of this surface treatment on titanium and its alloys. Moreover, TLIF is a promising standard procedure for the treatment of patients with unstable spinal disease. Given these encouraging results, we planned this small clinical trial as much as possible to test the efficacy and safety of porous bioactive titanium metal in a spinal fusion device. Fortunately, there was successful bony union without the need for autologous ICBG in this small series. However, implantation of metal devices has a potency to bring about several late complications such as stress shielding and adjacent segment disease during long-term implantation. Therefore, long-term clinical results are mandatory to reveal the true efficacy and safety of this device.
We consider that porous bioactive titanium metal is superior to other porous metal materials in terms of safety, osteoconductive and osteoinductive abilities, mechanical strength and controllable optimum microstructure [10, 24]. Another advantage of porous bioactive titanium metal is its potency for general purpose medical devices. First, our surface treatment can be applied not only to pure titanium but also to several types of titanium alloys. By changing materials, the mechanical characteristics can be optimized. Second, using our manufacturing technique, the pore structure, mechanical strength and biological characteristics can be controlled depending on the conditions. This material will be valuable not only for spinal fusion but also for reconstructive surgery to the skull, the maxillofacial region and in other orthopedic fields. Moreover, adjustments to the elastic modulus and bioactive abilities promise to produce new generations of devices for the treatment of osteoporotic bone.
Conclusions
We developed porous bioactive titanium device for spinal fusion. The optimal mechanical strength and interconnected structure of porous titanium metal were adjusted to the device. The whole surface of porous titanium was treated chemically and thermally to form the bioactive surface. Clinical trial was successfully performed and early bony union was achieved in all cases without ICBG by 6 months. Two specific findings including an anchoring effect and gap filling were evident radiologically. Although a larger and longer-term follow-up clinical study is mandatory to reach any firm conclusions, we consider this porous bioactive titanium metal is promising material for a spinal fusion device.
Acknowledgments
The authors thank Hisashi Kitagaki, Tsuneo Teraoka, of Osaka Yakin Co., for manufacturing and providing the porous titanium implants. They thank Seiji Yamaguchi, of Chubu University Biomedical Sciences, for treating the material chemically. They thank Shuji Higuchi, Masanori Fukushima, Satoshi Teramukai, Kenichi Yoshimura, Toshinori Murayama, Tomoko Yokota, Erika Hirata and Harue Tada, of Kyoto University’s translational research center, for help in protocol preparation, moderation of the clinical trial and data management. They also thank Takeshi Sakamoto, Makoto Yoshida and Masahiko Miyata for radiological assessments, and Masato Ota for surgical assistance.
This study was supported by a Grant in Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 19200039). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. This manuscript has not been previously published and is not under consideration for publication elsewhere. The first two authors contributed equally to this study and preparation of this manuscript.
References
- 1.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Bloebaum RD, Beeks D, Dorr LD, Savory CG, DuPont J, Hofmann AA. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin Orthop. 1994;298:19–26. [PubMed] [Google Scholar]
- 3.Boden SD, Zdeblick TA, Sandu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 4.Brantigan JW, Cunningham BW, Warden K, McAfee PC, Steffee AD. Compression strength of donor bone for posterior lumbar interbody fusion. Spine. 1993;18:1213–1221. doi: 10.1097/00007632-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 5.De Groot K, Geesink R, Klein CP, Serekian P. Plasma sprayed coatings of hydroxyapatite. J Biomed Mater Res. 1987;21:1375–1381. doi: 10.1002/jbm.820211203. [DOI] [PubMed] [Google Scholar]
- 6.Desogus N, Ennas F, Leuze R, et al. Posterior lumbar interbody fusion with PEEK cages: personal experience with 20 patients. J Neurosurg Sci. 2005;49:137–141. [PubMed] [Google Scholar]
- 7.Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287–2303. doi: 10.1016/S0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 8.Fujibayashi S, Shikata J, Tanaka C, Matsushita M, Nakamura T. Lumbar posterolateral fusion with biphasic calcium phosphate ceramic. J Spinal Disord. 2001;14:214–221. doi: 10.1097/00002517-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fujibayashi S, Nakamura T, Nishiguchi S, Tamura J, Uchida M, Kim HM, et al. Bioactive titanium: effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J Biomed Mater Res. 2001;56:562–570. doi: 10.1002/1097-4636(20010915)56:4<562::AID-JBM1128>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25:443–450. doi: 10.1016/S0142-9612(03)00551-9. [DOI] [PubMed] [Google Scholar]
