Abstract
No previous studies have reported 10-year follow-up results for double-door laminoplasty using hydroxyapatite (HA) spacers. The purpose of this study was therefore to explore the long-term results of double-door laminoplasty using HA spacers and to determine if non-union or breakage of HA spacers is related to restenosis of the enlarged cervical canal. The study group consisted of 68 patients with a minimum of 10 years of follow-up after double-door laminoplasty using HA spacers. The average postoperative Japanese Orthopaedic Association score improved significantly after surgery and was maintained until the final follow-up. The average range of motion decreased by 42.6% in patients with cervical spondylotic myelopathy (CSM) and 65.8% in patients with ossification of the posterior longitudinal ligament (OPLL). The enlarged cervical canal area was preserved almost until the final follow-up. The average non-union rates of HA spacers were 21% in CSM and 17% in OPLL, and the average breakage rates were 24 in CSM and 21% in OPLL at the final follow-up. Although non-union and breakage of HA spacers were common, neither of these factors were correlated with restenosis of the enlarged cervical canal.
Keywords: Cervical myelopathy, Double-door laminoplasty, Hydroxyapatite spacer, Long-term outcome
Introduction
Laminoplasty is a standard surgical option for the treatment of cervical myelopathy caused by multi-segmental cervical canal stenosis. Expansive open-door laminoplasty [1] and double-door laminoplasty [2] are two basic techniques used to enlarge a stenotic cervical canal, while preserving the posterior elements of the spine. Modifications of the various surgical techniques have included the use of hydroxyapatite (HA) spacers [3] or miniplates [4], the performance of an osteotomy using a thread-wire saw [5], and preservation of the muscular attachments to the spinous processes [6, 7]. The use of HA spacers instead of autologous bone grafts was developed to decrease the operative time and reduce blood loss and donor site morbidity [3].
There have only been a few long-term follow-up reports of open-door [1, 8, 9] and double-door laminoplasty [2], and these have focused on the use of autologous bone grafts [2, 9] or simple sutures [1, 8] to maintain an enlarged spinal canal; little is known regarding the long-term results of laminoplasty using HA spacers. In this study, we investigated the long-term outcomes over a 10-year follow-up period after double-door laminoplasty using HA spacers. We also investigated if non-union or breakage of the spacers was related to restenosis of the enlarged cervical canal.
Materials and methods
A total of 136 consecutive patients with compressive cervical myelopathy underwent double-door laminoplasty using HA spinous process spacers between 1995 and 1999. Of these 136 patients, 19 died of causes unrelated to cervical disease, 41 patients were lost to follow-up before completing the 10-year follow-up period, and 8 patients were excluded from the study because of hemiplegia due to brain infarction, concurrent anterior long fusion, or severe arteriosclerosis obliterans. The study group therefore included 68 patients who were followed for at least 10 years after surgery. The mean follow-up period was 12.2 years, ranging from 10 to 14 years. There were 39 patients with cervical spondylotic myelopathy (CSM) and 29 patients with ossification of the posterior longitudinal ligament (OPLL). The patients’ demographic data are shown in Table 1. Age, sex, and preoperative clinical status did not differ significantly between the study population and the patients excluded from the study (data not shown).
Table 1.
