Abstract
Blunt traumatic vertebral injury (TVAI) is frequently associated with head and neck injury and is being detected with increasing frequency due to improved imaging of the trauma patient. In a few cases, it can lead to potentially fatal posterior circulation ischaemia There is debate in the literature regarding whether TVAI should be actively screened for and, if so, how. Management of TVAI may be conservative, medical (antiplatelet agents or anticoagulation), endovascular or open surgery. We review the literature concerning the mechanisms and presentation of TVAI following blunt injury and the current screening recommendations. Management strategies proposed are based on the radiological grade and clinical severity of TVAI, where high-grade symptomatic injuries and high-grade injuries in patients where anticoagulation is contraindicated are treated endovascularly and asymptomatic or low-grade injuries are managed with anticoagulation where it is not contraindicated. Follow-up is via CT angiography to assess for resolution of the injury.
Keywords: Traumatic, Vertebral, Artery, Management
Introduction
Vertebral artery injury can be spontaneous or traumatic. Traumatic vertebral artery injury (TVAI) presents a clinical challenge since it is hard to detect, has a diverse presentation and there are no widely accepted guidelines on diagnosis and management. Most evidence available on TVAI is class 3, based on case series from individual institutions. Spontaneous vertebral artery dissection is well described and typically managed by anticoagulation [1]; however, traumatic dissection is a more common cause of posterior cerebral circulation stroke in patients under the age of 45 years than spontaneous dissection [2]. TVAI, although frequently asymptomatic [1], can have disastrous consequences of basilar territory infarction and death. TVAI may occur following blunt or penetrating trauma; this review considers blunt TVAI. We address the current evidence regarding screening and management strategies of TVAI.
Search strategy
We conducted a review of the literature trying to identify all articles related to traumatic vertebral artery injury. The following electronic databases were searched: PubMed, Web of Knowledge, EMBASE, Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials. All studies form 1 January 1950 till 31 December 2010 were considered. As we did not have stringent inclusion or exclusion criteria with regard to levels of evidence, we used a broad search strategy with the terms: “vertebral artery” AND (injury OR trauma OR dissection). We received 2,801 articles from PubMed, 305 from Web of Knowledge, 584 from EMBASE and 3 from Cochrane library. Only studies reported in English, regarding human subjects were included.
Three reviewers (MC, RD, NH) independently evaluated the abstract of each full-text article and determined the affinity of those studies to the purpose of our review with regard to incidence, diagnosis, management and complications of traumatic vertebral artery injury. Discrepancies between reviewers were resolved by consensus, data sets were merged and duplicates removed. Our referenced articles found in Tables 1, 2, 3, 4, 5 provide the evidence base for our recommendations.
Table 1.
The Denver criteria showing risk factors for blunt vertebral and carotid injuries and the Denver Radiological Criteria for Cerebrovascular Injury
| Denver screening criteria for blunt cerebrovascular injury (Cothren et al.) [32] | |
| Any cervical spine fracture | |
| Unexplained neurological deficit incongruous with imaging | |
| Basilar cranial fracture into carotid canal | |
| Le Fort II or III fracture | |
| Cervical haematoma | |
| Horner syndrome | |
| Cervical bruit | |
| Ischaemic stroke | |
| Head injury with Glasgow Coma Scale score<6 | |
| Hanging with anoxic injury | |
| Denver radiological grading scale of blunt cerebrovascular injury (Cothren et al.) [32] | |
| Grade I | Irregularity of vessel wall or dissection/intramural haematoma with <25% stenosis |
| Grade II | Intramural thrombus or raised intimal flap or dissection/intramural haematoma with >25% stenosis |
| Grade III | Pseudoaneurysm |
| Grade IV | Vessel occlusion |
| Grade V | Vessel transection |
Table 2.
Reports of screening modalities for diagnosing blunt traumatic vertebral artery injury
| Author and year | Title of study | Study design | Number of patients | Territory | Outcome | Comments |
|---|---|---|---|---|---|---|
| Doppler ultrasonography | ||||||
| Mutze et al. 2005 [37] | Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography | Retrospective case series | 1,471 | Carotid and Vertebral | 38.5% sensitivity, 100% specificity | |
| MR angiography | ||||||
| Levy et al. 1994 [36] | Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography | Prospective series | 5 | Vertebral | 20% sensitivity, 100% specificity | |
| Biffl et al. 2002 [51] | Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report | Prospective case series | 16 | Carotid and vertebral | 75% sensitivity, 67% specificity | |
| CT angiography | ||||||
| Biffl et al. 2002 [51] | Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report | Prospective case series | 46 | Carotid and vertebral | 68% sensitivity, 67% specificity | |
| Mutze et al. 2005 [37] | Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography | Retrospective case series | 407 | Carotid and vertebral | 100% sensitivity, 99.7% specificity | |
| Eastman et al. 2006 [7] | Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences | Prospective case series | 146 | Carotid and vertebral | 97.7% sensitivity, 100% specificity | |
| Utter et al. 2006 [49] | Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries | Retrospective case series | 82 | Carotid and vertebral | 92% negative predictive value | CTA negative, suspected vessel injury patients. |
Table 3.
Comparison of traumatic vertebral and carotid injuries
| TVAI | TCAI |
|---|---|
| Presenting features of posterior circulation ischaemia | Presenting features of anterior circulation ischaemia |
| Mortality rate 8–18% [40] | Mortality rate 17–38% [40] |
| Association with complex cervical spine fractures [39] | No association with complex cervical spine fractures [39] |
| No correlation between Colorado radiological severity and prognosis | Correlation between Colorado radiological severity and prognosis |
| Presence of traumatic aneurysms less common [40] | Higher incidence of traumatic aneurysms [40] |
| VA is difficult to access surgically | Easily accessible for surgery |
Table 4.
