Abstract
The aim of the current study was to evaluate changes in lumbar kinematics after lumbar monosegmental instrumented surgery with rigid fusion and dynamic non-fusion stabilization. A total of 77 lumbar spinal stenosis patients with L4 degenerative spondylolisthesis underwent L4–5 monosegmental posterior instrumented surgery. Of these, 36 patients were treated with rigid fusion (transforaminal lumbar interbody fusion) and 41 with dynamic stabilization [segmental spinal correction system (SSCS)]. Lumbar kinematics was evaluated with functional radiographs preoperatively and at final follow-up postoperatively. We defined the contribution of each segmental mobility to the total lumbar mobility as the percent segmental mobility [(sagittal angular motion of each segment in degrees)/(total sagittal angular motion in degrees) × 100]. Magnetic resonance imaging was performed on all patients preoperatively and at final follow-up postoperatively. The discs were classified into five grades based on the previously reported system. We defined the progress of disc degeneration as (grade at final follow-up) − (grade at preoperatively). No significant kinematical differences were shown at any of the lumbar segments preoperatively; however, significant differences were observed at the L2–3, L4–5, and L5–S1 segments postoperatively between the groups. At final follow-up, all of the lumbar segments with rigid fusion demonstrated significantly greater disc degeneration than those with dynamic stabilization. Our results suggest that the SSCS preserved 14% of the kinematical operations at the instrumented segment. The SSCS may prevent excessive effects on adjacent segmental kinematics and may prevent the incidence of adjacent segment disorder.
Keywords: Adjacent segment disorder, Dynamic non-fusion stabilization, Rigid fusion, Segmental spinal correction system (SSCS), Lumbar segmental mobility
Introduction
The lumbosacral and lumbar spine regions are those most often affected by degenerative disorders. These disorders lead to instability of the motion segments of the lumbar spine. Recently, spinal fusions using instrumentation, i.e., pedicle screw–rod system, have become widespread and accepted treatments to stabilize unstable motion segments of the spine. However, reducing the number of mobile lumbar segments may alter the biomechanical behavior of adjacent motion segments. Several experiments have shown increased mobility at the adjacent level of a fusion site [1–7]. Shono et al. [5] demonstrated that hypermobility at the adjacent levels was proportional to the length and rigidity of the instrumented constructs. The hypermobile level adjacent to a fusion site may be at an increased risk for lumbar intervertebral disc degeneration and is cited as a cause for adjacent segment disorder [3–5, 8–11]. Ghiselli et al. [8] demonstrated that postoperative symptomatic degeneration at an adjacent segment after rigid fusion was found in 16.5% after 5 years and 36.1% after 10 years. However, these experimental studies evaluated lumbar segmental mobility by means of sagittal segmental Cobb angle. Some factors, such as low back pain or leg pain, due to the dynamic motion of the spine, may influence the dynamic mobility of the lumbar spine for in vivo clinical study. We thought that more accurate kinematic evaluation of lumbar segmental motion might be allowed by the contribution of each lumbar segmental mobility to the total lumbar mobility, rather than the angular mobility of each segment.
The segmental spinal correction system (SSCS) (ulrich Gmbh & Co., KG, Ulm, Germany) is one of the dynamic non-fusion pedicle screw–rod systems used for stabilization of the lumbar spine. The hinged pedicle screw, provides the load to be shared between the implant and the vertebral column, provides high stability in relation to the rotational forces [12, 13]. Recently, several experiments have been devised to measure the positive effects of these dynamic stabilization techniques [10–21]. It has been proposed that non-fusion motion preservation techniques may prevent accelerated adjacent segment degeneration because of the protective effect of the persisting segmental motion. However, biomechanical and kinematical effects on instrumented and adjacent segments are still a matter of discussion. The aims of the current study were to evaluate changes in lumbar kinematics after lumbar monosegmental instrumented surgery with rigid fusion and dynamic non-fusion stabilization and to discuss the effects of these kinematic changes on the adjacent segments.
