Abstract
Some controversy still exists over the optimal treatment time and the surgical approach for cervical myelopathy due to ossification of the posterior longitudinal ligament (OPLL). The aim of the current study was first to analyze the effect of intramedullary spinal cord changes in signal intensity (hyperintensity on T2-weighted imaging and hypointensity on T1-weighted imaging) on magnetic resonance imaging (MRI) on surgical opportunity and approach for cervical myelopathy due to OPLL. This was a prospective randomized controlled study. Fifty-six patients with cervical myelopathy due to OPLL were enrolled and assigned to either group A (receiving anterior decompression and fusion, n = 27) or group P (receiving posterior laminectomy, n = 29). All the patients were followed up for an average 20.3 months (12–34 months). The clinical outcomes were assessed by the average operative time, blood loss, Japanese Orthopedic Association (JOA) score, improvement rate (IR) and complication. To determine the relevant statistics, we made two factorial designs and regrouped the data of all patients to group H (with hyperintensity on MRI, n = 31), group L (with hypointensity on MRI, n = 19) and group N (no signal on MRI, n = 25), and then to further six subgroups as well: AH (with hyperintensity on MRI from group A, n = 15), PH (with hyperintensity on MRI from group P, n = 16), AL (with hypointensity on MRI from group A, n = 10), PL (with hypointensity on MRI from group P, n = 9), AN (no signal intensity on MRI from group A, n = 12) and PN (no signal intensity on MRI from group P, n = 13). Both hyperintensity on T2-weighted imaging and hypointensity on T1-weighted imaging had a close relationship with the JOA score and IR. The pre- and postoperative JOA score and postoperative IR of either group H or group L was significantly lower than that of group N (P < 0.05), regardless of whether the patients had received anterior or posterior surgery. On the other hand, both the JOA score and IR of subgroup AH were higher than those of subgroup PH at 1 week, 6 and 12 months postoperatively (P < 0.05), as well as between subgroup AL and PL; but in group N, there was no difference between the subgroup AN and PN (P > 0.05). In conclusion, regardless of hyperintensity on T2-weighted imaging or hypointensity on T1-weighted imaging in patients with OPLL, severe damage to the spinal cord is indicated. Surgical treatment should be provided before the advent of intramedullary spinal cord changes in signal intensity on MRI. The anterior approach is more effective than posterior approach for treating cervical myelopathy due to OPLL characterized by intramedullary spinal cord changes in signal intensity on MRI.
Keywords: Anterior decompression and fusion, Laminectomy, Ossification of the posterior longitudinal ligament, Signal intensity on MRI
Introduction
Ossification of the posterior longitudinal ligament (OPLL) of the cervical spine results in compression of the spinal cord, which causes myelopathy. As conservative treatment is usually ineffective for severe myelopathy caused by OPLL, surgical treatment is chosen in most cases. There are two representative surgical procedures: anterior decompression and posterior decompression. The choice of surgical treatments has been conflicting. Anterior corpectomy and fusion seems to be a radical surgical option owing to direct decompression and satisfactory results [1, 2]. However, the anterior approach is relatively more difficult because posterior longitudinal ligament tissue lies just in front of the spinal cord, and it also carries potential risks of graft extrusion, cerebrospinal fluid (CSF) leakage, excessive venous bleeding, and dysphagia [3]. The advantage of posterior decompression is that the operation is relatively straightforward [4]. However, there exist the possibility of progression of OPLL, postlaminectomy membrane formation, and scars tissue into the spinal canal, kyphotic deformity, and instability of the cervical spine [5, 6]. Many factors affect the selection of the optimal therapeutic strategy for OPLL, such as age, OPLL type, the traverse area of the spinal cord, the position of OPLL, history of trauma, and the range of motion in the cervical spine, and so on. Whether the intramedullary spinal cord changes in signal intensity on magnetic resonance imaging (MRI) also affect the choice of surgical opportunity and approach remains unclear. Intramedullary spinal cord changes in signal intensity on MRI may indicate edema, inflammation, vascular ischemia, gliosis, or myelomalacia of the spinal cord. However, whether it indicates severe myelopathy caused by OPLL is conflicting. The purpose of the current study was to investigate whether intramedullary spinal cord changes in signal intensity on MRI affect surgical opportunity and approach for cervical myelopathy due to OPLL.