Current medical management for blunt traumatic vertebral artery injury
| Author and year | Title of study | Study design | Number of patients | Type of intervention | Outcome | Comments |
|---|---|---|---|---|---|---|
| Miller et al. 2001 [26] | Blunt cerebrovascular injuries: diagnosis and treatment. | Retrospective | 50 patients | 62% heparin, 26% aspirin, 8% no treatment, 4% low molecular weight dextran | Overall stroke rate 14%, heparin 0%, aspirin 9%. No treatment: 54% | 8% bleeding complications in heparin group |
| Biffl et al. 2002 [31] | Treatment-Related Outcomes From Blunt Cerebrovascular Injuries | Retrospective Trauma database | 171 patients combined carotid/vertebral |
29% of patients neurological deficit from TCVI 69% heparin 10% antiplatelets 18% none |
Heparin: 71% improved, 26% unchanged, 3% worsened Aspirin: 40% improved, 60% unchanged No treatment: 55% improved, 45% unchanged |
|
| Miller et al. 2002 [50] | Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes | Retrospective | 216 patients (43 with VAI) |
74% antiplatelets 18% heparin 8% none |
No evidence of stroke in either group | Heparin: 2 patients bleeding complications, antiplatelets: 1 patient GI bleed |
| Engelter et al. 2007 [52] | Antiplatelets versus anticoagulation in cervical artery dissection | Meta-analysis | 327 patients | Antiplatelets versus anticoagulation, dead or disabled outcome |
Dead or disabled: 23.7% antiplatelets 14.3%anticoagulants No significance |
|
| Cothen et al. 2009 [32] | Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents | Retrospective review of prospective database | 301, 282 of whom were treated medically |
Heparin, 192 aspirin, 67 aspirin and/or clopidogrel, 23 |
Injury healing rates (heparin, 39%; aspirin, 43%; aspirin/clopidogrel, 46%) and injury progression rates (12; 10; 15%) were equivalent between therapeutic regimens |
1 patient had a stroke (0.5%) |
| Stein et al. 2009 [24] | Blunt cerebrovascular injuries: does treatment always matter? | Retrospective | 147 patients TCVI |
22% endovascular 36% antiplatelets medications (10% anticoagulation 18% combination therapy antiplatelet/anticoagulation 30% no therapy primarily due to contraindications |
Stroke rate: 3.9% in treated patients 25.8% untreated patients |
TCVI traumatic cerebro vascular injury; GI gastroIntestinal; VAI vertebral artery injury; BCVI blunt cerebro vascular injury
Table 5.
Reports of endovascular management for blunt traumatic vertebral artery injury
| Author and year | Title of study | Study design | Number of patients and level of evidence | Type of intervention | Outcome | Comments |
|---|---|---|---|---|---|---|
| Price et al. 1998 [45] | Traumatic vertebral arterial dissection and vertebrobasilar arterial thrombosis successfully treated with endovascular thrombolysis and stenting | Case report | 1 | Endovascular thrombolysis and stenting followed by oral anticoagulation with warfarin | No neurological deficits, patient returned to employment | 3 months |
| Lee et al. 2007 [23] |
Therapeutic endovascular treatments for traumatic vertebral Artery injuries |
Retrospective analysis | 6 | 3 stented, 1 coil embolisation, 2 had stent-assisted coil embolisation | 3 improved neurologically, 3 stayed stable with no new neurological deficits | Follow-up 16-55 months |
| Wang and Orbach 2008 [17] | Traumatic dissecting aneurysm at the vertebrobasilar junction in a 3-month-old infant: evaluation and treatment strategies. | Case report | 1 | Coil embolisation | Full neurological recovery | 3 months |
| Stein et al. 2009 [24] | Blunt cerebrovascular injuries: does treatment always matter? | Two part study—retrospective analysis then prospective follow-up | 6 | Coil embolisation. 4 patients had anticoagulation afterwards. | Unspecified | Endovascular technique used in high-grade TVAI and where medical therapy was unsuitable |
Epidemiology
TVAI is well recognised in trauma and its incidence increases greatly in the presence of head or cervical spine injury. The reported incidence is highly variable in the literature (0.5–2% of all trauma patients) [3–7]. Some series have found coexisting TVAI in up to 20% of patients with head injuries. Data from the 1980s estimated TVAI to account for 3–19% of all cervical vascular injuries [8]. These figures have increased as the use of screening has increased detection rates, with recent series finding VAI in 17.2–25.5% in blunt cervical spine trauma [9–13].
Considerable variation in TVAI incidence is reported with cervical spine fractures, up to 70% of which have coexisting TVAI [14, 15]. This may be due to variations in screening protocols used in these individual studies. However, the subpopulation of all TVAI patients who are symptomatic is unclear. The accurate identification of this subpopulation, to distinguish them from those with a more benign natural history, is central to the correct management of these patients and represents the greatest challenge.
Anatomy
Blunt TVAI tends to occur where vessels are exposed to shearing forces, principally at junctions between fixed and mobile segments. The anatomical segments of the vertebral artery and its course are shown in Fig. 1. The V2 segment is the most commonly affected in adult TVAI [9, 16]. In infants and children, the V3 and upper V2 segment are more commonly affected [17, 53]. This may be related to the higher laxity of ligaments at the craniocervical junction in children associated with lower velocity trauma, while in the adult population most of the injuries relate to high-impact trauma with forced flexion/extension and lateral rotation involving the whole of the cervical spine. TVAI is unilateral or bilateral, with unilateral TVAI predominating (around two-thirds of cases) [6]. The artery is well protected inside the foramina transversaria in the V1 and V2 segments, although a recent MRI study of 250 patients’ vertebral arteries shows that 8% had segments lying outside the transverse foramen [18]. This segment is less surgically accessible. In the V3 and V4 segments, the artery has greater mobility and may therefore be more resistant to blunt trauma.
Fig. 1.
