The regulatory roles for NOD2 versus TLR signaling were explored in murine uveitis triggered by peptidoglycan.
Abstract
Purpose.
The innate immune receptor NOD2 is a genetic cause of uveitis (Blau syndrome). Intriguingly, in the intestine where polymorphisms of NOD2 predispose to Crohn's disease, NOD2 reportedly suppresses inflammation triggered by the bacterial cell wall component, peptidoglycan (PGN). Whether NOD2 exerts a similar capacity in the regulation of ocular inflammation to PGN has not been explored.
Methods.
NOD2, NOD1, or MyD88 knockout (KO) mice and their wild-type (WT) controls were administered an intravitreal injection of PGN (a metabolite of which is the NOD2 agonist, muramyl dipeptide), or synthetic TLR2/1 and TLR2/6 agonists, Pam3CSK4 and FSL-1. Ocular inflammation was assessed by intravital microscopy and histopathology. Cytokine production in eye tissue homogenates was measured by ELISA.
Results.
PGN triggered uveitis in mice. This inflammation was abolished in the absence of the TLR signaling mediator MyD88. NOD2 exerted a negative regulatory role because PGN-triggered eye inflammation was exacerbated in NOD2 KO mice. Increased intravascular response coincided with enhanced leukocytes within the aqueous and vitreous humors. The enhanced susceptibility of NOD2 KO mice to PGN uveitis coincided with increased cytokine production of IL-12p40, IL-17, and IL-23 but not IL-12p70, TNFα, or IFNγ. NOD1 deficiency did not result in the same sensitivity to PGN. Ocular inflammation induced by synthetic TLR2 agonists required MyD88 but not NOD2 or NOD1.
Conclusions.
NOD2 may serve differential roles in the eye to promote inflammation while also tempering cell responses to PGN akin to what has been reported in colitis.
Uveitis (or intraocular inflammation) describes a group of diseases with varying etiologies. Both genetic factors and infectious agents have been invoked as contributors to uveitis. Systemic diseases such as sarcoidosis, multiple sclerosis, Behçet's disease, or spondyloarthropathies may all present with anterior uveitis. Likewise, infectious agents such as viral or bacterial infections are well-recognized causes of uveitis. In animal models of autoimmune uveitis, an adjuvant that is usually a bacterial product is generally required to induce substantial disease. Indeed, it may be that there are overlapping mechanisms wherein in the setting of a genetically predisposed individual, an environmental infectious trigger could initiate disease. Cytokines such as TNFα, IL-6, and IL-1 have been proven therapeutic targets in systemic autoinflammatory or autoimmune diseases, but have not always yielded success as targets for the treatment of uveitis. As such, the focus has been on the initiating events by which inflammatory mediators are produced. Emerging evidence supports a role for innate immune receptors or pathogen recognition receptors (PRRs) that recognize conserved microbial structures or pathogen-associated molecular patterns (PAMPs). Examples of PAMPs include lipopolysaccharides (LPS), peptidoglycan (PGN), or microbial nucleic acids. Innate immune receptors have also been implicated as possible modulators of inflammation induced by noninfectious, endogenous danger signals further emphasizing their importance not only in microbial defense but also in inflammatory diseases with no obvious infection.
The Toll-like receptor (TLR) family is a class of PRRs. TLR4, which recognizes LPS, is strongly implicated in experimental models of uveitis triggered by LPS.1 Certain TLRs, for example, TLR2, TLR4, and TLR9, have been linked with inflammatory diseases that often present with uveitis.2 Historically, the TLR family was recognized for its role in host defense, but a second class of PRRs discovered approximately 10 years ago—the nucleotide binding oligomerization domain (NOD)-like receptor (NLR) family—has also since been established as an important component of innate immunity. The importance of the NLR family in regulation of inflammation is further underscored by the fact that polymorphisms or mutations within several NLR family members predispose to chronic, autoinflammatory human diseases including Muckle-Wells syndrome, familial cold autoinflammatory syndrome, vitiligo, allergic diseases such as asthma and atopic eczema, adult sarcoidosis, bare lymphocyte syndrome (which is clinically similar to severe combined immunodeficiency), Blau syndrome, and Crohn's disease.3 Despite the importance of NLRs, very few studies have addressed their contribution to ocular disease.
