Abstract
There are only few reports in literature about the treatment of traumatic lesions of the thoracic spine. They have been grouped together with thoracolumbar fractures, ignoring the particular biomechanics of the thoracic segment. The objective of this retrospective cohort is to describe the clinical presentation and outcomes of surgically treated patients with these injuries. Data were obtained from the institutional database of medical registries, identifying all the patients who had been treated for thoracic spine fractures, from January 1, 1995 through December 31, 2005 in our institution. The study group included the 51 surgically treated patients. General and surgery-related complications were considered as clinical outcomes and injury-related disability was also assessed. Statistical analysis evaluating possible associations with timing and type of surgery, neurological impairment and associated injuries was carried out. Motor vehicle accident was the most frequent mechanism of injury. Six patients had an incomplete neurological deficit, whereas 22 had a complete lesion. Thirty-two patients presented at least one complication. Five of the neurologically intact patients, while 20 of those with neurological impairment presented general complications (p = 0.0001). None of the patients’ neurological status deteriorated after surgery. All patients with complete spinal cord injury and those with incomplete cord injury with partial functional recovery received disability compensation. Short pedicle instrumentations should be used whenever possible, but also long instrumentations and mixed constructs may be necessary for the management of such unique fractures.
Keywords: Thoracic spine fractures, Spinal instrumentation, Thoracic trauma
Introduction
The thoracic spine has unique anatomical and biomechanical features. It is much stiffer than the lumbar spine due to the restraining effect of both the rib cage and its relatively thinner disks [1]. The radiate and costotransverse ligaments bind the ribs to their respective vertebrae, contributing with this region’s rigidity [2].
Another important difference between the thoracic and the lumbar spine is the presence of the spinal cord in the former. In this region, a narrower spinal canal implies less free space available between the cord and the osseous ring. This fact, together with the relatively sparse blood supply of the central thoracic spine could explain the occurrence of spinal cord injury with less compression or kyphotic deformity than in the lumbar spine [3].
Therefore, thoracic spine fractures differ in their clinical presentation compared to fractures at other levels and should be managed with distinct criteria.
There are only few reports in the literature regarding the treatment of traumatic injuries of the thoracic spine. They are usually grouped together with thoracolumbar fractures ignoring its distinctive biomechanical characteristics and its differences with the thoracolumbar junction and the lumbar spine [4].
In addition, there is no consensus regarding instrumentation type, construct, fusion length nor the need for anterior decompression of the spinal canal [5]. In this report, we describe the clinical presentation and outcomes of surgically treated patients with thoracic spine fractures (T2–T10). Based on our results, a treatment suggestion for these injuries is also proposed.
Patients and methods
We conducted a retrospective institutional database review of medical registries. All patients treated for thoracic spine fractures, from January 1, 1995 through December 31, 2005 in the Hospital del Trabajador de Santiago were identified. A total of 123 patients with traumatic non-pathological thoracic spine fractures were found. Fifty-three (43.1%) had surgical indications. One patient died secondary to hepatic and vascular (vena cava) injury before spine surgery and another patient refused surgery. The remaining 51 patients were included in our study cohort.
Information extracted from each patient’s medical record included demographics, injury mechanism, latency to surgery, fracture type, level, neurological impairment, associated injuries, type of surgical procedure and associated complications. Data were analyzed using a non-parametric test (χ2—Fischer’s exact test; according to distribution) to evaluate relationships between the studied categorical variables.
All fractures were classified according to AO/ASIF classification [6] using plain radiographs, complemented with a computerized tomography (CT) and magnetic resonance images (MRI). Patient’s assessment included the ASIA (American Spinal Injury Association) Impairment Scale [7] for neurological function.
Absolute indications for surgical treatment
Neurological impairment.
- Severe deformity:
- Canal encroachment >50%.
- Vertebral body wedging >50%.
- Kyphosis >25° at the injury level, measured between the upper and lower adjacent non-injured vertebrae.
Mechanical instability: posterior tension band disruption or rotational instability.
Relative indication
To avoid prolonged immobilization and rehabilitation periods, in order to achieve an early return to functional activities of daily living including gainful employment.