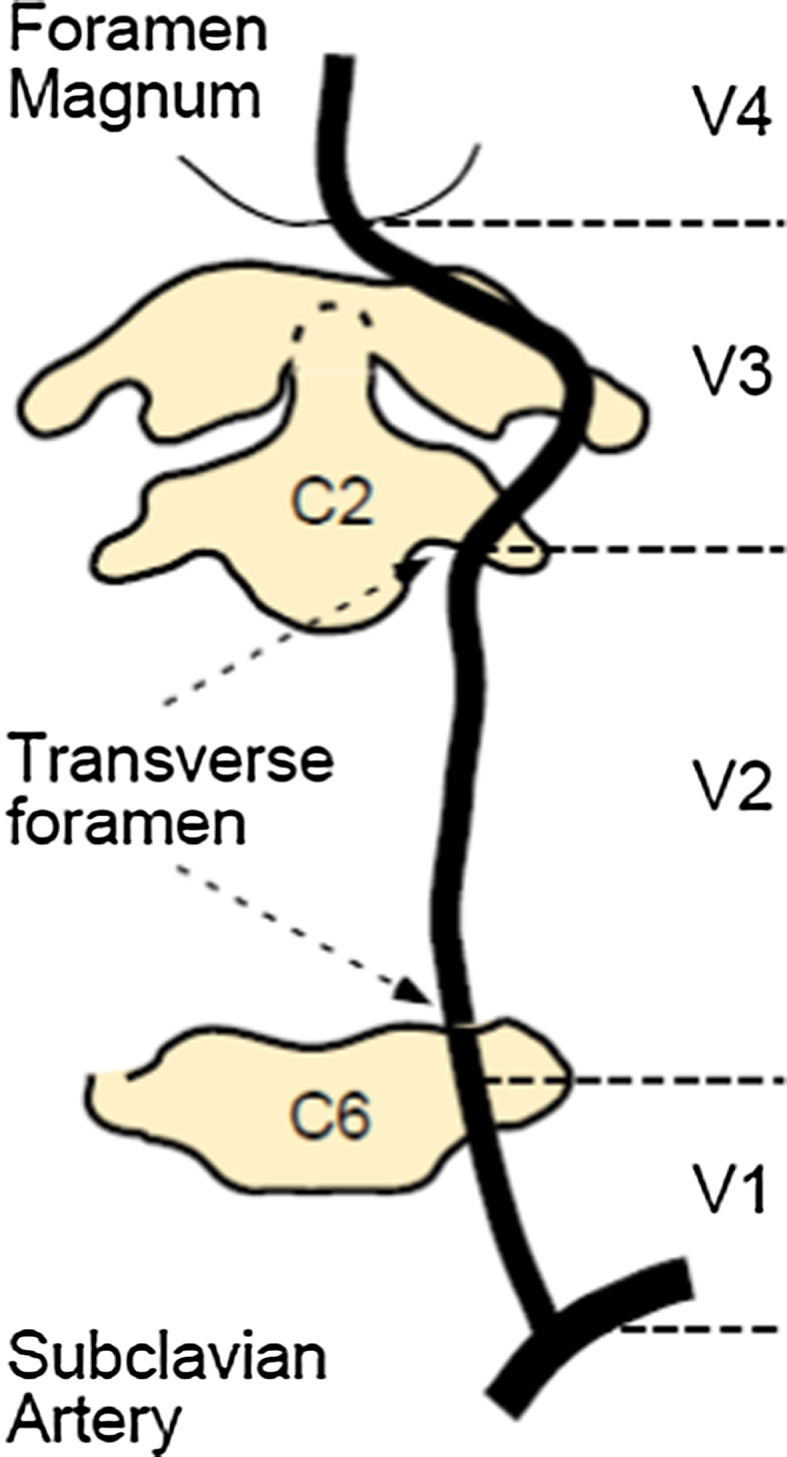
The vertebral arteries are composed of four segments. V1, the extraosseous segment, starts at the origin of the vertebral artery and runs to the transverse foramen of the C6. V2 is the foraminal segment where the artery passes through the transverse foramina from C6 to C1. V3, the extraspinal segment begins at the foramen transversarium of C2. The vertebral artery then passes along the superior aspect of the posterior ring of C1, then twists antero-superior towards the foramen magnum where it pierces the dura. Then begins V4, the intradural segment which courses to the pontomedullary junction, where the basilar artery is formed
Unilateral hypoplasia of the VA occurs in approximately 10% of individuals [18] and is compensated for by collateral flow most commonly from the contralateral vertebral or a foetal-type posterior communicating artery circulation. This is clinically central to determining the risk of an individual’s injury becoming symptomatic, or conversely the risks of a treatment strategy (which may risk or deliberately incur occlusion of the affected vessel) for individual patients.
Mechanisms
Mechanisms of cervical spine injury described in association with TVAI are hyperflexion, hyperextension, distraction, facet dislocation and fractures of the cervical spine. Higher risk injuries include fractures of the C1–C3 segment, foramen transversarium involvement and subluxation [3, 19].
Motor vehicle accidents are the leading causes of TVAI [20], whilst other mechanisms include direct assault, hanging and sporting injuries. An autopsy study of 16 patients with TVAI due to road traffic accidents sheds light on the factors, which increase the risk of TVAI. These include a major rotational component to the injury. Risk of artery rupture was associated with vertebral artery asymmetry [21].
Blunt TVAI without cervical spine fracture is described after neck manipulation by chiropractors and physiotherapists, and during sports involving repetitive neck movements, notably swimming [2, 6]. The risk of spontaneous vertebral artery dissection is also known to be increased in connective tissue disorders. Minor or seemingly innocuous supraclavicular trauma may also precipitate TVAI in such patients [22]. The pathophysiology of TVAI includes occlusion, dissection, thrombo-embolism, intimal damage, pseudoaneurysm, rupture, arteriovenous fistula and transection [3, 9, 23]. All these mechanisms can induce secondary vasospasm.
Natural history of TVAI detected by current screening procedures
The natural history of asymptomatic TVAI is relatively well understood since so many are treated conservatively. Typically, adequate collateral circulation ensures that there are no adverse clinical events, although patients who do experience vertebral artery territory ischaemia often suffer major neurological insults. These may be prevented if TVAI is confirmed by screening and the patient managed in a timely fashion. Recent data indicate that 90% of stenotic lesions resolve and 67% of occluded vessels recanalise [23]; Stein et al. [24] showed that 8 of 13 treated and untreated TVAIs improved radiologically after 1 month, with the other 5 remaining stable. However other conflicting reports suggest that 14–54% of untreated patients develop ischaemic events ranging from transient ischaemic attacks to full basilar territory strokes. Basilar occlusion and associated infarction has an 80% mortality, similar to the mortality rate reported by Matas, who first described TVAI in 1893 [15, 25]. The overall mortality of TVAI has been estimated at 4–8% [15, 26, 27]. Late presentation of TVAI may be due to delayed ischaemic deficit associated with vasospasm. There is no discussion of the recurrence rate in a series of pure TVAI (excluding carotid injuries) and neither is there data to suggest the time window during which patients with TVAI are at highest risk of ischaemic events. Some evidence is available from a study that reported a 10.4% rate of recurrent events in a series of 116 patients with both TVAI and a smaller number of traumatic carotid artery injury (TCAI) patients [2]. The authors do not specify how many of the recurrent events were in TVAI patients [2]. It is debatable whether TCAI and TVAI should be regarded as a single entity in this context, although they may rarely coexist.
Clinical presentation
Symptoms and features in the history suggestive of TVAI
Unilateral TVAI is estimated to be symptomatic in only up to 20% of cases due to extensive collateral supply within the posterior circulation [15].