Materials and methods
Study population
A total of 77 lumbar spinal canal stenosis patients (41 men and 36 women) with L4 degenerative spondylolisthesis (less than grade I in Meyerding four-grade classification [22]) were included in the study. Their average age was 61.19 years (range 21–82 years). All the patients had undergone L4–5 monosegmental posterior decompression and instrumented surgery with in situ internal pedicle screw–rod fixation during intraoperative prone position by two senior spine surgeons, and had a minimum follow-up of 24 months postoperatively (average 36.04 months; range 24–51 months).
Thirty-six patients (17 men and 19 women) with suspected foraminal stenosis showing reduction of fat shade at the intraforaminal region on parasagittal magnetic resonance imaging (MRI) images were treated with rigid fusion by means of unilateral transforaminal lumbar interbody fusion (TLIF) using local bone graft (average age of 63.0 years, average follow-up of 35.92 months), and 41 patients (24 men and 17 women) without suspected foraminal stenosis on parasagittal MRI images were treated with dynamic non-fusion stabilization by means of the SSCS (average age of 59.61 years, average follow-up of 36.15 months).
The following subjects were excluded from this study: patients who were re-operated for thoracolumbar regions, those showing failure of disc function at L4–5 segment (disc height loss) or at multiple segments (i.e., compression fracture in lumbar spine and severe degenerative scoliosis or kyphosis). One patient treated with dynamic non-fusion stabilization had developed ossification of the anterior longitudinal ligament bridged across the L4–5 segment during the follow-up period. This subject was also excluded from the study.
The details of this study were explained to the patients after obtaining their informed consent in advance. The experiment was performed only with those who gave their consent.
Clinical assessments
The clinical assessments were performed on the basis of the Japanese Orthopaedics Association (JOA) score [23] both preoperatively and at final follow-up postoperatively. The surgical outcomes of these patients were represented by the JOA score recovery ratio as calculated by the formula of Hirabayashi et al. [24].
Assessments of lumbar kinematics: radiographic analysis
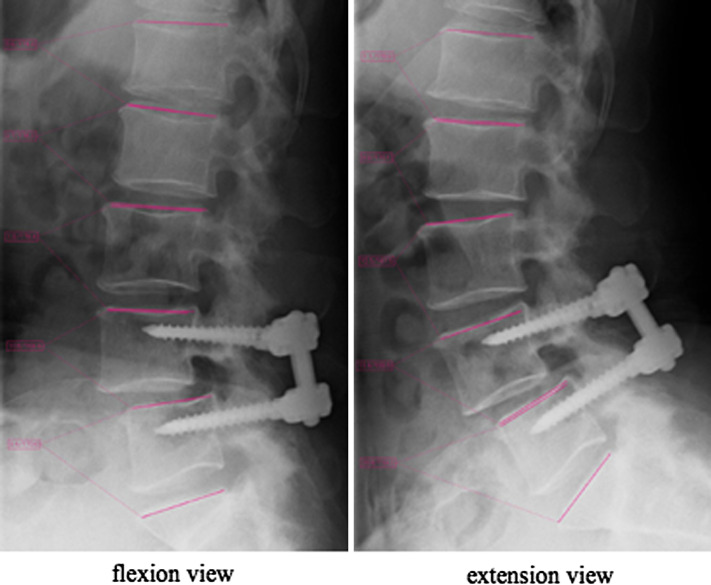
For all patients, we obtained plain radiographs (anterior-posterior and lateral standing) and functional radiographs with flexion and extension views both preoperatively and at final follow-up postoperatively. The sagittal angular motion through lumbar spine flexion to extension was measured with the Cobb technique for each segment at five lumbosacral segments L1–2, L2–3, L3–4, L4–5, and L5–S1 (angle between each upper vertebral endplate) (Fig. 1) using FORZ system (Excel Creates, Inc., Japan). We defined the total sagittal motion of the lumbar spine as the absolute total of the individual sagittal angular motions (L1–2 + L2–3 + L3–4 + L4–5 + L5–S1) in degrees, and the contribution of each segment’s mobility to the total angular mobility of the lumbar spine was defined as percent segmental mobility (%) [(sagittal angular motion of each segment in degrees)/(total sagittal angular motion in degrees) × 100].