Patient data
| CSM | OPLL | |
|---|---|---|
| No. of patients | 39 | 29 |
| Male/female | 24/15 | 21/8 |
| Average age (years) | 54.4 (35–77) | 56.2 (35–71) |
| Average follow-up period (years) | 12.4 (10–14) | 12.7 (10–14) |
| Surgical levels | ||
| C2–C7 | 23 | 22 |
| C3–C7 | 11 | 1 |
| C2–T1 | 5 | 4 |
| C3–T1 | 0 | 2 |
CSM cervical spondylotic myelopathy, OPLL ossification of the posterior longitudinal ligament
The procedure for double-door laminoplasty has been described in detail elsewhere [2]. In most patients, the cervical laminae from C3 to C7 were exposed laterally to the medial aspect of the facet joints through a conventional midline approach. For a C2 split, the semispinalis cervicis, rectus capitis posterior major, and obliquus capitis inferior muscles were detached transiently. Bilateral gutters were made using a high-speed burr at the transitional area between the facet joint and the laminae, and the spinous processes were then split sagittally with a high-speed burr. The spinal canal was enlarged by opening the split laminae bilaterally with a spreader. To maintain the expanded position, HA spacers (Apacerum®; Asahi Optical Co., Ltd., Tokyo, Japan) were placed between the split laminae and fixed with nonabsorbable sutures. In the case of C2 laminoplasty, detached muscles were repaired with nonabsorbable sutures. Patients wore a cervical orthosis for approximately 2 weeks.
The clinical results were assessed using the Japanese Orthopaedic Association scoring system (JOA score) for treatment of cervical myelopathy. The JOA score consists of several categories of function, i.e., upper- and lower-limb motor function, sensory function, and bladder function. Surgical complications and prevalence of residual axial symptoms were investigated. The range of motion (ROM) between C2 and C7 was measured on flexion–extension radiographs taken before and 1, 2, 3, 5, and 10 years after surgery.
Twenty-seven of 68 patients (13 CSM patients and 14 OPLL patients) were examined using computed tomography (CT) before and after surgery (1 month, 1 year, and at the final follow-up). The transverse area of the cervical canal was measured at the level of the vertebral pedicles using computer software (Scion Image, Scion Corp, Frederick, MD) before and after surgery (1 month and at the final follow-up). Non-union between HA spacers and split spinous processes, and breakage of HA spacers, were evaluated by CT at 1 year after surgery and at the final follow-up. Non-union was defined as a clear zone detected between an HA spacer and the split spinous process (Fig. 5b), and breakage was defined as fracture of an HA spacer (Fig. 5c).
Fig. 5.
Changes in cervical canal area in relation to the fate of HA spacers. Representative images of HA spacers with bone union (a), non-union (b), and breakage (c). Non-union was defined as a clear zone between the HA spacer and the split spinous process (white arrow), and breakage as fracture of an HA spacer (black arrowhead). Box-and-whisker plots demonstrate percent change in canal area. Neither non-union nor breakage of HA spacers correlated with restenosis of an enlarged cervical canal in either the CSM or OPLL groups (d, e)
Data were analyzed statistically using SPSS for Windows (version 17.0, SPSS Inc., Chicago, IL). Unpaired t tests or Mann–Whitney U tests were used to detect differences between the two groups. A P value <0.05 was considered to be significant.
Results
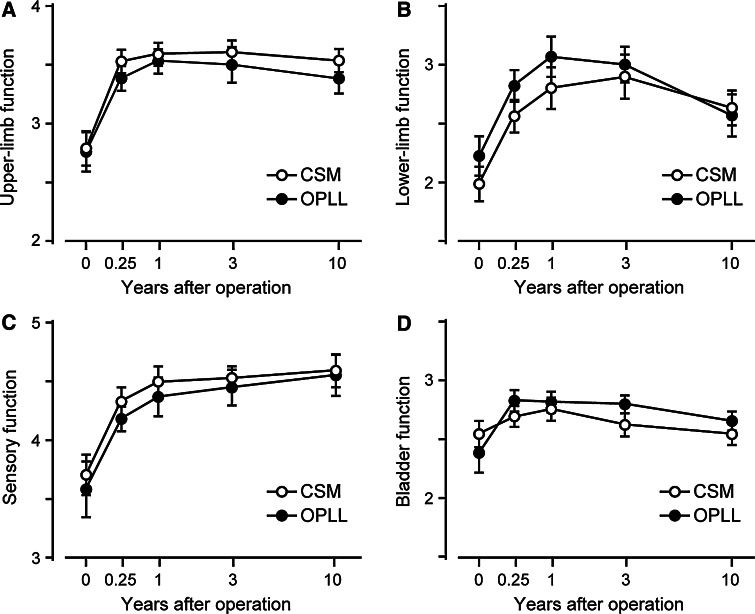