Materials and methods
Patient population
This is a prospective randomized and controlled study, approved by the local ethical committee and the institutional review board at our center. Between May 2005 and March 2008, 56 patients with OPLL were enrolled in our hospital. Written consent to participate in the study was obtained from all patients. The inclusion criterion was that the patient has cervical myelopathy on physical examination and the spinal cord compression was seen to be caused by OPLL in MRI. The exclusion criteria were the developmental stenosis, ossification of yellow ligament, previous history of cervical spine surgery, severe disc herniation, OPLL extending more than three vertebrae and previous history of cervical spine surgery.
Each case of the 56 patients was given a serial number according to the consecutive sequence of hospitalization, and assigned to group A (receiving anterior decompression and fusion) or group P (receiving posterior laminectomy) randomly by computer according to the serial number. Among the 56 patients, there were 45 males and 11 females with average age 58.4 years (range 49–74 years). All the 56 patients were operated by one surgeon. Data were collected prospectively by independent observers using standardized data collection forms. To determine the relevant statistics, we made two factorial designs and regrouped the data of all patients to group H (with hyperintensity on MRI), group L (with hypointensity on MRI) and group N (no signal intensity on MRI) (Tables 1, 2), and further to six subgroups: AH (with hyperintensity on MRI from group A), PH (with hyperintensity on MRI from group P), AL (with hypointensity on MRI from group A), PL (with hypointensity on MRI from group P), AN (no signal intensity on MRI from group A) and PN (no signal intensity on MRI from group P).
Table 1.
Patient group of factorial design
| Group | H | N | Total |
|---|---|---|---|
| A | 15 | 12 | 27 |
| P | 16 | 13 | 29 |
| Total | 31 | 25 | 56 |
A anterior decompression and fusion, P posterior laminectomy with fusion, H hyperintensity on MRI, N no signal intensity on MRI
Table 2.
Patient group of factorial design
| Group | L | N | Total |
|---|---|---|---|
| A | 10 | 12 | 22 |
| P | 9 | 13 | 22 |
| Total | 19 | 25 | 44 |
A anterior decompression and fusion, P posterior laminectomy with fusion, L hypointensity on MRI, N no signal intensity on MRI
Radiological evaluation
All patients had a preoperative plain radiograph, three-dimensional computed tomography construction (3D CT), and MRI of the cervical spine (Figs. 1, 2). The diameter of the spinal canal was measured from the middle of the posterior surface of the vertebral body to the nearest point on the corresponding spinolaminar line. The mean value of the measures at C2–C7 represented the spinal canal diameter for that patient. The occupying rate was defined as the greatest thickness of OPLL divided by the anteroposterior diameter of the bony spinal canal on the axial CT image. On the sagittal image, OPLL was classified into three types: segmental, continuous, or mixed. The extent of OPLL was also investigated. The intramedullary spinal cord changes in signal intensity were examined on axial and sagittal T2- and T1-weighted MRI.
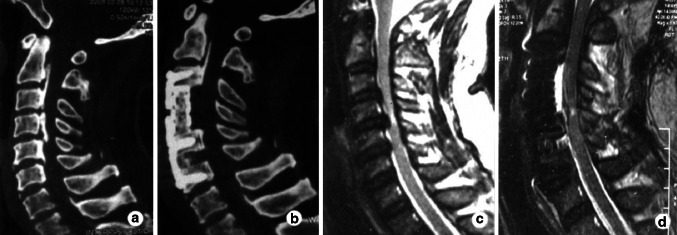
Fig. 1.
Case 1. Preoperative CT image (a) shows a mixed type OPLL and postoperative CT image (b) shows complete resection of OPLL using anterior corpectomy and fusion. Preoperative sagittal MRI of the cervical spine (c) shows signal intensity at C4–5 and postoperative sagittal MRI (d) shows the area of signal intensity decreased using anterior decompression
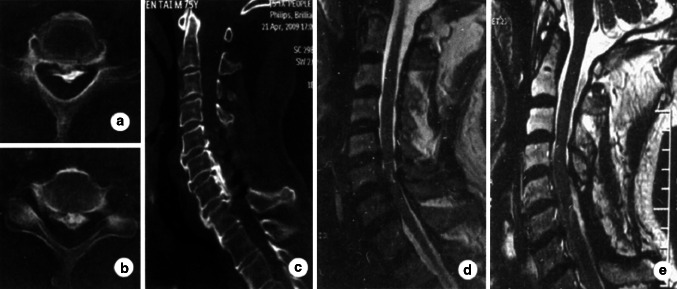
Fig. 2.