Treatment
Acute management
Patients were initially evaluated and managed according to advanced trauma life support (ATLS) protocol. The goals of initial treatment were early and aggressive hemodynamic stabilization and management of systemic trauma to provide optimal tissue perfusion. Associated skeletal injuries were treated with urgent or emergent stabilization as needed, with collaboration from a multidisciplinary team. Spine surgery was performed as soon as the patient’s medical condition allowed it.
Spine surgery
A posterior approach was used in all patients. When considering each injury’s characteristics (type, number and contiguity of fractures) and pedicle size. Different fixation lengths and combinations of pedicle screws and/or claws with laminar/pedicle hooks were used [8].
Rehabilitation
All patients initiated an early rehabilitation protocol managed by a multidisciplinary institutional group. The characteristics of this protocol differed between patients with and without neurological impairment.
Follow-up
Patients were evaluated at 2, 4, 6 and 12 weeks and then at 6 and 12 months after surgery. Annual clinical and radiographic (X-rays and/or CT) follow-up was carried out thereafter. Minimum follow-up was 3.4 years with a mean of 7.4 years.
At each follow-up, the following clinical outcomes were tracked:
Surgery-related complications (wound infection, pseudoarthrosis, sagittal collapse and instrumentation failure).
Recovery of neurological impairment.
General complications (pneumonia, urinary tract infection and thromboembolic disease).
Time out of work and/or compensation for disability.
Results
The study group included 51 patients with a surgically treated thoracic spine fracture. Table 1 shows demographic information of the study sample, including injury mechanism, neurological impairment and any additional vertebral fractures.
Table 1.
Demographic characteristics of patients (n = 51)
| Mean age at injury | 38 years | (19–72) (%) |
|---|---|---|
| Sex | ||
| Male | 44 | 86.3 |
| Female | 7 | 13.7 |
| Mechanism of trauma | ||
| Motor vehicle accidents | 25 | 48.0 |
| Fall from height | 22 | 43.2 |
| Direct trauma | 3 | 5.9 |
| Gunshot | 1 | 1.9 |
| Neurological intact | 23 | 45.1 |
| Incomplete spinal cord injury | 6 | 11.8 |
| Complete cord injury | 22 | 43.1 |
| Additional vertebral fractures | 15 | 28.3 |
There were 44 male (86.3%) and 7 female (13.7%) patients, with a mean age of 38 years (19–72 years). Motor vehicle accident was the most frequent injury mechanism (48%), followed by fall from a height (43.1%). The remaining four patients (7.9%) were injured by other mechanisms (direct trauma in three patients and gunshot in one patient).
Table 2 shows fracture type distribution, according to the AO/ASIF classification.
Table 2.
Fracture classification according to AO/ASIF
| A3 | 6 | 11.7% |
| B1 | 7 | 13.8% |
| B2 | 2 | 3.9% |
| C1 | 8 | 15.7% |
| C2 | 12 | 23.5% |
| C3 | 16 | 31.4% |
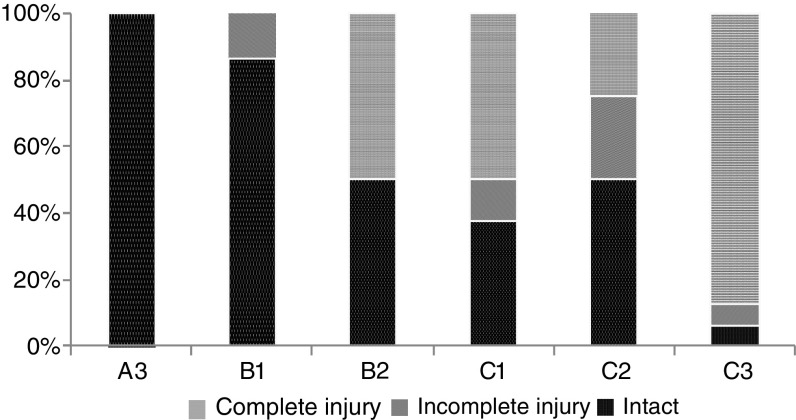
Spinal cord injury (Fig. 1): 23 patients (45.1%) were intact (ASIA E), 6 patients (11.8%) had an incomplete neurological injury (ASIA B–D) and 22 patients (43.1%) had a complete lesion (ASIA A). All patients with neurological impairment were treated according to the NASCIS II protocol [9].