The average JOA score improved significantly at 3 months after surgery and was maintained almost until the final follow-up in both the CSM and OPLL groups (Table 2). The time courses of the postoperative JOA scores for each category of function (upper- and lower-limb motor function, sensory function, and bladder function) are shown in Fig. 1. The average upper-limb motor and sensory scores increased significantly at 3 months after surgery and were both preserved until the final follow-up in both the CSM and OPLL groups. The lower-limb motor score also improved after surgery, but decreased after the third postoperative year. Twelve patients with CSM (31%) and 11 patients with OPLL (38%) showed declines in lower-limb motor scores of 1–2 points between 3 years after surgery and the final follow-up. Most of the patients who showed late deterioration in lower-limb function had complications that impaired ambulatory ability, such as osteoarthritis in the lower-limb joints (4 CSM patients and 2 OPLL patients), spinal canal stenosis at the thoracolumbar spine (3 CSM patients and 5 OPLL patients), degenerative disease of the brain (1 patient in each group), and arteriosclerosis obliterans (1 OPLL patient). In particular, spinal canal stenosis at the thoracolumbar spine was a major cause of reduction in lower-limb function in the OPLL group, and four patients required additional surgery of the thoracolumbar spine during the observation period. The average bladder function scores in both the CSM and OPLL groups increased after surgery, but had returned to preoperative levels at the final follow-up.
Table 2.
Chronological changes in JOA scores after surgery
| JOA score | ||||
|---|---|---|---|---|
| CSM | P* | OPLL | P* | |
| Preoperative | 11.0 ± 2.6 | 11.0 ± 2.5 | ||
| 3 months after surgery | 13.1 ± 1.8 | 0.0003 | 13.1 ± 1.7 | 0.0012 |
| 1 year after surgery | 13.6 ± 2.1 | <0.0001 | 13.8 ± 2.2 | 0.0002 |
| 3 years after surgery | 13.6 ± 2.2 | <0.0001 | 13.7 ± 2.0 | 0.0001 |
| More than 10 years after surgery | 13.1 ± 2.4 | 0.0008 | 13.0 ± 2.7 | 0.008 |
Values are mean ± SD
JOA Japanese Orthopaedic Association, CSM cervical spondylotic myelopathy, OPLL ossification of the posterior longitudinal ligament
* P by Mann–Whitney U test
Fig. 1.
Time-course of JOA score for each category of function. The average upper-limb motor and sensory scores improved significantly after surgery and were maintained almost until the final follow-up in both the CSM and OPLL groups. The lower-limb motor scores improved significantly, but gradually deteriorated after the third postoperative year in both groups. Bars indicate standard errors
The most common postoperative complication was segmental motor weakness at the C5 segment, which developed in 4 (6%) out of 67 patients, although the symptoms resolved spontaneously within 1 year after surgery in all patients. Other complications included leakage of cerebrospinal fluid in three patients and transient deterioration of lower-limb function in one patient, all of which resolved without sequelae. One patient had lamina closure on the left side at C5 level due to fracture of a split spinous process. This patient had concomitant severe canal stenosis of the thoracic spine and showed impaired recovery of lower-limb function after surgery. He underwent simultaneous posterior thoracic spine decompression and a laminectomy at C5 at 6 months after the primary operation, which led to improved lower-limb function. Axial pain was one of the most common subjective symptoms during the follow-up period [6]. The percentage of patients with axial pain decreased over time, but 17 patients (25%) still complained of axial pain at the final follow-up.