Case 2. Preoperative CT image (a, b) shows severe compression of the spinal cord, and postoperative CT image (c) shows satisfying decompression of the spinal cord using posterior Preoperative sagittal MRI of the cervical spine (d) shows signal intensity at C6–7 and postoperative sagittal MRI (e) shows the area of signal intensity decreased using posterior laminectomy
Surgical technique
Under general anesthesia, the patients were placed in the supine position with neck slightly extended. The cervical spine was exposed through a standard right-side anterior approach. The appropriate surgical level was confirmed by intraoperative radiography. After necessary discectomies, one or two vertebral bodies were partially removed using an appropriate rongeur until the posterior cortex of the vertebrae was exposed and thinned as much as possible. The OPLL was then separated from the dura using a specialized microdissector. The head of this dissector was a hook with a narrow slot that was inserted under the OPLL from the nonossified ligament, rotated 90°, and slightly lifted. The ligament was cut off by scalpel along the slot. The OPLL was then meticulously separated using the microdissector and removed using 1–2 mm Kerrison rongeur and microcurettes. If the OPLL was associated with dural ossification, this portion of the dura was carefully preserved using the anterior floating method to avoid dural tears. Titanium mesh cages were then used and filled with autologous bone fragments from the excised vertebrae to restore the bone defect. Finally, anterior cervical plates were applied across the segments to be fused.
For the patients of group P, the process of posterior laminectomy and fusion was performed as usual. Following complete decompression of neural elements, the lateral mass screw and plate instrumentation was performed. Peri-zygapophysial joints were filled with autologous bone fragments from the excised lamina. After surgery, all 56 patients were required to wear rigid cervical collars for an average 4 weeks.
Clinical assessment
Periprocedural parameters, including operative time and blood loss were collected. The Japanese Orthopedic Association (JOA) scoring system was used to evaluate the neurological status preoperatively and at 1 week, 6 months, and 12 months postoperatively. The improvement rate (IR) was calculated as IR = (postoperative JOA score − preoperative JOA score/17 − preoperative JOA score) × 100%. It was calculated at 1 week, 6 and 12 months postoperatively. The surgical outcome was defined by the IR as follows: excellent (IR ≥ 75%), good (75% > IR ≥ 50%), fair (50% > IR ≥ 25%), and poor (IR < 25%). The fine rate included both the excellent rate and good rate. This value was obtained at 12 months.
Statistical analysis
The Student’s t test and analysis of variance were used to perform statistical comparisons. The Fisher exact test was employed for categorical variables. P value less than 0.05 was considered to be statistically significant. Results are presented as mean ± standard deviation (SD).
Results
Of the 56 patients, 27 patients were in the group A and 29 were in the group P randomly. When the factorial designs were made, 31 patients were in group H, 19 in group L, and 25 in group N. There were 15, 16, 10, 9, 12, and 13 patients in subgroup AH, PH, AL, PL, AN, and PN, respectively (Tables 1, 2). Twenty-one patients underwent anterior cervical corpectomy and fusion (ACCF) to remove the discs, partial vertebral bodies and ossified ligament, 6 patients underwent anterior floating procedure, and 29 patients underwent laminectomy with fusion. All the 56 patients were followed up for a mean period of 20.3 months (range 12–34). There were no significant differences observed between group A and group P in patient age, duration of symptoms, mean spinal canal diameter, mean occupying rate, and type of OPLL (Table 3).
Table 3.