Symptomatic TVAI may present with ischaemic symptoms directly after the injury or may present with posterior circulation insufficiency in a delayed manner, up to 3-6 months in some cases [5, 28]. In the symptomatic subgroup of TVAI, neurological symptoms occurred in only 70% within the first 24 h post-injury according to a small series [2]. Delayed presentation and recurrent events may lead to sudden later deterioration, resulting in misdiagnosis and medico-legal concern surrounding the original trauma. Prior knowledge of TVAI, even if treated conservatively, is vital should the patient re-present at a later date with posterior circulation ischaemic symptoms.
TVAI may even be asymptomatic in the presence of serious radiological features of TVAI such as arteriovenous fistulae [29]. A recent series reports 79 patients with 97 clinical events attributed to TVAI over a 6-month period, 20% of these patients suffering a permanent deficit [5].
The key presenting complaints of symptomatic TVAI are due to ischaemia of the cerebellum, brainstem and the primary visual cortex. Symptoms include headache, neck pain, sensory and gait disturbance, dizziness, nausea and vomiting, altered consciousness, and speech and visual abnormalities. A case and characteristic imaging of a patient with symptomatic TVAI, presenting with posterior circulation infarction, is shown (Fig. 2).
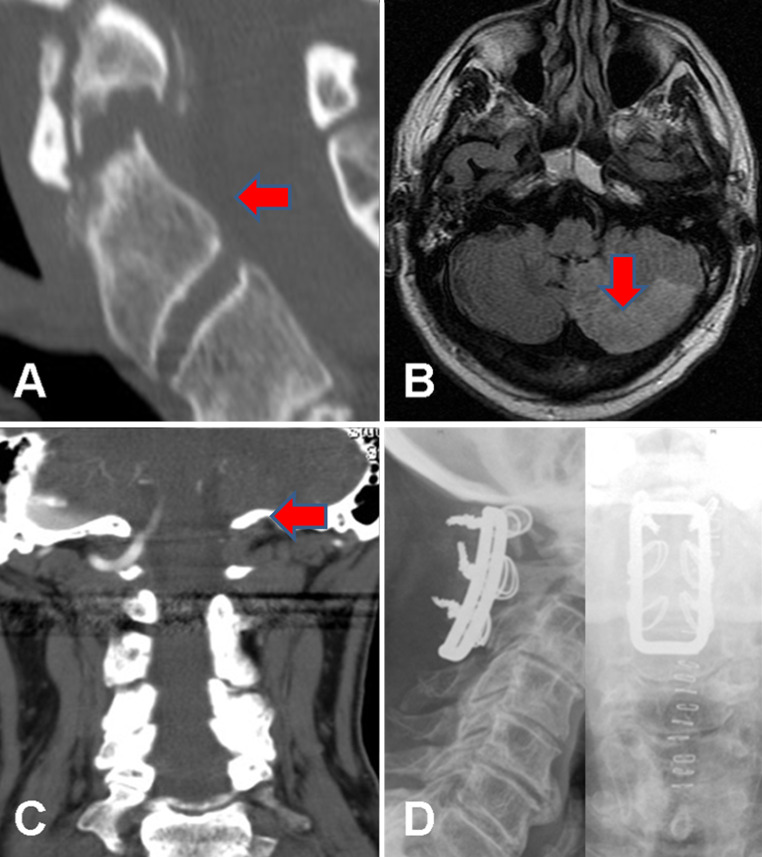
Fig. 2.
Images from a 64-year-old man who presented with acute vertigo and vomiting 2 weeks after a flexion–extension injury of his neck with continued neck pain. At the time of the injury, X-rays and a CT scan (a sagittal reformat) showed an unstable type 2 odontoid fracture, which was managed with a hard collar. An MRI (b axial FLAIR sequence) following admission with clinical features of a cerebellar ischaemic event 2 weeks later showed high signal in the left PICA territory compatible with acute infarction. A CT angiogram (c coronal maximum intensity projection) showed occlusion of the left vertebral artery arising from a dissection at the level of the C1/2 junction. He was managed with immediate anticoagulation using heparin, which was temporarily stopped after 3 days to allow surgery to stabilise the fracture via C1–C3 sublaminar wiring (d AP and lateral post-operative X-rays). He made a good recovery with rehabilitation and his anticoagulation was converted to warfarin, target INR 2–3, and then stopped after 6 months with no further adverse events
In view of the high proportion of TVAI that is initially asymptomatic, a high index of suspicion should be maintained for it based upon the mechanism of trauma and the nature of associated injuries. Associated injuries are seen in most (93%) patients [24]. Spinal cord injury coexists in 50–59% of patients with TVAI [12, 30] and complete cord injury is more commonly associated with TVAI (50%) than incomplete cord injury (12%) [11], presumably due to greater forces exerted on the neck at the time of injury. The Denver criteria highlight injuries frequently associated with TVAI and TCAI and suggest that the presence of these injuries (Table 1) should be used to prompt screening.
Physical examination
On physical examination, findings suggestive of TVAI can be classified into those directly due to posterior circulation ischaemia and those relating to traumatic injuries commonly associated with TVAI. Examination findings of posterior circulation ischaemia include dysarthria, impaired balance and coordination, ataxic gait, visual field defects, diplopia, nystagmus, Horner’s syndrome, hiccups, lateral or medial medullary syndrome, lower cranial nerve palsies, papillary abnormalities and impaired consciousness. Physical examination findings suggestive of associated trauma based upon the mechanisms suggested by the Denver criteria are neck tenderness, maxillofacial injuries, skull base fracture, head trauma, signs of hanging, high impact, flexion/extension or deceleration injury [4].
Laboratory studies
In addition to routine laboratory studies, in the setting of suspected TVAI it is important to obtain a coagulation screen and platelet count, especially in patients taking anticoagulant medications who may require radiological intervention, group and save, renal profile (for patients who may be receiving intravenous contrast) and pregnancy test.
Figure 3 provides a suggested algorithm for integrating symptoms, physical examination findings and mechanism of injury in cases of suspected TVAI to prompt further investigation.
Fig. 3.
Algorithm for assessment, screening and management of TVAIa
Screening for and diagnosing TVAI
As mentioned in the natural history of TVAI, there is little published data regarding the time frame of manifestation of neurological events after the trauma. The only data so far are from two large series [31, 32], which have reported combined results between the carotid and vertebral artery blunt injuries. The majority of events of blunt cerebrovascular injuries seem to develop within 10–72 h after the injury. If the people suffering an event in the early hours of the trauma (for which there is no time to institute effective treatment) are excluded, the need can be considered for an aggressive high-sensitivity and specificity screen, which at the same time would be easily accessible.