Fig. 1.
The sagittal angular motion between each adjacent upper vertebral endplate through lumbar spine flexion to extension was measured with the Cobb technique for each segment at five lumbosacral segments (L1–2, L2–3, L3–4, L4–5, and L5–S1)
Assessments of adjacent segments disorder: magnetic resonance imaging analysis
MRI of the lumbar spine was performed for all patients preoperatively and at final follow-up postoperatively. We used a comprehensive grading system for lumbar intervertebral disc degeneration that was previously reported and was based on the degenerative change in the functional spinal unit [25]. Accordingly, T2-weighted sagittal images of 308 lumbar intervertebral discs (L1–2, L2–3, L3–4, and L5–S1) of 77 subjects were classified into 5 grades (Table 1) by 3 independent blinded observers, and they were judged eligible for inclusion in the study. We defined the progress of intervertebral disc degeneration as (grade at final follow-up postoperatively) − (grade at preoperatively).
Table 1.
A comprehensive grading system for lumbar disc degeneration
| Grade | Structure | Distinction of nucleus and anulus | Signal intensity | Height of intervertebral disc |
|---|---|---|---|---|
| I | Homogeneous, bright wight | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| II | Inhomogeneous with or without horizontal bands | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| III | Inhomogeneous, gray | Unclear | Intermediate | Normal to slightly decreased |
| IV | Inhomogeneous, gray to black | Lost | Intermediate to hypointense | Normal to moderately decreased |
| V | Inhomogeneous, black | Lost | Hypointense | Collapsed disc space |
Cited from Pfirrmann et al. [25]
Statistical analysis
The computer software package StatMate III (ATMS Co., Ltd, Tokyo, Japan) was used. A kappa (κ) statistic was calculated as a measure of interobserver reliability of the MRI grading for the three independent blinded observers. Mann–Whitney U test was used for statistical analyses. A p value of less than 0.05 was considered statistically significant.
Results
Clinical findings
Instrumentation failure was not seen in any of the subjects throughout the follow-up period. There were no significant differences in age and follow-up period between the two groups of rigid fusion and dynamic stabilization.
The average values of the JOA score and recovery ratio are shown in Table 2. There were no significant differences in the JOA score (preoperatively and at final follow-up postoperatively) and the JOA recovery ratio between the rigid fusion and dynamic stabilization groups. However, the JOA recovery ratio for the dynamic stabilization group tended to show a higher value when compared with the rigid fusion group.
Table 2.
The JOA score and recovery ratio
| Pre-JOA score | Final-JOA score | Recovery ratio | |
|---|---|---|---|
| Rigid fusion | 14.25 ± 4.31 | 23.56 ± 3.38 | 61.05 ± 26.08 |
| Dynamic stability | 13.56 ± 4.47 | 24.83 ± 3.06 | 70.61 ± 22.64 |
Compared between rigid fusion and dynamic stabilization † p < 0.05, †† p < 0.01, ††† p < 0.001
Lumbar kinematics
The average values of percent segmental mobility are shown in Table 3. When preoperative and final follow-up values were compared for the rigid fusion group, significant differences were observed at the L4–5 (fused segment), L3–4, and L5–S1 segments (p < 0.001, p < 0.01, and p < 0.05, respectively). When preoperative and final follow-up values were compared for the dynamic stabilization group, significant differences were observed at the L4–5 (stabilized segment) and L3–4 segments (p < 0.001, and p < 0.01, respectively). When preoperative values of the rigid fusion and dynamic stabilization groups were compared, there were no significant differences in any of the percent segmental mobility. However, at final follow-up postoperatively, significant differences were observed at the L2–3, L4–5, and L5–S1 segments (p < 0.01, p < 0.001 and p < 0.05, respectively).