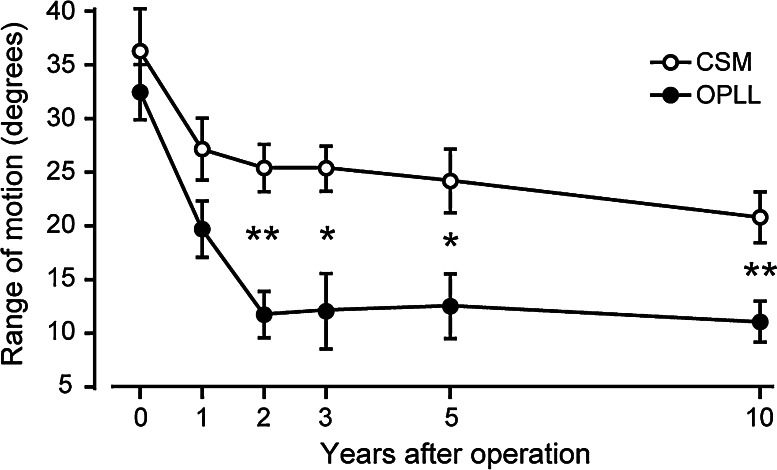
The cervical ROM decreased over time and reached a plateau by 2 years after surgery (Fig. 2). The average ROMs in CSM and OPLL patients decreased from preoperative values of 36.2° and 32.4° to postoperative values of 20.8° and 11.1°, respectively, at the final follow-up. The reduction in ROM was significantly larger in the OPLL group than in the CSM group (66 vs. 43%, P = 0.0052, 95% CI of the difference, 3.22–16.16). No patient in either group showed severe kyphotic deformity or instability at the final follow-up.
Fig. 2.
Changes in cervical ROM. The cervical ROM decreased over time and reached a plateau by 2 years after surgery. The reduction in ROM was significantly larger in the OPLL group compared to the CSM group (66 vs. 43%, P = 0.0052) at the final follow-up. Bars indicate standard errors: *P < 0.05, **P < 0.01
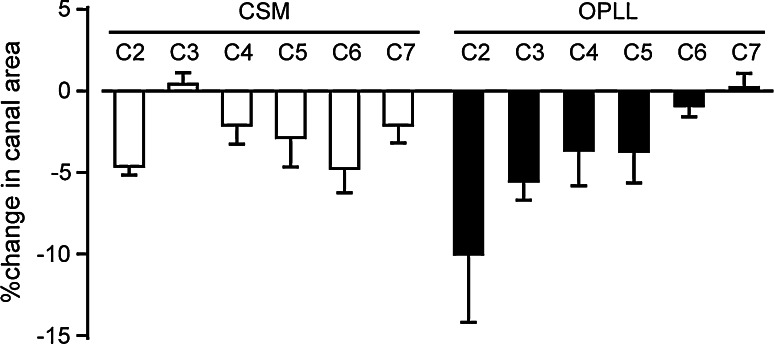
Chronological changes in cervical canal area, quantified by CT, are shown in Fig. 3. The average spinal canal area was significantly increased at every cervical level 1 month after surgery and was maintained almost until the final follow-up. The decrease in spinal canal area at the final follow-up was compared to that at 1 month after surgery to determine which cervical level was most susceptible to restenosis (Fig. 4). In patients with CSM, the decrease in canal area was smallest at the C3 level, while the canal area decreased preferentially in the upper cervical spine, with a maximum reduction of 9.9% at the C2 level, in OPLL patients.
Fig. 3.
Changes in spinal canal area quantified by CT. The average area of the spinal canal significantly increased at every cervical level 1 month after surgery and was maintained almost until the final follow-up (Pre preoperative, 1M 1 month after surgery, 10Y 10 years after surgery). Bars indicate standard errors
Fig. 4.