The advantages of actively screening for TVAI include treatment planning, with a view to anticoagulation, and preventing delayed presentation with ischaemic posterior circulation events. In addition, once TVAI lesions are detected, close follow-up can be instituted to ensure resolution of the lesion and to pick up the late complications of pseudoaneurysm formation, residual stenosis or arterio-venous fistula. It is important to avoid attributing depressed consciousness solely to concomitant TBI and hypoxia or hypovolaemia from associated trauma. The problems of searching for TVAI include the invasive nature and availability of the gold standard investigation, i.e. digital subtraction angiography (DSA), which cannot routinely be justified in head and neck injury patients. Other methods of screening for TVAI include CT and MR angiography (CTA and MRA). DSA is the gold standard, but itself carries 0.5% risk of stroke. The sensitivity of CTA in detecting VAI has improved over time, with one series showing 99% sensitivity of multislice CTA for angiographically proven VAI [7]. CTA-based screening was found to reduce the time to diagnosis 12-fold and the stroke rate due to the injury fourfold [33]. We believe that the literature so far supports the recommendation of CT angiography as the preferred method of choice for screening of TVAI (Table 2).
MRA has been shown to be a valid technique for imaging vertebral artery pathology and some centres have started using it for detecting TVAI [11, 34, 35]. Other series have reported less valuable results [31, 36]. In addition, it may be felt that in the context of acute polytrauma patients, MRA is unsuitable given the long scan times and challenges of safety within the scanning environment.
Duplex ultrasonography (USS), although the least invasive and most readily accessible modality, suffers from poor sensitivity. A recent large study reporting 1400 blunt carotid and vertebral artery injuries [37] quoted an overall sensitivity of USS in the region of 38.5%. As the authors mention, possible reasons may be that USS has technical limits in assessing vertebral arteries obscured by bone, stiff necks and central venous catheters.
The radiological features of TVAI include irregular “rat’s tail” stenosis, double lumen, haematoma, and segmental dilatation and occlusion [23]. These features may be used to grade severity of TVAI according to the Denver criteria (Table 1).
During the screening process, it is important to consider the individual patient’s premorbid state (atherosclerosis), collateral system and the vertebral artery dominance of the posterior circulation. These factors may be used to consider the risk of progression of an asymptomatic injury and the potential risks of invasive treatment [23]. Collateral circulation may derive from the anterior circulation via the posterior communicating arteries, from anastomoses via branches of the external carotid artery (e.g. the occipital arteries), the thyrocervical trunk, the costocervical trunk or via muscular and spinal branches of the more proximal vertebral arteries [3, 9]. The left vertebral artery is dominant in about 60% of people [38]. Knowledge of these factors must inform management strategy [23]. For example, a patient with a left dominant system and minimal collaterals with a high-grade left VA injury may merit earlier, more aggressive management than a young patient with minimal evidence of atherosclerotic disease and a low-grade injury to a relatively hypoplastic vessel. Features of the individual’s posterior circulation could be used to develop a risk stratification system for cases which require early management and careful follow-up. This system would be especially useful in the decision of whether to treat asymptomatic but high-risk TVAI.
Should TVAI be considered together with traumatic carotid injury?
Biffl et al. produced a detailed algorithm for the diagnosis and management of blunt cerebrovascular injuries in adults [5]. This algorithm used CTA for diagnosis and recommended anticoagulation for all but grade 5 (transection) injuries using the Denver Blunt Carotid and Vertebral Artery Grading Scale and the Denver Screening Criteria for blunt cerebrovascular injury [4]. However, the major limitation of this work is the inclusion in both sets of Denver criteria of carotid and vertebral injuries as a single entity. Anterior and posterior circulation injuries are different in terms of their time course to presentation, their mechanism, associated collateral circulation, surgical and endovascular access and their clinical consequences [32]. It has been suggested that risk factors for TCAI/TVAI do not entirely overlap. There are associations between TVAI and complex cervical spine fractures including foramen transversarium fractures, but this does not apply to TCAI [39]. A recent review highlights that in the Colorado grading scheme for radiological features of blunt TVAI and TCAI, the radiological severity of carotid injuries correlates with clinical presentation and prognosis, whereas in TVAI the radiological grading has no bearing on the clinical presentation [40, 41]. Stroke rates are higher with TCAI compared with TVAI according to a series of 147 patients where the stroke rate with TCAI was 12% and was 8% with TVAI [24]. Fusco and Harrigan, in their recent review, also point out that death rates are higher for TCAI than for TVAI [40]. Traumatic aneurysms are more common in TCAI than TVAI, which is important as these aneurysms tend to enlarge. This has implications for screening and follow-up time.
The differences between TVAI and TCAI are summarised in Table 3. Whilst undoubtedly TVAI and TCAI may be screened together owing to convenience, there needs to be a distinction between risk factors for symptomatic TVAI and TCAI to establish which subgroup of asymptomatic injuries are likely to progress and therefore require aggressive therapy. Due to these differences as well as the commonplace use of CTA and MRA, it may be useful to reconsider who to screen for TVAI and how.
Conventional treatment of TVAI
No level 1 or 2 evidence exists regarding the management of various grades of TVAI. The options for treatment include observation (most common), anticoagulation and surgery. Most of these lesions occur high at the base of the skull or within the foramen transversarium, making surgery difficult and unsafe. Anticoagulation is typically with heparin followed by 3 months of warfarin [42], the target INR based upon patient comorbidities and any relative contraindications to anticoagulation. Other options for anticoagulation in TVAI include antiplatelet agents such as aspirin and clopidogrel. There is no consensus on the choice of drug for anticoagulation with recent evidence suggesting equivalent effects of heparin and antiplatelet agents [32]. However, since unfractionated intravenous heparin infusions can be rapidly reversed whilst antiplatelet agents cannot be reversed so easily, there is a strong argument for using heparin in patients who may require surgery or a radiological procedure during their admission. Table 4 summarises the evidence for medical treatment of TVAI.
The actual risk reduction due to anticoagulation in TVAI is unknown. Proponents of anticoagulation have cited significant stroke risk and complications in patients who were not anticoagulated [26]. However, one paper showed only a nonsignificant trend toward deterioration and higher stroke risk in unheparinised patients [15]. Potential sources of bias in these studies relate to the inclusion of patients with severe head injury, lack of clear explanation regarding “subtherapeutic anticoagulation” and nonrandom allocation of patients to treatment versus conservative management groups [3]. The efficacy of heparin and/or antiplatelet agents in symptomatic versus asymptomatic TVAI is therefore not known. Absolute contraindications to anticoagulation in such patients include ongoing bleeding from any source, impending surgery and bleeding diatheses. Relative contraindications include the presence of an external ventricular drain (which may have been placed for associated cerebral trauma), recent intracerebral contusional injury and conservatively managed subdual haematomas. Recent data indicate that up to a third of TVAI patients receive no treatment owing to their moribund condition and 44% have evidence of cerebral infarction on admission before any treatment is started [24]. Heparin anticoagulation is likely to be of less benefit in patients who have already suffered a posterior circulation infarct.