Fig. 1.
Spinal cord injury according to fracture type
Based on the recent evidence [10], we considered 24 h after the accident as the time limit to differentiate early from delayed surgery.
Clinical outcomes
Associated injuries
We identified 36 patients (70.6%) with associated lesions. Twenty-three (45.1%) presented two or more (Table 3). Nineteen patients (37.2%) presented fractures of the thoracic cage (ribs, scapulae, clavicle and sternum); 17 of them had a hemothorax and/or pneumothorax, 5 had a pulmonary contusion, 2 a myocardial contusion and 1 had an esophageal perforation that required surgical treatment.
Table 3.
Associated injures
| Thorax | ||
| Fractures of thoracic cage | 19 | 37.2% |
| Hemothorax and/or pneumothorax | 17 | 33.3% |
| Pulmonary contusion | 5 | 9.8% |
| Myocardial contusion | 2 | 3.9% |
| Esophageal perforation | 1 | 1.9% |
| Abdominal | ||
| Pancreatic contusion | 1 | 1.9% |
| Splenic laceration | 1 | 1.9% |
| Spinal | ||
| Thoracic fractures | 9 | 17.6% |
| Other segments | 6 | 11.8% |
| Pelvis | 2 | 3.9% |
| Limb fractures | 13 | 25.4% |
Two patients (3.9%) presented an abdominal injury: one pancreatic contusion and one splenic laceration.
Nine patients (17.6%) had multiple thoracic spine fractures; 6 (11.8%) had spine fractures of others segments and 2 (3.9%) had a pelvic fracture. Finally, 13 patients (25.4%) presented limb fractures.
Complications
Thirty-two patients (62.7%) presented 39 complications, 24 of them had only one complication, while 8 had 2 or more. We subdivided these as follows:
General complications
Thirty general complications occurred in 25 (49%) patients. Three types of general complications were identified: Urinary tract infections in 21 cases (41.2%), pneumonia in 5 cases (9.8%) and thromboembolic disease in 4 cases (7.8%): 3 deep venous thromboses and 1 fat embolism.
Only 5 (20%) of the neurologically intact patients presented general complications while 20 (71.4%) of those with neurological impairment (complete or incomplete) developed at least one general complication (p = 0.0001). There was no difference between the incidence of general complications among patients operated within the first 24 h from admission when compared with those operated afterwards (p = 0.18).
Surgery-related complications
We reported no cases of pseudoarthrosis and only 1 (1.9%) of sagittal dis-balance with instrumentation failure. There were 5 (9.8%) cases of superficial wound infection and 3 (5.9%) cases of deep infection. We analyzed a possible relationship between surgery-related complications and time of surgery. Complications occurred in 2 of the 15 patients that were operated during the first 24 h (13.3%) and in 9 of the 36 patients that were operated after (25%). Although clinically significant, it was not statistically significant (p = 0.3). Instrumentation removal was indicated in nine patients (17.6%) due to hardware-related back pain and/or to free non-fused segments included in the construct, to recover their mobility, based on the previous reports by our study group [11]. This procedure was carried out only after fusion confirmation with a CT scan was obtained. None of the patients presented loss of reduction or spinal alignment imbalance during the follow-up period after the instrumentation removal.