Patients demographics and radiographic outcomes
| Group A (n = 27) | Group P (n = 29) | P | |
|---|---|---|---|
| Mean age (years) | 57.5 ± 8.6 | 59.3 ± 7.9 | NS |
| Duration of symptoms (months) | 9.8 ± 2.1 | 9.4 ± 3.2 | NS |
| Mean spinal canal diameter (mm) | 14.7 ± 1.2 | 14.2 ± 1.7 | NS |
| Mean occupying rate (%) | 38.9 ± 9.4 | 41.2 ± 8.2 | NS |
| Type of OPLL | |||
| Segmental (%) | 5 (18.5%) | 4 (13.8%) | NS |
| Mixed (%) | 8 (29.6%) | 6 (20.7%) | NS |
| Continuous (%) | 14 (51.9%) | 19 (65.5%) | NS |
Values are mean ± SD
NS not significant
Radiological findings
The mean diameter of the spinal canal on preoperative plain radiographs was 14.7 mm (range 12–16 mm) in group A, and 14.2 mm in group P (range 11–17 mm). Sagittal 3D CT scans of the cervical spine showed segmental type OPLL in 9 patients, mixed type OPLL in 14 patients, and continuous type OPLL in 33 patients. Preoperative CT scans showed that the mean occupying rate reached 38.9% (range 21–78%) in group A, and 41.2% (range 20–80%) in group P. On the sagittal images, OPLL extended from one to three vertebrae (mean 2.1 ± 0.9). Preoperative MRI showed an alteration of the intramedullary signal in 31 cases. In 19 of these cases both hypointensity on T1-weighted images and hyperintensity on T2-weighted images were observed, whereas in 12 cases, only hyperintensity on T2-weighted imaging was present. At 1 week postoperative, all patients underwent plain radiographs, CT, and MRI scans. On the postoperative sagittal T2- or T1-weighted MRI, the intramedullary signal did not disappear in all 31 cases, but the areas decreased in 28 cases at 6 months postoperatively. Three patients showed postoperative abnormal expansion of signal intensity area, but the areas decreased at 12 months postoperatively. There were no hardware complications in both groups. In group A, 23 patients obtained fusion at 6 months postoperatively, and the remaining 4 patients achieved fusion at 12 months. In group P, 5 patients did not achieved fusion at 6 months postoperatively, and all patients obtained fusion at 12 months postoperatively. The difference in fusion time between the two groups was not significant (P > 0.05).
Surgical outcomes
The surgical approach and intramedullary spinal cord changes in signal intensity on MRI had no relationship to operative time and blood loss. However, both hyperintensity on T2-weighted imaging and hypointensity on T1-weighted imaging were closely related with the JOA score and IR. The pre- and postoperative JOA score and postoperative IR of either group H or group L was significantly lower than that of group N (P < 0.05), regardless of whether the patients had received anterior or posterior surgery. The surgical approach was also related with postoperative JOA score and IR, and there were significant differences between group A and group P (P < 0.05).
The surgical outcomes of the subgroups are depicted in Table 4. There was no difference between subgroups AH and PH in term of operative time, blood loss and preoperative JOA score, as well as between subgroup AN and PN, and subgroup AL and PL. However, at 1 week, 6 and 12 months postoperatively, the JOA score in subgroup AH was 10.1, 12.1 and 13, which were significantly higher than those in subgroup PH (P < 0.05), 8.9, 10.8 and 11.9, respectively. Similarly, at every data collection time, the postoperative JOA score of subgroup AL was significantly higher than those in subgroup PL (P < 0.05). On the other hand, there was no significant difference between subgroup AN and PN in JOA score after surgery (P > 0.05). There were significant differences between subgroups AH and PH in IR at every data collection time (P < 0.05), but no differences between subgroup AN and PN (P > 0.05). The IR of subgroup AL at 1 week and 6 months, but at 12 months postoperatively was higher than those of subgroup PL. However, the fine rate was 93.3 and 62.5% in subgroup AH and PH, respectively, thus indicating no difference between the two subgroups (P > 0.05), as well as between subgroup AL and PL, which the fine rate was 90 and 77.8%, respectively. Similar statistical result occurred between subgroup AN and PN, for which the fine rate was 91.7 and 92.3%, respectively.
Table 4.