The head injured patient will often have coexisting TVAI—Biffl et al. [15] reported traumatic brain injury in 41% of 79 patients with TVAI. Thromboprophylaxis in the setting of intracranial or spinal trauma is well discussed [43]. The incidence of intracranial haemorrhage after thromboprophylaxis 72 h post-admission in head injured patients is no greater than in control patients using prevention dosage. The effect of treatment dose heparin in TVAI is unknown, although it should not be instituted in the first 24 h and not before progression to frank vertebral artery rupture has been excluded. In polytrauma cases with TVAI, it is important to establish early on the nature of other injuries and if these preclude early anticoagulation.
The extent of medium and small vessel vasospasm in TVAI is unknown. If present, it may contribute to brainstem ischaemia, analogous to the role of vasospasm in aneurysmal subarachnoid haemorrhage. The frequency of vasospasm after TVAI and its contribution to ischaemia are unknown. If vasospasm is found to be a common and clinically relevant problem, there may be a role for prophylactic calcium channel antagonists to reduce it.
Supportive evidence for conservative management of TVAI in the presence of associated cervical spine injury can be found in a short series of eight patients with asymptomatic TVAI with cervical spine fractures and six with spinal cord injury. This study reports that no treatment for the TVAI, until the associated cervical spine fracture had been operated upon and then starting anticoagulation, was not associated with any ischaemic events [44].
What are the treatment options for TVAI when anticoagulation is contraindicated?
As above, coexisting TBI or polytrauma means that anticoagulation may be temporarily contraindicated. If TVAI is discovered, especially if high grade, symptomatic or in a patient with poor collateral supply, alternative treatment options may be required. The other key form of treatment for TVAI, besides anticoagulation, is endovascular. Endovascular techniques include stenting, vertebral artery occlusion and pseudoaneurysm coil embolisation, either individually or in combination. The choice of technique is dependent on the grade of the TVAI, the site of the injury and presence of collateral circulation/dominant vertebral artery. For example, a TVAI causing distal embolisation in the presence of good collateral supply is more likely to be treated with proximal occlusion in contrast to a pseudoaneurysm in a dominant vertebral artery causing symptoms despite therapeutic anticoagulation, which might be managed with a covered stent [6].
Endovascular management strategies have been extensively evaluated in spontaneous VAI, but not in the setting of the specific challenges presented in TVAI—including the effect of other head and neck injuries. There are no large clinical trials comparing endovascular therapy with conservative or medical management. Table 5 summarises the current case series and reports of endovascular management for blunt TVAI. It is worth noting that the endovascular procedure itself requires the patient to be temporarily heparinised, typically with a single dose. Many institutions follow endovascular therapy with 3 months of antiplatelet agents, depending on the device(s) deployed. Endovascular management is more challenging in young children owing to small diameter of vessels, resulting in increased associated risk of stent thrombosis [17]. A case of local intra-arterial thrombolysis in a moribund patient with TVAI and clot that occluded the entire right vertebral artery as far as the basilar tip reports successful outcome, with the patient returning to employment [45]. The current experience with thrombolysis is limited and its use is currently as a last resort in patients with a large demonstrable intravascular clot and poor neurological condition [46, 47]. As experience of the technique increases, it may be possible to develop a list of radiological findings predictive of effective thrombolysis. Complications and adverse events of endovascular intervention in TVAI are anecdotal. The Denver studies provide data on complications of carotid artery stenting after TCAI [4, 32], but this cannot be extrapolated to TVAI, which should be examined as a separate entity as the mechanism, symptoms and ease of surgical and radiological access to the carotid and vertebral arteries differ [19]. Currently, the only long-term follow-up of endovascular treatment is with regard to carotid artery injuries [48]. Long-term studies are needed to focus on outcomes and complications of endovascular treatment for TVAI.
The VA is more difficult to access surgically than the CA and surgery is seldom attempted in the setting of TVAI. Open surgical management should be a last resort treatment for patients unresponsive to medical or endovascular therapy. Surgery may have a role in complete VA transactions that have failed embolisation. Rapid neurological deterioration in spontaneous VAI has been managed by anticoagulation with or without a stent. In the patient where anticoagulation is absolutely contraindicated and endovascular treatment is unavailable or has failed, surgery may be appropriate. There is no consensus on the most suitable surgical approach and procedure, although bypasses and grafting have been attempted. Surgery to the VA is associated with high morbidity and mortality, which is likely to be increased further if there is coexisting traumatic brain injury.
The only definite indication for open surgical treatment is packing and surgical ligation in the event of uncontrollable haemorrhage. The endovascular option in this case would be vessel sacrifice with coil embolisation/detachable balloons, although emergency provision of interventional radiological services is not a standard of care in many centres.
Follow-up CT angiography can be performed after treatment, although an optimal time for repeat imaging has not been established, as TVAI radiologically evolves or resolves in the 7–10 days post-injury [31]. Clinical deterioration should prompt earlier scanning, otherwise the patient can be reimaged approximately 2 weeks post-injury to assess radiological change and for the presence of stenosis or aneurysms.
Conclusion
TVAI is a common finding in trauma patients with increased incidence in head and neck injuries. Although usually asymptomatic, it can lead to cerebellar and brainstem infarction in a minority of patients. TVAI is best screened for using CT angiography. Screening criteria are based upon a likely mechanism of injury or features in the history and examination suggestive of neck trauma or posterior circulation insufficiency. Radiologically proven TVAI may be graded according to clinical presentation and radiological severity. Taking these factors into account, treatment may either be medical with antiplatelet agents or heparin, or can be endovascular. We recommend high-grade and symptomatic injuries to be treated by endovascular approaches. Asymptomatic injuries with a low-radiological grade can be managed medically if there are no contraindications. Follow-up CT angiography may be performed a few weeks post-presentation to assess for resolution or progression of the injury.
Conflict of interest
The authors have no disclosures to make or conflicts of interest.