We are particularly interested in the NLR family member NOD2 (also known as NLRC2 or caspase activation and recruitment domain 15 [CARD15]) as it appears to play an important role in regulating inflammatory responses of the eye. Mutations in the nucleotide oligomerization domain of NOD2 cause an inherited form of uveitis (called Blau syndrome) that is accompanied by arthritis and dermatitis.4 Interestingly, polymorphisms in the LRR domain of NOD2 are also associated with Crohn's disease,5,6 an intestinal inflammatory disease that can be accompanied by uveitis. Despite the link between the gene NOD2 and uveitis, very little is known regarding functions of NOD2 in the eye that relate to the development of uveitis.
NOD2 is considered an important aspect of immune surveillance of intracellular pathogens and detects fragments of peptidoglycan (PGN) from bacteria.7–9 NOD2 is activated by muramyl dipeptide (MDP), a PGN motif present in all Gram-negative and Gram-positive bacteria, and activates downstream signaling events involving the serine/threonine kinase RIP2 (also known as RICK, CARDIAK), MAPK, and NF-κB.10 NOD2 works in concert with NOD1, which detects diaminopimelic acid-type peptidoglycan7,8 found predominantly in Gram-negative bacteria. Our laboratory has previously demonstrated that NOD2 or NOD1 agonists initiate ocular inflammation in mice,11–13 supporting an inflammatory role for these NLRs in the eye. Intriguingly, NOD2 is thought to play a secondary, regulatory role in enhancing or diminishing TLR responses in other systems.14–19 In the intestine, where NOD2 polymorphisms predispose to Crohn's disease, NOD2 is thought to exert a negative regulatory role in PGN-triggered inflammation.20–22 The potential interplay between NOD2 and TLRs in regulating inflammation of the eye in vivo has not been investigated. Here we sought to examine the contribution of TLR signaling and NOD2 or NOD1 in PGN-triggered uveitis.
Methods
Reagents
PGN purified from Staphylococcus aureus or the synthetic lipopeptides (FSL-1 and Pam3CSK4) and D-γ-Glu-mDAP (iE-DAP) were purchased from Invitrogen (Carlsbad, CA). All compounds were dissolved in pyrogen-free, sterile saline. PGN was thoroughly vortexed and sonicated at 30% amplitude for 5 seconds.
Mice
NOD2 knockout (KO) mice (previously reported) were backcrossed onto BALB/c background.11 NOD1 KO mice were crossed with tyrosinase mutant mice (Jackson Laboratories, Bar Harbor, ME) to reduce pigment in the eye since this interferes with the intravital microscopic examination. MyD88 KO mice on BALB/c background were gifted by Shizuo Akira (Osaka University, Japan). All mice were housed in a facility approved by the Association of Assessment and Accreditation of Laboratory Animal Care International. Age- and gender-matched (8–10-week-old) congenic controls were purchased from Jackson Laboratories. Procedures were carried out in accordance with National Institutes of Health and guidelines designated by Oregon Health & Science University Institutional Animal Care and Use policies and with ARVO guidelines.
Uveitis Model and Intravital Microscopy
For induction of uveitis, mice were administered an intravitreal (i.v.t.) injection of the indicated compounds as previously described.13 The contralateral eye was administered an injection of an equal volume of saline. The cellular response within the iris was assessed by intravital microscopy as previously described in detail,11 wherein the leukocytic response within the vasculature and extravascular tissue of the iris is directly visualized using an intravital microscope. Digital images were captured from anesthetized mice (1.7% isofluorane) with a black-and-white video camera (Kappa Scientific, Gleichen, Germany) for off-line quantification of leukocyte rolling, adherence, and infiltration.