Surgical outcomes of subgroups
| AH (n = 15) | pH (n = 16) | P* | AL (n = 10) | PL (n = 9) | P † | AN (n = 12) | PN (n = 13) | P ‡ | |
|---|---|---|---|---|---|---|---|---|---|
| Operative time (min) | 132.7 ± 53.5 | 112.2 ± 23.5 | NS | 136 ± 59.5 | 115 ± 28.9 | NS | 133.3 ± 48.5 | 127.7 ± 71.5 | NS |
| Blood (ml) | 394.7 ± 350.2 | 278.1 ± 225.9 | NS | 324 ± 340.1 | 282.2 ± 217.4 | NS | 302.5 ± 205.5 | 276.5 ± 229.8 | NS |
| JOA score (point) | |||||||||
| Preoperative | 7.4 ± 1.6 | 7.3 ± 1.6 | NS | 6.6 ± 1.2 | 6.2 ± 0.8 | NS | 8.3 ± 0.9 | 8.1 ± 0.9 | NS |
| Postoperative | |||||||||
| 1 week | 10.1 ± 1.6 | 8.9 ± 1.6 | <0.05 | 9.5 ± 1.6 | 8.0 ± 1.1 | <0.05 | 11.3 ± 1.1 | 10.8 ± 0.9 | NS |
| 6 months | 12.1 ± 1.4 | 10.8 ± 1.3 | <0.05 | 11.4 ± 1.0 | 10.1 ± 0.8 | <0.05 | 13.5 ± 1.3 | 12.8 ± 1.3 | NS |
| 12 months | 13 ± 1.5 | 11.9 ± 1.4 | <0.05 | 12.3 ± 0.8 | 11.4 ± 0.9 | <0.05 | 14.3 ± 1.2 | 13.5 ± 1.3 | NS |
| IR (%) | |||||||||
| 1 week | 28.3 ± 8.3 | 16.4 ± 5.9 | <0.01 | 28.6 ± 9.9 | 16.7 ± 4.7 | <0.05 | 34.4 ± 8.2 | 30.4 ± 5.9 | NS |
| 6 months | 49.5 ± 7.8 | 35.6 ± 8.2 | <0.01 | 46.4 ± 4.1 | 36.1 ± 5.0 | <0.05 | 60.1 ± 13.9 | 53.2 ± 11.1 | NS |
| 12 months | 59.2 ± 13 | 48.1 ± 12.3 | <0.05 | 54.5 ± 8.1 | 48.6 ± 5.9 | NS | 69.8 ± 12 | 60.7 ± 13.5 | NS |
| Fine rate of IR (%) | 93.3 | 62.5 | NS | 90 | 77.8 | NS | 91.7 | 92.3 | NS |
Values are mean ± SD
JOA Japanese Orthopedic Association, IR improvement rate, NS not significant
* P compared between subgroup AH and PH; † P compared between subgroup AL and PL; ‡ P compared between subgroup AN and PN
Complications
In this series, the complications included CSF leakage in three cases, haematoma in one case, and postoperative expansion of intramedullary high-intensity area on T2-weighted MRI in one case after anterior corpectomy and fusion and C5 palsy in three cases, postoperative expansion of intramedullary high-intensity area on T2-weighted MRI in two cases after posterior laminectomy. CSF leakage occurred after a dural tear during the operation due to tight adhesion to the dura. There was no significant dural defect in these cases, because dural ossification was preserved by careful dissection. CSF leakage stopped after approximately a week of conservative treatment of local pressure. The patient who experienced haematoma recovered neurological function after an emergency operation. C5 palsy developed at 8 h postoperatively and the strength of the related deltoid and biceps muscles of the three patients decreased separately to grade 1, 2, and 3 in a manual muscle test (MMT). The patients were treated conservatively including neurotrophy drugs, high-pressure oxygen therapy, and functional exercises. The strength of the paralyzed muscles of all the 3 patients recovered to grade 4 in MMT after 2 months. Postoperative expansion of intramedullary high-intensity area on T2-weighted MRI was found at 6 months postoperatively because there were no special clinical symptoms. All the three patients who suffered postoperative expansion of intramedullary high-intensity area on T2-weighted MRI did not achieve fusion at 6 months postoperatively. The patients were required to wear rigid cervical collars until they obtained fusion. At 12 months postoperatively, the intramedullary high-intensity area decreased, but did not disappear.