References
- 1.Kim YK, Schulman S. Cervical artery dissection: pathology, epidemiology and management. Thromb Res. 2009;123(6):810–821. doi: 10.1016/j.thromres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Beletsky V, Nadareishvili Z, Lynch J, Shuaib A, Woolfenden A, Norris JW, Canadian Stroke Consortium Cervical arterial dissection: time for a therapeutic trial? Stroke. 2003;34(12):2856–2860. doi: 10.1161/01.STR.0000098649.39767.BC. [DOI] [PubMed] [Google Scholar]
- 3.Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252–258. doi: 10.1097/BSD.0b013e3180cab162. [DOI] [PubMed] [Google Scholar]
- 4.Cothren CC, Moore EE. Blunt cerebrovascular injuries. Clinics (Sao Paulo) 2005;60(6):489–496. doi: 10.1590/s1807-59322005000600011. [DOI] [PubMed] [Google Scholar]
- 5.Biffl WL, Cothren CC, Moore EE, Kozar R, Cocanour C, Davis JW, McIntyre RC, Jr, West MA, Moore FA. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma. 2009;67(6):1150–1153. doi: 10.1097/TA.0b013e3181c1c1d6. [DOI] [PubMed] [Google Scholar]
- 6.Berne JD, Norwood SH. Blunt vertebral artery injuries in the era of computed tomographic angiographic screening: incidence and outcomes from 8,292 patients. J Trauma. 2009;67(6):1333–1338. doi: 10.1097/TA.0b013e31818888c7. [DOI] [PubMed] [Google Scholar]
- 7.Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006;60(5):925–929. doi: 10.1097/01.ta.0000197479.28714.62. [DOI] [PubMed] [Google Scholar]
- 8.Meier DE, Brink BE, Fry WJ. Vertebral artery trauma: acute recognition and treatment. Arch Surg. 1981;116(2):236–239. doi: 10.1001/archsurg.1981.01380140082021. [DOI] [PubMed] [Google Scholar]
- 9.Taneichi H, Suda K, Kajino T, Kaneda K. Traumatically induced vertebral artery occlusion associated with cervical spine injuries: prospective study using magnetic resonance angiography. Spine (Phila Pa 1976) 2005;30(17):1955–1962. doi: 10.1097/01.brs.0000176186.64276.d4. [DOI] [PubMed] [Google Scholar]
- 10.Vaccaro AR, Klein GR, Flanders AE, Albert TJ, Balderston RA, Cotler JM. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine (Phila Pa 1976) 1998;23(7):789–794. doi: 10.1097/00007632-199804010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Friedman D, Flanders A, Thomas C, Millar W. Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences. AJR Am J Roentgenol. 1995;164(2):443–447. doi: 10.2214/ajr.164.2.7839986. [DOI] [PubMed] [Google Scholar]
- 12.Willis BK, Greiner F, Orrison WW, Benzel EC. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34(3):435–441. doi: 10.1227/00006123-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Parbhoo AH, Govender S, Corr P. Vertebral artery injury in cervical spine trauma. Injury. 2001;32(7):565–568. doi: 10.1016/S0020-1383(00)00232-1. [DOI] [PubMed] [Google Scholar]
- 14.Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE, Jr, Johnson JL, Moore JB, Burch JM. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811–813. doi: 10.1097/01.TA.0000092700.92587.32. [DOI] [PubMed] [Google Scholar]
- 15.Biffl WL, Moore EE, Elliott JP, Ray C, Offner PJ, Franciose RJ, Brega KE, Burch JM. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231(5):672–681. doi: 10.1097/00000658-200005000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera DA, Vargas SA, Dublin AB. Endovascular treatment of traumatic injuries of the vertebral artery. AJNR Am J Neuroradiol. 2008;29(8):1585–1589. doi: 10.3174/ajnr.A1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Orbach DB. Traumatic dissecting aneurysm at the vertebrobasilar junction in a 3-month-old infant: evaluation and treatment strategies. Case report. J Neurosurg Pediatr. 2008;1(5):415–419. doi: 10.3171/PED/2008/1/5/415. [DOI] [PubMed] [Google Scholar]
- 18.Eskander MS, Drew JM, Aubin ME, Marvin J, Franklin PD, Eck JC, Patel N, Boyle K, Connolly PJ (2010) Vertebral artery anatomy: a review of two hundred fifty magnetic resonance imaging scans. Spine (Phila Pa 1976) 35(23):2035–2040 [DOI] [PubMed]
- 19.Kerwin AJ, Bynoe RP, Murray J, Hudson ER, Close TP, Gifford RR, Carson KW, Smith LP, Bell RM. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma. 2001;51(2):308–314. doi: 10.1097/00005373-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Biffl WL, Moore EE, Offner PJ, Burch JM. Blunt carotid and vertebral arterial injuries. World J Surg. 2001;25(8):1036–1043. doi: 10.1007/s00268-001-0056-x. [DOI] [PubMed] [Google Scholar]
- 21.Hiraiwa K, Sato T, Sasaki T, Mizusawa I, Nata M, Kodama N. Medico-legal aspects of traumatic injury of the vertebrobasilar artery. Neurol Med Chir (Tokyo) 2005;45(11):549–555. doi: 10.2176/nmc.45.549. [DOI] [PubMed] [Google Scholar]
- 22.Haldeman S, Kohlbeck FJ, McGregor M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine (Phila Pa 1976) 1999;24(8):785–794. doi: 10.1097/00007632-199904150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Lee YJ, Ahn JY, Han IB, Chung YS, Hong CK, Joo JY. Therapeutic endovascular treatments for traumatic vertebral artery injuries. J Trauma. 2007;62(4):886–891. doi: 10.1097/01.ta.0000209398.07973.60. [DOI] [PubMed] [Google Scholar]
- 24.Stein DM, Boswell S, Sliker CW, Lui FY, Scalea TM. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66(1):132–143. doi: 10.1097/TA.0b013e318142d146. [DOI] [PubMed] [Google Scholar]
- 25.Matas R. Traumatisms and traumatic aneurysms of the vertebral artery and their surgical treatment, with report of a cured case. Ann Surg. 1893;18:477–521. doi: 10.1097/00000658-189307000-00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, Croce MA. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279–285. doi: 10.1097/00005373-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Reid JD, Weigelt JA. Forty-three cases of vertebral artery trauma. J Trauma. 1988;28(7):1007–1012. doi: 10.1097/00005373-198807000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Tulyapronchote R, Selhorst JB, Malkoff MD, Gomez CR. Delayed sequelae of vertebral artery dissection and occult cervical fractures. Neurology. 1994;44(8):1397–1399. doi: 10.1212/wnl.44.8.1397. [DOI] [PubMed] [Google Scholar]