Recovery of neurological impairment
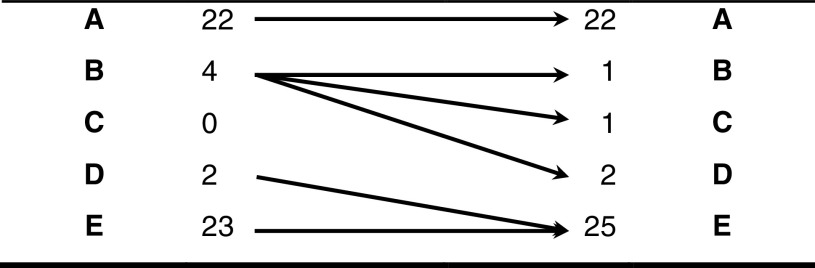
According to the previous reports [12, 13], we considered the spinal cord injury as stable after completing 1 year from the accident. During this period of time, none of the patients with ASIA A improved their neurological status. Three of the four patients with ASIA B improved at least one ASIA grade during postsurgical follow-up, while two patients with ASIA D evolved to full recovery (Table 4). Overall, 5 (83.3%) of the 6 patients with an incomplete cord injury demonstrated neurologic recovery during their whole follow-up (52–159 months).
Table 4.
AIS evolution
In this cohort, different types of posterior stabilization constructs were used, according to each injury’s characteristics and pedicle size. Short instrumentation, which under our criteria in the thoracic spine consider two levels above and two levels below the lesion, were used when possible, while longer instrumentations were indicated when a larger lever arm for reduction was needed, and/or to include multiple injured levels [14–16], to avoid additional external immobilization. Mixed constructs, using hooks configuring claws in the cranial levels were used when pedicle diameter above the fracture was <4 mm, also longer instrumentations were used, when the diameter of the pedicles of the adjacent segments below the injury did not allowed the use of pedicle screws (Table 5).
Table 5.
Patients managed with long and mixed instrumentations
| Case | Level | Fracture | Construct | Fixation | Fusion | Explanation |
|---|---|---|---|---|---|---|
| 1 | T5–T6–T7 | C2 | PL | T3–T9 | T4–T8 | Contiguous thoracic fracture |
| 5 | T5–T6 | C1 | MC | T4–T9 | T5–T7 | Small pedicles above the fracture |
| 7 | T3–T4–T6–T7 | C3–C2 | PL | T2–T9 | T2–T9 | Non-contiguous thoracic fracture |
| 12 | T5–T6–T7 | C1 | MC | T3–T10 | T4–T10 | Contiguous thoracic fracture—small pedicles adjacent to the fracture |
| 17 | T4–T5 | B1 | MC | T2–T8 | T3–T6 | Small pedicles—restoration of thoracic alignment |
| 18 | T4–T5 | C3 | PL | T3–T7 | T3–T7 | Unable to place screws at T5 |
| 19 | T7–T8–T9 | C2 | MC | T5–T11 | T6–T10 | Small pedicles at T5 and T6 |
| 20 | T2–T3 | C2 | MC | T1–T9 | T1–T9 | Small pedicles above the fracture—lever arm needed for realignment |
| 21 | T6–T7 | C3 | PL | T5–T9 | T5–T8 | Unable to place screws at T7 |
| 22 | T4–T5–T6 | C3 | MC | T2–T8 | T4–T6 | Small pedicles above the fracture—contiguous fractures |
| 24 | T4 | B2 | MC | T3–T9 | T3–T6 | Small pedicles adjacent to the fracture |
| 25 | T5–T6–T7 | C1 | MC | T4–T10 | T5–T8 | Contiguous fractures—large distal pedicles |
| 27 | T6–T7 | C3 | PL | T2–T9 | T3–T7 | Small pedicles at T4 and T5—unable to place screws at T7 |
| 32 | T8 | A3 | MC | T6–T11 | T6–T11 | Small pedicles adjacent to the fracture |
| 33 | T5–T6 | C3 | PL | T3–T8 | T3–T8 | Unable to place screws at fractured levels |
| 38 | T2–T3–T4 | C3 | PL | T1–T7 | T1–T5 | Unable to place screws at fractured levels—restoration of thoracic alignment |
| 40 | T4–T5 | C1 | MC | T3–T8 | T3–T8 | Small pedicles adjacent to the fracture |
| 44 | T4–T5 | C1 | MC | T2–T8 | T3–T8 | Small pedicles adjacent to the fracture |
| 48 | T6–T7; T9–T10 | B1–C3 | PL | T5–T11 | T5–T11 | Non-contiguous thoracic fracture |
| 49 | T7 | A3 | MC | T5–T11 | T5–T11 | Small pedicles at T5 and T6 |
| 50 | T4–T5 | B1 | MC | T1–T11 | T3–T6 | Small pedicles—restoration of thoracic alignment |
| 51 | T5; T9 | A3; A3 | PL | T3–T10 | T3–T10 | Non-contiguous thoracic fracture |
PL posterior long pedicle instrumentation, MC mixed construct with proximal claws/hooks and distal pedicle screws
We identified one hardware failure, secondary to deep infection. No association between construct length and complication incidence was identified.