Table 3.
The percent segmental mobility (%)
| L1–2 | L2–3 | L3–4 | L4–5 | L5–S1 | |
|---|---|---|---|---|---|
| Rigid fusion | |||||
| Pre-op | 13.9 ± 6.99 | 17.97 ± 10.18 | 13.21 ± 8.71 | 25.11 ± 11.9 | 29.81 ± 15.95 |
| Final | 15.03 ± 9.04 | 22.23 ± 11.59 | 22.87 ± 14.32** | 0*** | 39.87 ± 18.27* |
| Dynamic stabilization | |||||
| Pre-op | 11.56 ± 7.53 | 15.32 ± 9.81 | 14.81 ± 10.29 | 24.14 ± 10.07 | 34.17 ± 14.99 |
| Final | 15.2 ± 9.74 | 15.2 ± 9.87†† | 23.01 ± 11.47** | 14.03 ± 8.68***, ††† | 32.56 ± 16.1† |
Compared between preoperatively and final follow-up postoperatively * p < 0.05, ** p < 0.01, ***p < 0.001
Compared between rigid fusion and dynamic stabilization † p < 0.05, †† p < 0.01, ††† p < 0.001
Adjacent segments disc degeneration
Consistent agreement (κ = 0.874) was noted among the three independent observers, who graded the MR images. The average values of preoperative lumbar intervertebral disc degeneration grades and lumbar intervertebral disc degenerative changes are shown in Table 4. When preoperative lumbar intervertebral disc degeneration grades of the rigid fusion and dynamic stabilization groups were compared, there were no significant differences in any of the adjacent segments. While all of the lumbar segments in the rigid fusion group demonstrated significantly greater degenerative changes than those in the dynamic stabilization group at final follow-up postoperatively (p < 0.05 at L1–2, p < 0.001 at L2–3, and p < 0.05 at L3–4, and p < 0.05 at L5–S1 segment).
Table 4.
The adjacent segment disc degeneration
| L1–2 | L2–3 | L3–4 | L5–S1 | |
|---|---|---|---|---|
| Rigid fusion | ||||
| Pre-op grades | 2.14 ± 0.83 | 2.33 ± 0.93 | 2.42 ± 0.84 | 2.53 ± 0.84 |
| Progress | 0.19 ± 0.4 | 0.36 ± 0.49 | 0.31 ± 0.47 | 0.19 ± 0.4 |
| Dynamic stabilization | ||||
| Pre-op grades | 2.12 ± 0.9 | 2.49 ± 0.87 | 2.56 ± 0.78 | 2.78 ± 0.99 |
| Progress | 0.02 ± 0.16† | 0.02 ± 0.16††† | 0.12 ± 0.33† | 0.05 ± 0.22† |
Compared preoperative disc degeneration grades between rigid fusion and dynamic stabilization * p < 0.05, ** p < 0.01, *** p < 0.001
Compared progress of disc degeneration between rigid fusion and dynamic stabilization †p < 0.05, †† p < 0.01, ††† p < 0.001
Discussion
The dynamic stabilization of the lumbar spine is a relatively new conceptualized system. It is a non-fusion stabilization device that unloads the intervertebral disc without the complete loss of motion at the treated motion segment. Several dynamic non-fusion stabilization systems have been developed for the treatment of degenerative lumbar instability to preserve kinematic operations of the instrumented segment. Kanayama et al. [18], in their minimum 10-year follow-up study of Graf ligamentoplasty, reported that lordosis of the operative segment was maintained in 90% and segmental motion was preserved in 70% of the patients, and the clinical outcome was satisfactory. However, Hadlow et al. [26] reported that Graf ligamentoplasty achieve a worse surgical outcome and a significantly higher revision rate was shown rather than in the posterolateral fusion with pedicle screw instrumentations. Moreover, Rigby et al. [27] reported, according to mid-term clinical results, that complications occurred in 23.5% of patients, and additional fusion surgery was required in 13.7%. In our unpublished data, from a long-term (minimum 10-year follow-up) review of 68 patients who underwent Graf ligamentoplasty, 35.3% of patients showed adjacent segment disorder, and 7.4% required additional fusion surgery, including 2.9% at the instrumented segment. Moreover, several experimental studies have been performed using the Dynesys, another dynamic stabilization device. Most of these studies reported sufficiently satisfactory clinical outcomes with the Dynesys. However, Schaeren et al. [28] reported in their 4-year follow-up study that 47% of the patients showed some degeneration at adjacent segments. Schnake et al. [20] also demonstrated 29% adjacent segmental disorder at 2-year follow-ups. In addition, Kumar et al. [15] reported that significant intervertebral disc degeneration (56%) at the instrumented segment was observed in the MRI findings, and the anterior disc height was significantly reduced. Schmoelz et al. [29] found that the Dynesys is comparable to internal fixation in restricting spinal flexion, but allows more extension and lateral bending. These systems do not have high stability in relation to the rotational or lateral bending forces.