Discussion
Correlation between signal intensity on MRI and spinal cord lesion
Takahashi et al. [7] were first to describe the MRI findings of intramedullary high signal intensity in cervical spondylosis myelopathy. They considered intramedullary high signal intensity to be an indicator of poor prognosis, as they speculated that this change reflected myelomalacia or cord gliosis secondary to longstanding compression of the spinal cord. However, this is controversial as other studies have had conflicting results. Mehalic et al. [8] concluded that high signal changes on T2-weighted images were nonspecific and indicated edema, inflammation, vascular ischemia, gliosis, or myelomalacia. Ramanauskas et al. [9] divided myelomalacia into three stages and speculated that in early and intermediate stages, the spinal cord showed hyperintensity on T2-weighted sequences, and that in the late stage, a low-signal intensity on T1-weighted image and high signal intensity on T2-weighted sequences were noted. Al-Mefty et al. [10] reported that intramedullary changes of the spinal cord on MRI coincided with pathologic changes in the spinal cord, which included remarkable changes in the gray matter: vascular morphologic changes, loss of motor neurons, necrosis, and cavitation in experimental chronic compressive myelopathies of canines. It was reported that low-signal intensity changes on T1-weighted sequences indicated a poor prognosis [11]. However, Matsuda et al. [12] found that the patients who demonstrated hyperintensity on T2-weighted images before surgery had more severe damage to the spinal cord than those who showed normal signal intensity on T2-weighted images before surgery, and that these patients had a worse rate of recovery from clinical symptoms after surgery. Some investigators have insisted that hyperintensity changes on T2-weighted images before surgery correlated with the surgical outcome and indicated a poor prognosis by quantifying the high signal intensity [8, 13]. In the current study, through the analysis of factorial design, we observed that both hyperintensity on T2-weighted imaging and hypointensity on T1-weighted imaging were closely related with the JOA score and IR. The preoperative JOA score and IR were significantly lower in patients with signal intensity on MRI than those in patients without signal intensity on MRI. Unluckily, we could not compare the JOA score and IR between group H and group L since there was no random sampling. The results showed that intramedullary spinal cord changes in signal intensity on MRI indicated severe damage to the spinal cord, which resulted in worse rate of recovery from clinical symptoms after surgery.
Choice of surgical opportunity
OPLL is characterized by chronic and insidious pressure on the spinal cord and long course to show up clinical symptom. The optimal treatment time for patients with cervical OPLL has been controversial. Some authors have advocated early surgery in patients with mild myelopathy [14], and some have even suggested prophylactic surgery in patients without myelopathy [15]. In contrast, other authors have recommended conservative treatment [16]. In view of Macondo et al. [17] and other findings [14, 18, 19], surgical treatment is indicated if (1) the C1–7 ROM is ≥35°; (2) OPLL type is segmental; (3) the patient is younger than 50 years of age and has activated growth activity of the ossified mass; and (4) a high signal intensity is seen in the spinal cord and there is a risk of myelopathy or aggravation of existing myelopathy. As discussed above, no matter hyperintensity on T2-weighted imaging or hypointensity on T1-weighted imaging on MRI in patients with OPLL indicated severe damage to the spinal cord, and resulted in worse rate of recovery from clinical symptoms after surgery. Thus, as Macondo et al. devised that surgical treatment should be performed before the advent of signal intensity on MRI. Of course, the signal intensity was not the sole factor to determine the surgical treatment time.
Choice of surgical procedure
Some controversy still exists over the surgical approach for cervical myelopathy due to OPLL. Anterior corpectomy and resection of OPLL is a radical surgical option due to direct decompression, and provides satisfactory results. The overall fusion rate in a large number of patients evaluated in a comprehensive literature review was 92% [20]. In this study, we observed that the surgical approach was closely related with postoperative JOA score and IR. The postoperative JOA and IR of group A were significant higher than those of group P. However, the anterior approach is relatively more difficult when attempting to remove the OPLL. In most cases, the anterior floating method without resection of OPLL was usually used to avoid dural tears and injury to the spinal cord. The results of anterior procedures varied due to insufficient decompression resulting from dural ossification or massive bleeding from the epidural space. Posterior decompression is an alternative choice for OPLL when the anterior approach threatens iatrogenic deterioration of the neurological status.