Changes in cervical canal area at the final follow-up compared to 1 month after surgery. The decrease was smallest at the C3 level in patients with CSM. In patients with OPLL, the canal area decreased preferentially in the upper cervical spine, which showed a maximum reduction of 9.9% at the C2 level. Bars indicate standard errors
The average rates of non-union between HA spacer and split spinous process were 27% in CSM and 25% in OPLL patients at 1 year after surgery (Table 3). The rates of non-union decreased over time, but remained at 21% in CSM and 17% in OPLL patients at the final follow-up. The average breakage rates of HA spacers in the CSM and OPLL groups were 8 and 4%, respectively, at 1 year after surgery, which increased to 24 and 21% at the final follow-up. However, all the bilateral gutters examined by CT demonstrated bone union by the final follow-up, and no spacers were significantly displaced from their original location. We also investigated the changes in spinal canal area between 1 month after surgery and the final follow-up to determine if non-union or breakage of HA spacers accelerated restenosis of the spinal canal. The percent changes in spinal canal area were comparable for both bony union and non-union in both the CSM and OPLL groups (Fig. 5d). In addition, no significant differences in the cervical canal area were detected for patients with intact HA spacers compared to those with broken HA spacers in either the CSM or OPLL groups (Fig. 5e).
Table 3.
Incidence of non-union and breakage of HA spacers
| No. of evaluated spacers | Non-union | Breakage | |||
|---|---|---|---|---|---|
| 1 year | Final follow-up | 1 year | Final follow-up | ||
| CSM (n = 13) | 71 | 19 (27%) | 15 (21%) | 6 (8%) | 17 (24%) |
| OPLL (n = 14) | 77 | 19 (25%) | 13 (17%) | 3 (4%) | 16 (21%) |
HA hydroxyapatite, CSM cervical spondylotic myelopathy, OPLL ossification of the posterior longitudinal ligament
Discussion
The results of the current study demonstrated satisfactory long-term outcomes following double-door laminoplasty using HA spacers for compressive myelopathy. The average JOA score improved significantly at 3 months after surgery and was largely maintained during a minimum 10-year follow-up period in both CSM and OPLL patients. When the JOA score was divided into each functional category, the average score for lower-limb function gradually decreased over time, whereas upper-limb and sensory functions were stable during the observation period. The late deterioration of lower-limb function was largely attributable to degenerative changes, such as spinal canal stenosis at the thoracolumbar spine, osteoarthritis of the knee, and other general complications associated with aging. In particular, spinal canal stenosis at the thoracolumbar spine was the major cause of late deterioration of lower-limb function in patients with OPLL.
Several long-term studies of cervical laminoplasty found that the average ROM of the cervical spine substantially decreased over time [1, 2, 8, 9]. Seichi et al. [2] reported a 77% loss in the average ROM (36°–8°) over a 10-year follow-up of double-door laminoplasty using autologous bone grafts. Chiba et al. [1] reported that the pre- and postoperative ROMs were 44° and 14° in CSM and 32° and 11° in OPLL, respectively (a decrease of 68% in the CSM group and 66% in the OPLL group), over an average 14-year follow-up period after expansive open-door laminoplasty. The reductions in ROM in the current series (decreases of 43% in the CSM group and 66% in the OPLL group) were generally low, compared to these other long-term studies. The relative preservation of the ROM in the current study is attributable to the difference in the duration of cervical orthosis after surgery. Our patients wore a cervical orthosis for approximately 2 weeks, whereas patients in the previous studies wore an orthosis for 2–3 months after surgery. Several authors have proposed contracture of the paraspinal muscles and the facet joint as a potential cause of reduced cervical ROM after laminoplasty [7, 10, 11]. Early removal of the cervical orthosis and postoperative neck exercise are recommended to prevent atrophy and dysfunction of the paraspinal muscles. In addition, modified surgical techniques have been developed to preserve the paraspinal muscles, not only to maintain cervical ROM, but also to reduce axial symptoms [11, 12]. Roselli et al. [12] reported well-preserved cervical ROM after open-door laminoplasty using unilateral muscle dissection, while Takeuchi et al. [11, 13] maintained cervical ROM following double-door laminoplasty by preserving the semispinalis cervicis inserted into C2. Because bilateral muscle dissection is inevitable during double-door laminoplasty, further modifications to the surgical techniques are desirable to preserve the integrity of the cervical muscles.