Histology
Mouse eyes were enucleated and prepared for histologic assessment as previously described11 Briefly, eyes were fixed in 10% neutral-buffered formalin overnight and then in 80% ethanol for embedding in paraffin and subsequent tissue sectioning. Seven-micrometer tissue sections obtained through the papillary-optic nerve axis at four different depths were stained with hematoxylin and eosin. Four sections from different levels of each eye were examined by an observer who was masked to the treatments, and the numbers of cells within the anterior and posterior segments were quantified. Values are mean ± SE across the four representative sections examined. Slides were photographed at 200× using a microscope (DM500B; Leica, Wetzlar, Germany) and a digital camera (CD500; Leica).
Cytokine Measurements
Protein was extracted from eye tissue as described13 in the presence of a protease inhibitor cocktail (Roche, Manneheim, Germany). Equal amounts of protein for each sample were measured for the cytokines IFNγ, IL-12p40, IL-12p70, IL-23 (p19), and IL-17 using commercially available ELISA kits (R&D Systems, Minneapolis, MN).
Statistics
Data are represented as mean ± SE. Mean differences between treatment and genotypes were analyzed using two-way and one-way analysis of variance with t-test post hoc analyses. Differences were considered statistically significant when P < 0.05.
Results
TLR Signaling Plays an Essential Role in the Induction of PGN-Induced Uveitis
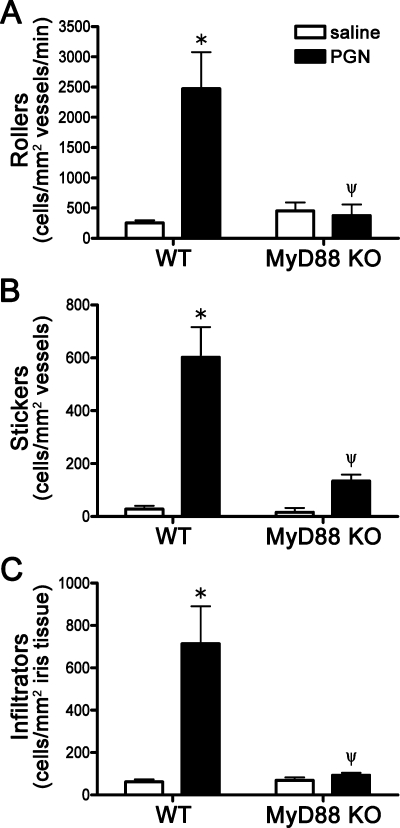
PGN is a putative trigger of ocular inflammation, but the mechanisms by which it could trigger uveitis are not entirely understood. We assessed the initial cellular responses within the vasculature of the iris tissue by intravital microscopy in response to local, intraocular PGN injection. Intravital microscopy enables us to assess in real-time the cellular responses within the microvasculature of the iris in vivo as previously described,11 thereby quantifying the number of rolling, adhering, and infiltrating leukocytes. We find that local PGN administration of 5 μg results in a significant increase in early cellular responsiveness such as leukocyte rolling and adherence as well as subsequent infiltration into the iris itself within an early time frame, with maximal intravascular inflammation observed consistently at 6 hours post-treatment (Figs. 1A–C). The contribution of TLR signaling was assessed with the use of MyD88 KO mice. The adaptor molecule MyD88 is required by all TLRs except for TLR3 for the activation of MAPK and NF-κB signaling pathways. We find that deficiency in MyD88 results in complete resistance to PGN-induced uveitis (Fig. 1), as the increase in rolling, adhering, and infiltrating leukocytes was abolished. Since PGN can be degraded to MDP, which signals through NOD2 independently of MyD88, the observations with MyD88 knockout mice also indicate that this quantity of PGN does not activate NOD2 sufficiently for inflammation to be detectable.
Figure 1.
Toll-like receptor signaling is essential for PGN-induced uveitis. MyD88 KO mice or WT controls were administered an i.v.t. injection of 5 μg PGN or saline, and ocular inflammation was assessed by intravital microscopy 6 hours later. The number of rolling (A), sticking (B), and infiltrating (C) leukocytes within the iris was quantified. Values are mean ± SE (n = 10–14 mice per group). *P < 0.05 saline vs. PGN within a genotype; ψP < 0.05 KO vs. WT mice treated with PGN.