Interestingly, though the postoperative JOA and IR of group A were significant higher than those of group P, there were two different statistical outcomes between subgroups. The postoperative JOA and IR of subgroup AH were higher than those of subgroup PH, as well as between AL and PL, but no differences were observed between subgroup AN and PN. The reason may be that the patients with signal intensity on MRI had severe spinal cord lesion and needed direct decompression. Although anterior approach and posterior approach share the same goal of relieving neurological compression, the anterior approach can provide a good surgical outcome for radical decompression by directly removing the OPLL and other anterior pathogenic structures. However, in terms of the patients with mild OPLL, such as those in group N, both anterior approach and posterior approach get the same surgical outcome in the short term. On the other hand, the results of this study demonstrated the advantages of anterior approach for the patients with severe OPLL such as those in group H and group L. Intramedullary spinal cord changes in signal intensity on MRI may be an important factor in choosing the surgical approach. Of course, there are many factors that affect the choice of surgical approach such as developmental stenosis, ossification of yellow ligament, previous history of cervical spine surgery, severe disc herniation, extent of OPLL and previous history of cervical spine surgery, and so on, just as the exclusion criteria of this study.
Complications
CSF leakage and C5 palsy were the most common complications after decompression surgery for cervical myelopathy. The incidence rate of CSF leakage varied from 4.5 to 32%. Avoiding CSF leakage depends on the preoperative identification of dural ossification and meticulous dissection during the operation. In cases where it was difficult to remove the OPLL, the floating method was an alternative choice in which the OPLL was only separated from the vertebral wall. Of 27 patients treated with the anterior approach in this current study, the floating method was applied to 6 patients. In our series, CSF leakage resulting from dural tears occurred in 3 patients. But the area of dural defect was limited and CSF leakage could be cured simply by conservative treatment. C5 palsy after decompression and multilevel corpectomy was noted first in the English literature by Shinomiya, et al. [21]. The incidence of postoperative C5 palsy has been reported at 4.6% after surgery for cervical compression myelopathy and this value has not varied with different surgical procedures or disease etiologies. The pathogenesis of postoperative C5 palsy remains unclear at the present time. Patients with postoperative C5 palsy generally have a good prognosis for functional recovery [22]. In our series, C5 palsy occurred in three patients after posterior laminectomy and all were treated conservatively. After 2 months, they recovered well. Postoperative expansion of intramedullary high-intensity area on T2-weighted MRI, although not common, is an unpreventable complication after surgery. It has been reported in a few articles as a complication either after anterior or posterior decompression surgery [23–26]. This complication has not been considered a technical problem. In these reports, some cases showed a postoperative neurologic complication and others did not. In view of Yagi et al. [27] the cervical instability was a risk factor for the postoperative expansion of intramedullary high-intensity area on T2-weighted MRI. In our series, all the 3 patients who suffered this complication did not achieve fusion at 6 months postoperatively. The reason may be the cervical instability just as Yagi et al. had advocated. After fusion at 6 months postoperatively, the intramedullary high-intensity area decreased, but did not disappear. For avoiding postoperative expansion of intramedullary high-intensity area on T2-weighted MRI, the cervical instability should be carefully treated.
In conclusion, regardless of hyperintensity on T2-weighted imaging or hypointensity on T1-weighted imaging in patients with OPLL, severe damage to the spinal cord is indicated. Surgical treatment should be provided before the advent of intramedullary spinal cord changes in signal intensity on MRI. The anterior approach is more effective than posterior approach for treating cervical myelopathy due to OPLL characterized by intramedullary spinal cord changes in signal intensity on MRI.