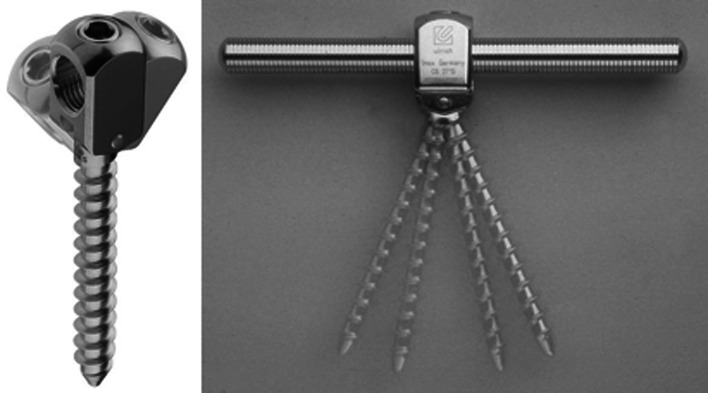
The SSCS provides the load to be shared between the implant and the vertebral column by a hinged screw, allows for high stability in relation to the rotational or lateral bending forces, and preserves segmental motion only in a sagittal direction (Fig. 2) [12, 13].
Fig. 2.
The hinged pedicle screw provides the load to be shared between the implant and the vertebral column. It allows high stability in relation to the rotational forces and preserves segmental motion only in a sagittal direction
In our results, the L4–5 segment completely lost segmental mobility by rigid fusion. The loss of segmental mobility at the L4–5 segment might affect the biomechanical behavior of the remaining adjacent lumbar segments. Cunningham et al. [30] demonstrated in their in vitro experiment that lumbar intradiscal pressure at the cranial adjacent segment to the instrumented segment significantly increased with axial loading and anterior flexion stresses. Increasing mobility at the adjacent segments may lead to increasing mechanical stresses on all the adjacent lumbar segments. Consequently, lumbar intervertebral disc degeneration at the adjacent lumbar segments may deteriorate with increasing mechanical stresses postoperatively. With respect to SSCS, segmental mobility at the L4–5 segment was significantly reduced, but 3.38 ± 2.5° of segmental mobility, 14% of the kinematic operations for lumbar mobility, was preserved. Although the cranial segmental mobility significantly increased after surgery, the effects on the other segments were less than those with rigid fusion. With TLIF, we found intervertebral disc degeneration in 19.4% of patients (7/36) at the L1–2, 36.6% (13/36) at the L2–3, 30.6% (11/36) at the L3–4, and 19.4% (7/36) at the L5–S1 segments. On the other hand, with the SSCS, 2.4% of patients (1/41) at the L1–2, 2.4% (1/41) at the L2–3, 12.2% (5/41) at the L3–4, and 4.9% (2/41) at the L5–S1 segments demonstrated increased disc degeneration. All of the adjacent segments with TLIF and the cranial adjacent segment (L3–4) with the SSCS might be affected biomechanically by kinematical changes of the lumbar spine. We hypothesized that the disc degeneration at the other segments with the SSCS might be due to natural disease progression, rather than an effect of stabilization. Our results suggest that the surgical management of lumbar spinal instability with the SSCS may prevent excessive kinematical effects on the adjacent segments, and it may also prevent the incidences of adjacent segmental disorder.