- 29.Beaujeux RL, Reizine DC, Casasco A, Aymard A, Rüfenacht D, Khayata MH, Riché MC, Merland JJ. Endovascular treatment of vertebral arteriovenous fistula. Radiology. 1992;183(2):361–367. doi: 10.1148/radiology.183.2.1561336. [DOI] [PubMed] [Google Scholar]
- 30.Torina PJ, Flanders AE, Carrino JA, Burns AS, Friedman DP, Harrop JS, Vacarro AR. Incidence of vertebral artery thrombosis in cervical spine trauma: correlation with severity of spinal cord injury. AJNR Am J Neuroradiol. 2005;26(10):2645–2651. [PMC free article] [PubMed] [Google Scholar]
- 31.Biffl WL, Ray CE, Jr, Moore EE, Franciose RJ, Aly S, Heyrosa MG, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235(5):699. doi: 10.1097/00000658-200205000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cothren CC, Biffl WL, Moore EE, Kashuk JL, Johnson JL. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144(7):685. doi: 10.1001/archsurg.2009.111. [DOI] [PubMed] [Google Scholar]
- 33.Eastman AL, Muraliraj V, Sperry JL, Minei JP. CTA-based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma. 2009;67(3):551–556. doi: 10.1097/TA.0b013e3181b84408. [DOI] [PubMed] [Google Scholar]
- 34.Weller SJ, Rossitch E, Malek AM. Detection of vertebral artery injury after cervical spine trauma using magnetic resonance angiography. J Trauma. 1999;46(4):660–666. doi: 10.1097/00005373-199904000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Bok AP, Peter JC. Carotid and vertebral artery occlusion after blunt cervical injury: the role of MR angiography in early diagnosis. J Trauma. 1996;40(6):968–972. doi: 10.1097/00005373-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Lévy C, Laissy JP, Raveau V, Amarenco P, Servois V, Bousser MG, et al. Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology. 1994;190(1):97–103. doi: 10.1148/radiology.190.1.8259436. [DOI] [PubMed] [Google Scholar]
- 37.Mutze S, Rademacher G, Matthes G, Hosten N, Stengel D. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex doppler US and early CT angiography. Radiology. 2005;237(3):884. doi: 10.1148/radiol.2373042189. [DOI] [PubMed] [Google Scholar]
- 38.Hong JM, Chung CS, Bang OY, Yong SW, Joo IS, Huh K. Vertebral artery dominance contributes to basilar artery curvature and peri-vertebrobasilar junctional infarcts. J Neurol Neurosurg Psychiatry. 2009;80(10):1087–1092. doi: 10.1136/jnnp.2008.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoit DA, Schirmer CM, Weller SJ, Lisbon A, Edlow JA, Malek AM. Angiographic detection of carotid and vertebral arterial injury in the high-energy blunt trauma patient. J Spinal Disord Tech. 2008;21(4):259–266. doi: 10.1097/BSD.0b013e318141fce8. [DOI] [PubMed] [Google Scholar]
- 40.Fusco MR, Harrigan MR (2011) Cerebrovascular dissections: a review. Part II: Blunt cerebrovascular injury. Neurosurgery 68(2):517–530 [DOI] [PubMed]
- 41.Biffl WL, Moore EE, Elliott JP, Brega KE, Burch JM. Blunt cerebrovascular injuries. Curr Probl Surg. 1999;36(7):505–599. doi: 10.1016/s0011-3840(99)80807-7. [DOI] [PubMed] [Google Scholar]
- 42.http://www.aans.org/en/Education%20and%20Meetings/Clinical%20Guidelines.aspx
- 43.Browd SR, Ragel BT, Davis GE, Scott AM, Skalabrin EJ, Couldwell WT (2004) Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurg Focus 15;17(4):E1 [DOI] [PubMed]
- 44.Sack JA, Etame AB, Shah GV, La Marca F, Park P. Management and outcomes of patients undergoing surgery for traumatic cervical fracture-subluxation associated with an asymptomatic vertebral artery injury. J Spinal Disord Tech. 2009;22(2):86–90. doi: 10.1097/BSD.0b013e318167a81e. [DOI] [PubMed] [Google Scholar]
- 45.Price RF, Sellar R, Leung C, O’Sullivan MJ. Traumatic vertebral arterial dissection and vertebrobasilar arterial thrombosis successfully treated with endovascular thrombolysis and stenting. AJNR Am J Neuroradiol. 1998;19(9):1677–1680. [PMC free article] [PubMed] [Google Scholar]
- 46.Sugrue PA, Hage ZA, Surdell DL, Foroohar M, Liu J, Bendok BR. Basilar artery occlusion following C1 lateral mass fracture managed by mechanical and pharmacological thrombolysis. Neurocrit Care. 2009;11(2):255–260. doi: 10.1007/s12028-008-9159-7. [DOI] [PubMed] [Google Scholar]
- 47.Bissay F, Allard S, Van Tussenbroek F, Stadnik T, Michotte A. Vertebral artery dissection complicated by a thrombosis of the basilar artery was successfully treated with endovascular thrombolysis. Eur Neurol. 2004;51(2):110–113. doi: 10.1159/000076790. [DOI] [PubMed] [Google Scholar]
- 48.Edwards NM, Fabian TC, Claridge JA, Timmons SD, Fischer PE, Croce MA. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg. 2007;204(5):1007–1013. doi: 10.1016/j.jamcollsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 49.Utter GH, Hollingworth W, Hallam DK, Jarvik JG, Jurkovich GJ. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203(6):838–848. doi: 10.1016/j.jamcollsurg.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236(3):386. doi: 10.1097/00000658-200209000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biffl WL, Ray CE, Jr, Moore EE, Mestek M, Johnson JL, Burch JM. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53(5):850. doi: 10.1097/00005373-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Engelter ST, Brandt T, Debette S, Caso V, Lichy C, Pezzini A, Abboud S, Bersano A, Dittrich R, Grond-Ginsbach C, Hausser I, Kloss M, Grau AJ, Tatlisumak T, Leys D, Lyrer PA, for the Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605–2611. doi: 10.1161/STROKEAHA.107.489666. [DOI] [PubMed] [Google Scholar]
- 53.Hasan I, Wapnick S, Tenner MS, Couldwell WT. Vertebral artery dissection in children: a comprehensive review. Pediatr Neurosurg. 2000;37(4):168–177. doi: 10.1159/000065391. [DOI] [PubMed] [Google Scholar]