NOD2 Deficiency Results in Increased Susceptibility to PGN-Induced Uveitis
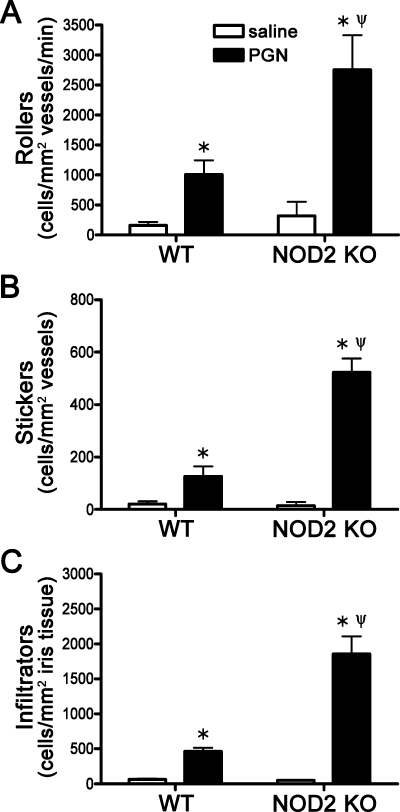
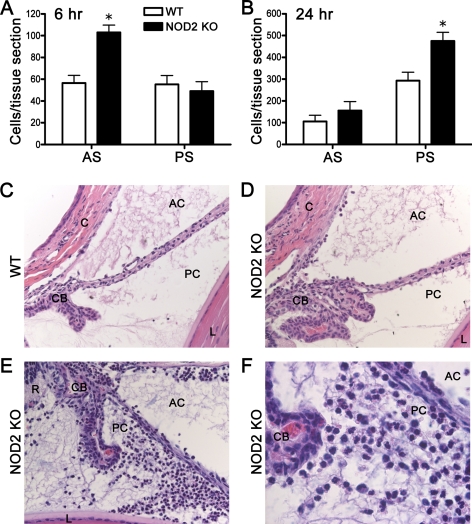
We next tested whether NOD2 deficiency alters the sensitivity of the eye to PGN exposure. NOD2 KO mice were injected with PGN and inflammation was assessed by intravital microscopy. We find that NOD2 deficiency results in increased sensitivity to PGN as the number of rolling, adhering, and infiltrating leukocytes was significantly increased compared to wild-type (WT) mice treated with PGN (Fig. 2). Consistent with the intravital data, histopathology revealed a quantitative increase in the number of leukocytes within the aqueous and vitreous of both the anterior and posterior segments from NOD2 KO eyes compared with WT controls (Figs. 3A, 3B). The exacerbated inflammation in NOD2 KO mice can be visualized in the representative photos (Figs. 3C–F), wherein an increased mixed population of mononuclear and polymorphonuclear leukocytes are observed within the anterior chamber, posterior chamber, ciliary body, and posterior segment.
Figure 2.
NOD2 deficiency results in increased susceptibility to PGN-induced uveitis. NOD2 KO or WT controls were administered an i.v.t. injection of 5 μg PGN or saline, and ocular inflammation was assessed by intravital microscopy 6 hours later. The number of rolling (A), sticking (B), and infiltrating (C) leukocytes within the iris was quantified. Values are mean ± SE (n = 10–16 mice per group). *P < 0.05 saline vs. PGN within a genotype; ψP < 0.05 KO vs. WT mice treated with PGN.
Figure 3.
NOD2 deficiency results in exacerbated histopathlogy during PGN-triggered uveitis. NOD2 KO or WT controls were administered an i.v.t. injection of 5 μg PGN or saline, and ocular inflammation was assessed histologically at 6 and 24 hours. The number of leukocytes within the anterior and posterior segments were quantified (A, B); values are mean ± SE (n = 5 mice per group); *P < 0.05 KO vs. WT mice treated with PGN. (C, D) Representative photos taken at 400× of WT and NOD2 KO mice injected with PGN and collected 6 hours later; (E, F) images of NOD2 KO eyes at 24 hours post-PGN treatment at 400× and 1000×, respectively.