On comparing the clinical outcomes between the two groups, we found that there were no significant differences in the JOA score or the JOA recovery ratio. Both groups demonstrated sufficiently satisfactory clinical outcomes. However, the outcomes with TLIF tended to be lower than those with the SSCS. We hypothesized that the clinical outcomes might deteriorate gradually though disc degeneration may progress with rigid fusion.
However, some issues remain unanswered even in the current study. We discussed the effects of instrumented surgery on adjacent segments only kinematically, not biomechanically, and long-term effects with the SSCS are still unknown. Therefore, further research using larger patient populations and long-term follow-ups may help to resolve the unclear results obtained in this study. Moreover, the details of using the SSCS for protecting adjacent segment disorder can be investigated further.
References
- 1.Panjabi M, Henderson G, Abjornson C, et al. Multidirectional testing of one- and two-level ProDisc-L versus simulated fusions. Spine. 2007;32:1311–1319. doi: 10.1097/BRS.0b013e318059af6f. [DOI] [PubMed] [Google Scholar]
- 2.Panjabi M, Malcolmson G, Teng E, et al. Hybrid testing of lumbar CHARITE discs versus fusions. Spine. 2007;32:959–966. doi: 10.1097/01.brs.0000260792.13893.88. [DOI] [PubMed] [Google Scholar]
- 3.Okuda S, Iwasaki M, Miyauchi A, et al. Risk factors for adjacent segment degeneration after PLIF. Spine. 2004;29:1535–1540. doi: 10.1097/01.BRS.0000131417.93637.9D. [DOI] [PubMed] [Google Scholar]
- 4.Throckmorton T, Hilibrand A, Mencio G, et al. The impact of the adjacent level disc degeneration on health status outcomes following lumbar fusion. Spine. 2003;28:2546–2550. doi: 10.1097/01.BRS.0000092340.24070.F3. [DOI] [PubMed] [Google Scholar]
- 5.Shono Y, Kaneda K, Abumi K, et al. Stability of posterior spinal instrumentation and its effects on adjacent motion segments in the lumbosacral spine. Spine. 1998;23:1550–1558. doi: 10.1097/00007632-199807150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Chow DH, Luk KD, Evans JH, et al. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine. 1996;21:549–555. doi: 10.1097/00007632-199603010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Esses SI, Doherty BJ, Crawford MJ, et al. Kinematics evaluation of lumbar fusion techniques. Spine. 1996;21:676–684. doi: 10.1097/00007632-199603150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86:1497–1503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 9.McAffee PC, Farey ID, Suttelin CE, et al. 1989 Volvo award in basic science. Device-related osteoporosis with spinal instrumentation. Spine. 1989;14:919–926. doi: 10.1097/00007632-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cakir B, Carazzo C, Schmidt R, et al. Adjacent segment mobility after rigid and semirigid instrumentation of the lumbar spine. Spine. 2009;34:1287–1291. doi: 10.1097/BRS.0b013e3181a136ab. [DOI] [PubMed] [Google Scholar]
- 11.Korovessis P, Papazisis Z, Koureas G, et al. Rigid, semirigid versus dynamic instrumentation for degenerative lumbar spinal stenosis. A correlative radiological and clinical analysis of short-term results. Spine. 2004;29:735–742. doi: 10.1097/01.BRS.0000112072.83196.0F. [DOI] [PubMed] [Google Scholar]
- 12.Strempel AV, Neekritz A, Muelenaere PD, et al. Dynamic versus rigid spinal implants. In: Gunzburg R, Szpalski M, et al., editors. Lumbar Spinal Stenosis. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 275–285. [Google Scholar]