One of the postoperative complications of cervical laminoplasty is restenosis of the enlarged spinal canal as a result of hinge closure [14]. One patient in our study required additional surgery because of hinge closure caused by fracture of the split spinous process. The constitutionally thin spinous process fractured spontaneously on the left side at the contact face with an HA spacer. This is a rare complication, but great care must be taken to avoid hinge closure resulting from fracture of a split spinous process. The incidences of other complications, such as segmental motor paresis, leakage of cerebrospinal fluid, and axial pain were similar to those demonstrated in long-term follow-ups of open-door laminoplasty [1, 9] and double-door laminoplasty using autologous bone grafts [2].
In this study, we focused on chronic changes in the cervical canal area over a 10-year period. The average cross-sectional area at each spinal level was significantly increased after surgery and was maintained almost until the final follow-up. The percent change in spinal canal area (comparison between the area at final follow-up and 1 month after surgery) revealed that the changes were smallest at the C3 spinal level in patients with CSM. This result may be related to the limited segmental motion between C2 and C3 caused by spontaneous lamina fusion, which occurs most commonly at the C2–C3 level [2]. The spinal canal area decreased preferentially in the upper cervical spine in patients with OPLL, especially at the C2 level. This can be attributed to the longitudinal growth of OPLL, which occurs preferentially in mixed and continuous types of OPLL after laminoplasty [8, 15]. Two patients showed obvious growth of the OPLL towards C2 and a mildly narrowed spinal canal in the upper cervical spine. However, neither of these patients showed deterioration of acquired function. The preserved function may have been attributed to restricted segmental motion in the upper cervical spine as a result of bridge formation by continuous type-OPLL.
Non-union and breakage of HA spacers at the final follow-up were not rare. However, none of the spacers were significantly displaced from their original location during the observation period. No patient in this study developed delayed dural damage or myelopathy due to displacement of HA spacers, as reported in other recent studies [16, 17]. Furthermore, neither non-union nor breakage of HA spacers correlated with restenosis of the enlarged spinal canal. These results suggest that rigid fusion between the HA spacer and the split spinous process is not essential for maintaining an enlarged spinal canal, even in the long-term. We speculate that HA spacers have fulfilled most of their roles by the time bone fusion has occurred in the lateral gutters, and that surgical techniques aimed at preventing the early displacement of HA spacers are essential for ensuring a good outcome after double-door laminoplasty. Kaito et al. [16] suggested that installing HA spacers at the base of the spinous process can help to prevent postoperative HA displacement. However, further prospective studies are needed to determine the most suitable surgical techniques.
This study had several limitations. It was a retrospective analysis involving a relatively small number of patients. In particular, limited CT data were available because of the retrospective nature of this study; however, functional outcomes estimated by JOA scores were comparable between patients who completed periodical CT scans and those who did not. Prospective controlled studies using patient-based outcomes are required to verify the efficacy of double-door laminoplasty using HA spacers.
Conclusions
The long-term results of double-door laminoplasty using HA spacers were satisfactory. Although non-union and breakage of HA spacers was not rare, an enlarged cervical canal was maintained over a 10-year period. Given the favorable long-term results with no donor site morbidity, the use of HA spacers for double-door laminoplasty is justified for the treatment of cervical myelopathy caused by multisegmental cervical canal stenosis. Further modifications of surgical technique are desirable to reduce postoperative complications, such as reduction in cervical ROM, segmental motor paresis, and axial symptoms.
Acknowledgments
Conflict of interest
None.