Differential Functions for NOD2 and NOD1 in PGN-Triggered Uveitis
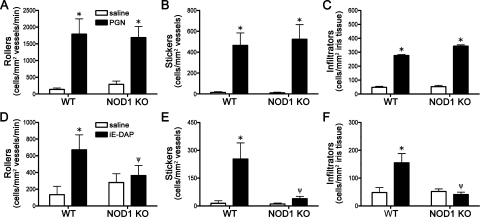
We have previously shown that NOD1 is expressed in murine eye tissue and that it promotes ocular inflammation when triggered by its synthetic agonist, γ-D-glutoamyl-meso-diaminopimelic acid (iE-DAP).23 As such we tested whether NOD1 might have the same regulatory capacity as NOD2 in PGN-triggered ocular inflammation. We find that in contrast to NOD2 KO mice, NOD1 KO mice did not show a significant alteration in the cellular response to intraocular PGN injection as assessed by intravital microscopy (Figs. 4A–C). This would support differential roles for NOD2 and NOD1 in regulation of ocular inflammation. As would be expected, however, NOD1 KO mice do show abolished ocular inflammation triggered by its agonist, iE-DAP (Fig. 4D–F), as the number of rolling, adhering, and infiltrating leukocytes was abrogated in mice lacking NOD1.
Figure 4.
Differential functions for NOD2 and NOD1 in PGN-triggered uveitis. NOD1 KO mice or WT controls were administered an i.v.t. injection of 5 μg PGN or saline, and ocular inflammation was assessed by intravital microscopy 6 hours later. The number of rolling (A), sticking (B), and infiltrating (C) leukocytes within the iris was quantified. Values are mean ± SE (n = 8–12 mice per group). * P< 0.05 saline vs. PGN within a genotype; ψP < 0.05 KO vs. WT mice treated with PGN. (D–F) Quantification of rolling, sticking, and infiltrating leukocytes within the iris in response to 100 μg iE-DAP i.v.t. injection. * P < 0.05 saline vs. iE-DAP within a genotype; ψP < 0.05 KO vs. WT mice treated with iE-DAP.
NOD2 Deficiency Does Not Alter Uveitis Triggered by Synthetic TLR2 Agonists
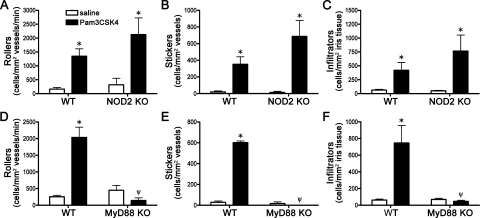
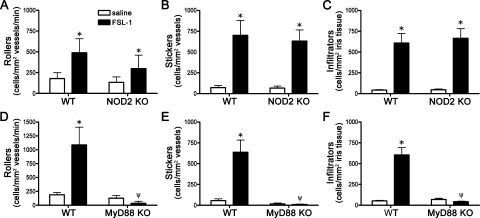
PGN contains MDP as part of its structure, which could be directly sensed by NOD2 in the context of simultaneous TLR activation by PGN. To further assess the direct versus indirect capacity of NOD2 to regulate TLR2 responses in the eye, we used synthetic lipopeptides to activate TLR2 hetero-dimers in the absence of any MDP. The diacylated lipopeptide, FSL-1, or the triacylated lipopeptide, Pam3CSK4, were used to activate TLR2/6 or TLR2/1 receptor complexes, respectively. As shown in Figures 5A–C, NOD2 deficiency does not alter responsiveness to the TLR2/1 agonist, Pam3CSK4. Although there was a trend toward enhanced inflammation in NOD2 KO mice, this did not achieve statistical significance with the number of mice (8 to 12 per group). Of course, we cannot exclude the possibility that the absence of NOD2 would show a statistically significant effect on activation of a TLR2 agonist if we studied a larger number of animals. However, we have been able to show statistical significance for the absence of NOD2 using smaller groups of mice with PGN as an agonist. In contrast, MyD88 deficiency did significantly impair Pam3CSK4 responsiveness (Figs. 5D–F), indicating a TLR-dependent and NOD2-independent response. NOD2 deficiency does not appear to modulate uveitis triggered by the diacetylated lipopeptide and TLR2/6 agonist FSL-1 (Figs. 6A–C). As would be expected, MyD88 deficiency significantly impairs responsiveness to FSL-1 stimulation (Figs. 6D–F).