- 11.Fujibayashi S, Neo M, Takemoto M, Ota M, Nakamura T. Paraspinal-approach transforaminal lumbar interbody fusion for the treatment of lumbar foraminal stenosis. J Neurosurg Spine. 2010;13:500–508. doi: 10.3171/2010.4.SPINE09691. [DOI] [PubMed] [Google Scholar]
- 12.Hench LL. Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res. 1998;41:511–518. doi: 10.1002/(SICI)1097-4636(19980915)41:4<511::AID-JBM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Jost B, Cripton PA, Lund T, Oxland TR, Lippuner K, Jaeger P, et al. Compressive strength of interbody cages in lumbar spine: the effect of cage shape, posterior instrumentation and bone density. Eur Spine J. 1998;7:132–141. doi: 10.1007/s005860050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawanabe K, Ise K, Goto K, Akiyama H, Nakamura T, Kaneuji A, et al. A new cementless total hip arthroplasty with bioactive titanium porous-coating by alkaline and heat treatment: average 4.8-year results. J Biomed Mater Res Part B: Appl Biomater. 2009;90B:476–481. doi: 10.1002/jbm.b.31309. [DOI] [PubMed] [Google Scholar]
- 15.Kim HM, Kokubo T, Fujibayashi S, Nishiguchi S, Nakamura T. Bioactive macroporous titanium surface layer on titanium substrate. J Biomed Mater Res. 2000;52:553–557. doi: 10.1002/1097-4636(20001205)52:3<553::AID-JBM14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12:155–163. doi: 10.1016/0142-9612(91)90194-F. [DOI] [PubMed] [Google Scholar]
- 17.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 18.Kokubo T, Miyaji F, Kim HM, Nakamura T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Ceram Soc. 1996;79:1127–1129. doi: 10.1111/j.1151-2916.1996.tb08561.x. [DOI] [Google Scholar]
- 19.McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2) J Spinal Disord Tech. 2006;19:483–486. doi: 10.1097/01.bsd.0000211231.83716.4b. [DOI] [PubMed] [Google Scholar]
- 20.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 21.Morscher EW, Hefti A, Aebi U. Severe osteolysis after third body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg Br. 1998;80:267–272. doi: 10.1302/0301-620X.80B2.8316. [DOI] [PubMed] [Google Scholar]
- 22.Nachemson AL. Disc pressure measurements. Spine. 1981;6:93–97. doi: 10.1097/00007632-198101000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki B, Takemoto M, Fujibayashi S, Neo M, Kokubo T, Nakamura T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006;27:5892–5900. doi: 10.1016/j.biomaterials.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Takemoto M, Fujibayashi S, Neo M, Suzuki J, Kokubo T, Nakamura T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials. 2005;26:6014–6023. doi: 10.1016/j.biomaterials.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto M, Fujibayashi S, Neo M, Suzuki J, Matsushita T, Kokubo T, Nakamura T. Osteoinductive porous titanium implants: effect of sodium removal by dilute HCl treatment. Biomaterials. 2006;27:2682–2691. doi: 10.1016/j.biomaterials.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Takemoto M, Fujibayashi S, Neo M, So K, Akiyama N, Matsushita T, et al. A porous bioactive titanium implant for spinal interbody fusion: an experimental study using a canine model. J Neurosurg Spine. 2007;7:435–443. doi: 10.3171/SPI-07/10/435. [DOI] [PubMed] [Google Scholar]
- 27.Toth JM, Boden SD, Burkus JK MD, Badura JM, Peckham SM, McKay WF. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine. 2009;34:539–550. doi: 10.1097/BRS.0b013e3181952695. [DOI] [PubMed] [Google Scholar]
- 28.Tullberg T, Brandt B, Rydberg J, Fritzell P. Fusion rate after posterior lumbar interbody fusion with carbon fiber implant: 1-year follow-up of 51 patients. Eur Spine J. 1996;5:178–182. doi: 10.1007/BF00395510. [DOI] [PubMed] [Google Scholar]
- 29.Tullberg T. Failure of a carbon fiber implant: a case report. Spine. 1998;23:1804–1806. doi: 10.1097/00007632-199808150-00016. [DOI] [PubMed] [Google Scholar]
- 30.Vaccaro AR, Lawrence JP, Patel T, Katz LD, Anderson DG, Fischgrund JS, et al. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis. Spine. 2008;33:2850–2862. doi: 10.1097/BRS.0b013e31818a314d. [DOI] [PubMed] [Google Scholar]
- 31.Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557–562. doi: 10.1097/BSD.0b013e31815ea897. [DOI] [PubMed] [Google Scholar]
- 32.Wen CE, Mabuchi M, Yamada Y, Shimojima K, Chino Y, Asahina T. Processing of biocompatible porous Ti and Mg. Scripta Mater. 2001;45:1147–1153. doi: 10.1016/S1359-6462(01)01132-0. [DOI] [Google Scholar]