- 13.Strempel AV, Stoss C, Moosmann D, et al. Non-fusion stabilization of the lumbar spine in the case of degenerative diseases with a dynamic pedicle screw rod. Coluna/Columna. 2006;5:27–34. [Google Scholar]
- 14.Wilke HJ, Heuer F, Schmidt H. Prospective design delineation and subsequent in vitro evaluation of a new posterior dynamic stabilization system. Spine. 2009;34:255–261. doi: 10.1097/BRS.0b013e3181920e9c. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Beastall J, Hughes J, et al. Disc changes in the bridged and adjacent segments after dynesys dynamic stabilization system after two years. Spine. 2008;33:2909–2914. doi: 10.1097/BRS.0b013e31818bdca7. [DOI] [PubMed] [Google Scholar]
- 16.Niosi CA, Wilson DC, Zhu Q, et al. The effect of dynamic posterior stabilization on facet joint contact forces. An in vitro investigation. Spine. 2008;33:19–26. doi: 10.1097/BRS.0b013e31815e7f76. [DOI] [PubMed] [Google Scholar]
- 17.Cheng BC, Gordon J, Cheng J, et al. Immediate biomechanical effects of lumbar posterior dynamic stabilization above a circumferential fusion. Spine. 2007;32:2551–2557. doi: 10.1097/BRS.0b013e318158cdbe. [DOI] [PubMed] [Google Scholar]
- 18.Kanayama M, Hashimoto T, Shigenobu K, et al. A minimum 10-year follow-up of posterior dynamic stabilization using Graf artificial ligament. Spine. 2007;32:1992–1996. doi: 10.1097/BRS.0b013e318133faae. [DOI] [PubMed] [Google Scholar]
- 19.Beastall J, Karadimas E, Siddiqui M, et al. The Dynesys lumbar spinal stabilization system. A preliminary report on positional magnetic resonance imaging findings. Spine. 2007;32:685–690. doi: 10.1097/01.brs.0000257578.44134.fb. [DOI] [PubMed] [Google Scholar]
- 20.Schnake KJ, Schaeren S, Jeanneret B, et al. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolithesis. Spine. 2006;31:442–449. doi: 10.1097/01.brs.0000200092.49001.6e. [DOI] [PubMed] [Google Scholar]
- 21.Grob D, Benini A, Junge A, et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine. Spine. 2005;30:324–331. doi: 10.1097/01.brs.0000152584.46266.25. [DOI] [PubMed] [Google Scholar]
- 22.Meyerding HW. Spondylolisthesis. J Bone Joint Surg Am. 1931;13:39–48. [Google Scholar]
- 23.Izumida S, Inoue S. Japanese Orthopedic Association Assessment of surgical treatment of low back pain (in Japanese) J Jpn Orthop Assoc. 1986;60:391–394. [Google Scholar]
- 24.Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354–365. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Hadlow SV, Fagan AB, Hillier TM, et al. The Graf ligamentoplasty procedure: comparison with posterolateral fusion in the management of low back pain. Spine. 1998;23:1172–1179. doi: 10.1097/00007632-199805150-00020. [DOI] [PubMed] [Google Scholar]
- 27.Rigby MC, Selmon GP, Foy MA, et al. Graf ligament stabilization: mid- to long-term follw-up. Eur Spine J. 2001;10:234–236. doi: 10.1007/s005860100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine. 2008;33:E636–E642. doi: 10.1097/BRS.0b013e31817d2435. [DOI] [PubMed] [Google Scholar]
- 29.Schmoelz W, Huber JF, Nydegger T, et al. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech. 2003;16:418–423. doi: 10.1097/00024720-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham BW, Kotani Y, McNulty PS, et al. The effect of spinal destabilization and instrumentation on lumbar intradiscal pressure. An in vitro biomechanical analysis. Spine. 1997;22:2655–2663. doi: 10.1097/00007632-199711150-00014. [DOI] [PubMed] [Google Scholar]