Figure 5.
NOD2 deficiency does not alter uveitis triggered by synthetic TLR2/1 agonist Pam3CSK4. NOD2 KO mice or WT controls were administered an i.v.t. injection of 1 μg Pam3CSK4 or saline, and ocular inflammation was assessed by intravital microscopy 6 hours later. The number of rolling (A), sticking (B), and infiltrating (C) leukocytes within the iris was quantified. (D–F) Quantification of rolling, sticking, and infiltrating leukocytes within the iris in response to Pam3CSK4 injection in MyD88 KO vs. WT controls. Values are mean ± SE (n = 8–12 mice per group). *P < 0.05 saline vs. Pam3CSK4 within a genotype; ψP < 0.05 KO vs. WT mice treated with Pam3CSK4.
Figure 6.
NOD2 deficiency does not alter uveitis triggered by synthetic TLR2/6 agonist FSL-1. NOD2 KO mice or WT controls were administered an i.v.t. injection of 1 μg FSL-1 or saline, and ocular inflammation was assessed by intravital microscopy 6 hours later. The number of rolling (A), sticking (B), and infiltrating (C) leukocytes within the iris was quantified. (D–F) Quantification of rolling, sticking, and infiltrating leukocytes within the iris in response to FSL-1 injection in MyD88 KO vs. WT controls. Values are mean ± SE (n = 9–10 mice per group). *P < 0.05 saline vs. FSL-1 within a genotype; ψP < 0.05 KO vs. WT mice treated with FSL-1.
NOD2 Deficiency Results in Skewed Cytokine Responses to PGN
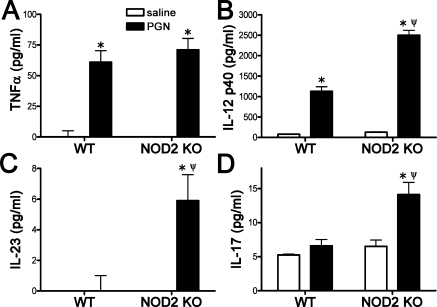
In addition to the effect of NOD2 deficiency on the cellular responses, we further examined how NOD2 deficiency might alter local cytokine production in response to PGN. Our findings indicate that NOD2 deficiency did not alter PGN-induced TNFα production (Fig. 7A) but did exacerbate IL-12p40 production (Fig. 7B). The IL-12p40 subunit of IL-12 dimerizes with the IL-12p35 subunit to activate the IL-12 receptor. Alternatively, the IL-12p40 subunit can dimerize with the p19 subunit of IL-23 to activate the IL-23 receptor and thereby promote IL-17 production. Hence we also measured IL-12p70, IL-23p19, and IL-17 cytokine levels to determine the extent to which NOD2 deficiency influences their production after PGN exposure. We find that NOD2 deficiency results in exacerbated production of IL-23 and IL-17 (Figs. 7C, 7D) but PGN treatment did not significantly increase IL-12p70 production in either genotype (data not shown here). Cytokine production of IFN-γ was undetectable by this assay at this time point. At 24 hours post-PGN injection, cytokine production had markedly reduced compared with 6 hours with no measurable production of IL-17 or IL-23, suggesting a resolution in cytokine production at this time. TNFα and IL-12p40 induction were statistically different from saline controls with TNFα levels at 48.84 pg/mL ± 4.97 in WT-PGN-treated eyes versus 42.95 pg/mL ± 2.8 in NOD2 KO-PGN-treated eyes and IL-12p40 levels at 112.72 ± 0.72 in WT-PGN-treated eyes versus 419.93 pg/mL ± 66.6 in NOD2 KO-PGN-treated eyes (P = 0.07, n = 6). No statistical difference was observed in the NOD2 KO mice, supporting a role for NOD2 in shaping the initiating events to PGN responses within the eye.