Disability
All patients with complete spinal cord injury and those with an incomplete injury without neurologic recovery received worker’s compensation. Patients with an incomplete cord injury that evolved to full recovery did not receive any compensation and returned to full-time work. Four patients (7.8%) received compensation for chronic lumbar pain (15–25% of disability).
We identified no statistical associations between chronic lumbar pain and age (p = 0.26, using the median of 36 years to divide the cohort into 2 subgroups), time of surgery (p = 0.66) and length of the fixation (p = 0.39).
Among the patients that were able to work after the accident, those requiring great physical activity had an inferior job performance.
Discussion
We describe the results of the surgical treatment of a cohort of patients with thoracic spine fractures. The available literature describes these fractures as severe injuries, with an important incidence of associated lesions, caused by a high energy trauma [17]. In our study group, 70.6% of the patients had associated injuries, including thoracic and abdominal viscera, thoracic cage, limbs and other spinal segments and systems. Forty-five percent of the patients had two or more of these associated injuries, confirming the fact that thoracic spine fractures may be related to life-threatening conditions [18]. This way, a thoracic spine fracture must be suspected in any patient with multiple injuries after a high-energy trauma. Given the anatomically congested area that surrounds the thoracic spine, fractures in this segment may be difficult to identified using only plain X-rays. To avoid under diagnosis, a CT scan should be obtained [19, 20]. In addition, complementary MRI allows an accurate evaluation of the soft tissues (inter-vertebral discs, ligaments and spinal cord) [21].
A higher incidence of neurological impairment has been associated with thoracic spine fractures, due to the decreased cross-sectional area of the spinal canal (thoracic canal to spinal cord ratio close to 1) compared with other spinal segments [22]. In our cohort, 54.9% of the patients presented some degree of spinal cord injury. We reported no cases of neurological recovery in patients with complete deficit, while the majority (83.3%) of those with an incomplete injury recovered at least one degree in the ASIA scale. In our analysis, neurological impairment was statistically associated with a higher complication rate after spine surgery. In a systematic review, Verlaan et al. [23] also described this finding in surgically managed thoracic and lumbar spine fractures.
When planning the surgical treatment of these fractures one must consider the particular anatomy of the thoracic spine, specifically the physiological kyphosis and the smaller pedicle size of the middle thoracic vertebrae.
The lever arm needed for reduction usually implies the inclusion of a greater number of healthy segments in the fixation. This issue may be irrelevant in the thoracic spine, due to its intrinsic and extrinsic rigidity: relatively shorter disk height (20% of vertebral body height vs. 40% in the lumbar spine), trapezoidal vertebral body, facet joint orientation [24] and the restraining effect of the thoracic cage [25–27]. Particularly, we observed that sternum fracture adds instability to this type of injuries.
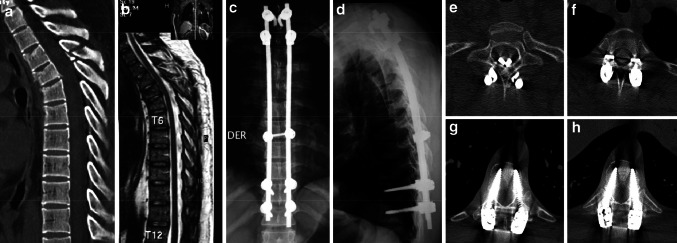
Twenty-two patients (49%) in this cohort required instrumentations of five or more segments. On the other hand, hooks configuring claws were used in 13 patients (25.5%), due to a smaller pedicle width (<4 mm) in the cephalic levels (Fig. 2).