Conflict of interest
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
References
- 1.Epstein N. Anterior approaches to cervical spondylosis and ossification of the posterior longitudinal ligament: review of operative technique and assessment of 65 multilevel circumferential procedures. Surg Neurol. 2001;55:313–324. doi: 10.1016/S0090-3019(01)00464-5. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno J, Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J. 2006;6:282S–288S. doi: 10.1016/j.spinee.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990–997. doi: 10.3171/jns.1997.86.6.0990. [DOI] [PubMed] [Google Scholar]
- 4.Tomita K, Nomura S, Umeda S, et al. Cervical laminoplasty to enlarge the spinal canal in multilevel ossification of the posterior longitudinal ligament with myelopathy. Arch Orthop Trauma Surg. 1988;107:148–153. doi: 10.1007/BF00451594. [DOI] [PubMed] [Google Scholar]
- 5.Epstein N (2002) Posterior approaches in the management of cervical spondylosis and ossification of the posterior longitudinal ligament. Surg Neurol 58:194–207 (discussion 207–208) [DOI] [PubMed]
- 6.Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow-up. J Neurosurg. 2002;96:180–189. [PubMed] [Google Scholar]
- 7.Takahashi M, Sakamoto Y, Miyawaki M, et al. Increased MR signal intensity secondary to chronic cervical cord compression. Neuroradiology. 1987;29:550–556. doi: 10.1007/BF00350439. [DOI] [PubMed] [Google Scholar]
- 8.Mehalic TF, Pezzuti RT, Applebaum BI (1990) Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery 26:217–226 (discussion 226–227) [DOI] [PubMed]
- 9.Ramanauskas WL, Wilner HI, Metes JJ, et al. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1998;13:399–404. doi: 10.1097/00004728-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mefty O, Harkey LH, Middleton TH, et al. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg. 1988;68:217–222. doi: 10.3171/jns.1988.68.2.0217. [DOI] [PubMed] [Google Scholar]
- 11.Morio Y, Teshima R, Nagashima H, et al. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine. 2001;26:1238–1245. doi: 10.1097/00007632-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda Y, Miyazaki K, Tada K, et al. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg. 1991;74:887–892. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 13.Okada Y, Ikata T, Yamada H, et al. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine. 1993;18:2024–2029. doi: 10.1097/00007632-199310001-00016. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa Y, Chiba K, Matsumoto M, et al. Long-term results after expansive open-door laminoplasty for the segmental-type of ossification of the posterior longitudinal ligament of the cervical spine: a comparison with nonsegmental-type lesions. J Neurosurg Spine. 2005;3:198–204. doi: 10.3171/spi.2005.3.3.0198. [DOI] [PubMed] [Google Scholar]
- 15.Epstein N. Diagnosis and surgical management of cervical ossification of the posterior longitudinal ligament. Spine J. 2002;2:436–449. doi: 10.1016/S1529-9430(02)00394-7. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Toyama Y, Ishikawa M, et al. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy. Does it predict the outcome of conservative treatment? Spine. 2000;25:677–682. doi: 10.1097/00007632-200003150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki M, Aiba A, Hashimoto M, et al. Cervical myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2009;10:122–128. doi: 10.3171/2008.10.SPI08480. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga S, Kukita M, Hayashi K, et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;96:168–172. doi: 10.3171/spi.2002.96.2.0168. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka T, Yamaura I, Kurosa Y, et al. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine. 2001;26:241–248. doi: 10.1097/00007632-200102010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Cauthen JC, Kinard RE, Vogler JB, et al. Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine. 1998;23:188–192. doi: 10.1097/00007632-199801150-00008. [DOI] [PubMed] [Google Scholar]
- 21.Shinomiya K, Kurosa Y, Fuchioka M, et al. Clinical study of dissociated motor weakness following anterior cervical decompression surgery. Spine. 1989;14:1211–1214. doi: 10.1097/00007632-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine. 2003;28:2447–2451. doi: 10.1097/01.BRS.0000090833.96168.3F. [DOI] [PubMed] [Google Scholar]
- 23.Itoh T, Ohshima Y, Hayashi M, et al. Two cases of cervical myelopathy showing spinal cord swelling after operation in MRI. Rinsho Seikei Geka. 1995;30:755–759. [Google Scholar]
- 24.Nagashima H, Morio Y, Teshima R. Re-aggravation of myelopathy due to intramedullary lesion with spinal cord enlargement after posterior decompression for cervical spondylotic myelopathy: serial magnetic resonance evaluation. Spinal Cord. 2002;40:137–141. doi: 10.1038/sj.sc.3101262. [DOI] [PubMed] [Google Scholar]
- 25.Okumura H, Homma T. Tumor-like findings in MRI after decompression for cervical spondylotic myelopathy. Seikeigeka. 1992;43:41–46. [Google Scholar]
- 26.Sato T, Kojima T, Ohnuma H, et al. Intramedullary enhanced lesion in MRI of cervical spondylotic myelopathy. Ortho Surg Traumatol. 1993;36:917–922. [Google Scholar]
- 27.Yagi M, Ninomiya K, Kihara M, et al. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on magnetic resonance imaging. J Neurosurg Spine. 2010;12:59–65. doi: 10.3171/2009.5.SPINE08940. [DOI] [PubMed] [Google Scholar]