References
- 1.Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31:2998–3005. doi: 10.1097/01.brs.0000250307.78987.6b. [DOI] [PubMed] [Google Scholar]
- 2.Seichi A, Takeshita K, Ohishi I, et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine. 2001;26:479–487. doi: 10.1097/00007632-200103010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Nakano K, Harata S, Suetsuna F, et al. Spinous process-splitting laminoplasty using hydroxyapatite spinous process spacer. Spine. 1992;17(Suppl 3):S41–S43. doi: 10.1097/00007632-199203001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Tsuzuki N. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization: a computerized morphometric analysis. Spine. 1997;22:926–927. doi: 10.1097/00007632-199704150-00022. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Benlloch JA, Maruenda-Paulino JI, Barra-Pla A, et al. Expansive laminoplasty as a method for managing cervical multilevel spondylotic myelopathy. Spine. 2003;28:680–684. doi: 10.1097/01.BRS.0000051913.55259.5F. [DOI] [PubMed] [Google Scholar]
- 6.Hosono N, Sakaura H, Mukai Y, et al. The source of axial pain after cervical laminoplasty—C7 is more crucial than deep extensor muscles. Spine. 2007;32:2985–2988. doi: 10.1097/BRS.0b013e31815cda83. [DOI] [PubMed] [Google Scholar]
- 7.Kotani Y, Abumi K, Ito M, et al. Minimum 2-year outcome of cervical laminoplasty with deep extensor muscle-preserving approach: impact on cervical spine function and quality of life. Eur Spine J. 2009;18:663–671. doi: 10.1007/s00586-009-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96(Suppl 2):180–189. [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Kanamori M, Ishihara H, et al. Minimum 10-year follow-up after en bloc cervical laminoplasty. Clin Orthop Relat Res. 2003;411:129–139. doi: 10.1097/01.blo.0000069889.31220.62. [DOI] [PubMed] [Google Scholar]
- 10.Iizuka H, Nakagawa Y, Shimegi A, et al. Clinical results after cervical laminoplasty: differences due to the duration of wearing a cervical collar. J Spinal Disord Tech. 2005;18:489–491. doi: 10.1097/01.bsd.0000154447.83084.b2. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi K, Yokoyama T, Ono A, et al. Cervical range of motion and alignment after laminoplasty preserving or reattaching the semispinalis cervicis inserted into axis. J Spinal Disord Tech. 2007;20:571–576. doi: 10.1097/BSD.0b013e318046363a. [DOI] [PubMed] [Google Scholar]
- 12.Roselli R, Pompucci A, Formica F, et al. Open-door laminoplasty for cervical stenotic myelopathy: surgical technique and neurophysiological monitoring. J Neurosurg. 2000;92:38–43. doi: 10.3171/spi.2000.92.1.0038. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi K, Yokoyama T, Ono A, et al. Limitation of activities of daily living accompanying reduced neck mobility after laminoplasty preserving or reattaching the semispinalis cervicis into axis. Eur Spine J. 2008;17:415–420. doi: 10.1007/s00586-007-0553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu HL, Xiang LB, Liu J, et al. Laminoplasty using Twinfix suture anchors to maintain cervical canal expansion. Eur Spine J. 2010;19:1795–1798. doi: 10.1007/s00586-010-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament progress after cervical laminoplasty? Spine. 2006;31:2807–2812. doi: 10.1097/01.brs.0000245870.97231.65. [DOI] [PubMed] [Google Scholar]
- 16.Kaito T, Hosono N, Makino T, et al. Postoperative displacement of hydroxyapatite spacers implanted during double-door laminoplasty. J Neurosurg Spine. 2009;10:551–556. doi: 10.3171/2009.2.17680. [DOI] [PubMed] [Google Scholar]
- 17.Kanemura A, Doita M, Iguchi T, et al. Delayed dural laceration by hydroxyapatite spacer causing tetraparesis following double-door laminoplasty. J Neurosurg Spine. 2008;8:121–128. doi: 10.3171/SPI/2008/8/2/121. [DOI] [PubMed] [Google Scholar]