Fig. 2.
a, b A 35-year-old male with a T4–T5 type B1 (AO/ASIF) fracture, ASIA E secondary to a motor vehicle accident. c, d A long mixed construct was used. e Laminar hooks at T1. f Pedicle hooks at T2. g, h Polyaxial pedicle screws at T10 and T11
There is no level I evidence to support a specific construct or instrumentation for the management of these fractures. When considering that our incidence of surgery-related complications is the lowest one reported in the literature, with the largest group of patients [23, 28] and with no significant differences between short and long instrumentations (including mixed ones with pedicle screws and hooks), we recommend the use of short instrumentations with pedicle screws whenever possible. Hooks configuring claws should be considered as an alternative when the pedicle diameter is <4 mm. Longer instrumentations may be used to facilitate and improve reduction.
No patient in our study group presented neurological deterioration due to the surgical procedure or fixation technique. We report no cases of pseudoarthrosis during our follow-up and only one fixation collapse. This patient achieved consolidation, after a deep infection was treated with antibiotics together with surgical debridement and irrigation.
Neurological impairment was the only element related with disability. An inferior performance was reported among the patients that returned to great physical activity jobs after the accident. No other relationships with functional outcomes could be established when analyzing other factors, such as age, time to surgery and instrumentation length.
Conclusion
Thoracic spine fractures that require surgical treatment occur more frequently in polytrauma patients and are often associated with neurological impairment and such patients may have associated life-threatening injuries and should be addressed via a multidisciplinary team approach. Neurological impairment was found to be the most important predictor of complications and disability in our study group. In addition, the restoration of physiologic kyphosis and fracture reduction may require larger lever arms. We recommend the use of short pedicle instrumentations whenever possible, considering long instrumentations, together with mixed constructs, when necessary, for the management of such unique fractures.
Acknowledgments
The authors did not receive grants or outside founding support of their research or for preparation of this article.
References
- 1.El-Khoury GY, Whitten CG. Trauma to the upper thoracic spine: anatomy, biomechanics, and unique imaging features. AJR Am J Roentgenol. 1993;160:95–102. doi: 10.2214/ajr.160.1.8416656. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi T, Schultz A, Belytschko T, et al. A model for studies of mechanical interactions between the human spine and rib cage. J Biomech. 1974;7:497–507. doi: 10.1016/0021-9290(74)90084-0. [DOI] [PubMed] [Google Scholar]
- 3.Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360–369. [PubMed] [Google Scholar]
- 4.Schweighofer F, Hofer H, Wildburger R, et al. Unstable fractures of the upper thoracic spine. Langebecks Arch Chir. 1997;382:25–28. doi: 10.1007/BF02539304. [DOI] [PubMed] [Google Scholar]
- 5.Yue J, Jossan A, Selgrath C, et al. The treatment of unstable thoracic spine fractures with transpedicular screw instrumentation. A 3-year consecutive series. Spine. 2002;27:2782–2787. doi: 10.1097/00007632-200212150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Magerl F, Aebi M, Gertzbein S, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 7.American Spine Injury Association (ASIA) International standards for neurological classification of SCI, Revised 2002 American Spine Injury Association Booklet
- 8.Coe J, Warden K, Herzig M, et al. Influence of bone mineral density on the fixation of thoracolumbar implants: a comparative study of transpedicular screws, laminar hooks and spinous process wires. Spine. 1990;15:902–907. doi: 10.1097/00007632-199009000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Bracken M, Shepard M, Collins W, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. Results of the second national acute spinal cord injury study. N Eng J Med. 1999;20:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 10.Fehlings M, Perrin R. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28–S35. doi: 10.1097/01.brs.0000217973.11402.7f. [DOI] [PubMed] [Google Scholar]
- 11.Yurac R, Marré B, Urzúa A, et al. Residual mobility of instrumented and non-fused segments in thoracolumbar spine fractures. Eur Spine J. 2006;15:864–875. doi: 10.1007/s00586-005-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland JW, Hawryluk GW, Kwon B, et al. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2–E18. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 13.Wuermser LA, Ho CH, Chiodo AE, et al. Spinal cord injury medicine. 2. Acute care management of traumatic and nontraumatic injury. Arch Phys Med Rehabil. 2007;88:S55–S61. doi: 10.1016/j.apmr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 14.McLain R, Burkus K, Benson D. Segmental instrumentation for thoracic and thoracolumbar fractures: prospective analysis of construct survival and five-year follow-up. Spine J. 2001;1:310–323. doi: 10.1016/S1529-9430(01)00101-2. [DOI] [PubMed] [Google Scholar]
- 15.Bransford R, Bellabarba C, Thompson J, et al. The safety of fluoroscopically assisted thoracic pedicle screw instrumentation for spine trauma. J Trauma. 2006;60:1047–1052. doi: 10.1097/01.ta.0000215949.95089.18. [DOI] [PubMed] [Google Scholar]
- 16.Potter BK, Lehman RA, Kuklo TR. Anatomy and biomechanics of thoracic pedicle screw instrumentation. Curr Opin Orthop. 2004;15:133–141. doi: 10.1097/01.bco.0000120511.04726.d6. [DOI] [Google Scholar]
- 17.Cotton B, Pryor J, Chinwalla I, et al. Respiratory complications and mortality risk associated with thoracic spine injury. J Trauma. 2005;6:1400–1409. doi: 10.1097/01.ta.0000196005.49422.e6. [DOI] [PubMed] [Google Scholar]
- 18.McHenry T, Mirza S, Wang J, et al. Risk factors for respiratory failure following operative stabilization of thoracic and lumbar spine fractures. J Bone Joint Surg Am. 2006;88:997–1005. doi: 10.2106/JBJS.E.00560. [DOI] [PubMed] [Google Scholar]
- 19.Brandt M, Wahl W, Yeom K, et al. Computed tomographic scanning reduces cost and time of complete spine evaluation. J Trauma. 2004;56:1022–1028. doi: 10.1097/01.TA.0000124304.68584.2C. [DOI] [PubMed] [Google Scholar]
- 20.Antevil L, Sise M, Sack D, et al. Spiral computed tomography for the initial evaluation of spine trauma: a new standard of care? J Trauma. 2006;61:382–387. doi: 10.1097/01.ta.0000226154.38852.e6. [DOI] [PubMed] [Google Scholar]
- 21.Van Beek E, Been H, Ponsen K, Maas M. Upper thoracic spinal fractures in trauma patients—a diagnostic pitfall. Injury. 2000;31:219–223. doi: 10.1016/S0020-1383(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 22.Vialle L, Vialle E. Thoracic spine fractures. Injury. 2005;36:S-B65–S-B72. doi: 10.1016/j.injury.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Verlaan J, Diekerhof C, Buskens E, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine. Spine. 2004;7:803–814. doi: 10.1097/01.BRS.0000116990.31984.A9. [DOI] [PubMed] [Google Scholar]
- 24.Goh S, Price R, Leedman p, Singer K. The relative influence of vertebral body and inter-vertebral disc shape on thoracic kyphosis. Clin Biomech. 1999;14:439–448. doi: 10.1016/S0268-0033(98)00105-3. [DOI] [PubMed] [Google Scholar]
- 25.IV WatkinsR, Williams L, Ahlbrand S, et al. Stability provided by the sternum and rib cage in the thoracic spine. Spine. 2005;1:1283–1286. doi: 10.1097/01.brs.0000164257.69354.bb. [DOI] [PubMed] [Google Scholar]
- 26.Horton W, Kraiwattanapong C, Akamaru T, et al. The role of the sternum, costo-sternal articulations, intervertebral disc and facets in thoracic sagittal plane biomechanics. Spine. 2005;18:2014–2023. doi: 10.1097/01.brs.0000180478.96494.88. [DOI] [PubMed] [Google Scholar]
- 27.Feiertag M, Horton W, Norman J, et al. The effect of different surgical releases on thoracic spinal motion. Spine. 1995;20:1604–1611. doi: 10.1097/00007632-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 28.McLain R. Functional outcomes after surgery for spinal fractures: return to work and activity. Spine. 2004;4:470–477. doi: 10.1097/01.BRS.0000092373.57039.FC. [DOI] [PubMed] [Google Scholar]