Figure 7.
NOD2 deficiency results in skewed cytokine responses to PGN. NOD2 KO or WT controls were administered an i.v.t. injection of 5 μg PGN or saline, and cytokine production in eye tissue homogenates was assessed by ELISA 6 hours later. Values are mean ± SE (n = 5–7 mice per group). *P < 0.05 saline vs. PGN within a genotype; ψP < 0.05 KO vs. WT mice treated with PGN.
Discussion
The pathogenesis of uveitis is incompletely understood, although innate immune receptors have been implicated in regulating ocular inflammation. TLRs are expressed in the eye, and their inflammatory function is an active area of ophthalmology research. In contrast, very minimal research has focused on NLR family members or the possible interplay between the two receptor family types within the eye. In the present study, we investigated the interplay between TLR signaling and NOD2 or NOD1 in ocular inflammation resulting from an intraocular PGN injection. Our findings suggest opposing roles for NOD2 and TLR signaling in that MyD88 is required for optimal inflammation. In contrast, we have found that the inflammatory response to PGN is not reduced in the absence of NOD2 expression but is, in fact increased, indicating that the presence of NOD2 is critical for proper suppression of PGN-triggered uveitis. The greater susceptibility of NOD2 KO mice to PGN-triggered uveitis was associated with increased cellular responses within the iris vasculature, aqueous and vitreous humors along with increased cytokine production of IL-12p40, IL-17, and IL-23. The absence of NOD2 did not influence ocular inflammation in response to synthetic lipopeptides that trigger TLR2/1 or TLR2/6 heterodimers but that do not contain MDP. Interestingly, NOD1 deficiency did not result in the same sensitivity to PGN as NOD2 deficiency did, thereby identifying differential functions of these two NLR receptors in the eye.
On activation by their synthetic agonists, NOD1 and NOD2 will self-oligomerize, recruit the kinase RIP2, and signal NF-κB and MAPK pathways and transcription of various inflammatory cytokines and chemokines.10 However, despite the identification of the PGN moieties sensed by these two NLRs, their roles in vivo in response to iE-DAP or MDP are not clear. Recently our laboratory has described the inflammatory effects of the NLR members, NOD1 and NOD2, in the eye using murine models of uveitis triggered by their synthetic agonists, iE-DAP and MDP, respectively.11–13,23 These studies have demonstrated the essential role for NOD2 in MDP-triggered uveitis and cytokine production. However, our studies and others have commented on the relatively low inflammatory potential of iE-DAP or MDP in vivo. In contrast, they do exhibit marked inflammatory activities when examined in combination with various TLR agonists, which has led to the hypothesis that NOD2 and NOD1 may play a secondary role in modulating TLR inflammatory responsiveness even though they do not themselves signal by way of MyD88. Many of the TLR and NLRs converge to common pathways such as NF-κB activation and IL-1β production, which could be envisioned as a protective response to infection and would be consistent with the synergy observed between TLR and NOD1 or NOD2 agonists in certain situations.14,15,17,18,24–26
In addition to enhancing inflammatory responses, several NLRs also exert negative regulatory roles.27–29 In the intestine where commensal bacteria-derived PGN is thought to trigger inflammation, data support a negative regulatory role for NOD2. NOD2 deficiency renders the intestine more sensitive to TLR2 activation by PGN, thereby resulting in exacerbated colitis, and increased NF-κB activation and production of IL-12 and IL-23.20–22 Conversely, transgenic mice that overexpress NOD2 were protected from colitis.30 These experimental observations would be consistent with the heightened Th1 or Th17 type of immune responses observed in patients with Crohn's disease.31 Our studies would suggest a similar scenario for NOD2 in possibly influencing Th1 versus Th17 responses in the setting of uveitis.
How NOD2 exerts differential functions is not entirely clear. In the intestine, the suppressive function of NOD2 reportedly involves IFN regulatory factor 4 (IRF4),22 which is known to interact with MyD88 to negatively regulate TLR signaling.32 We have found that IRF4 protein is expressed in eye tissue, and its expression is rapidly upregulated by PGN treatment (data not shown here). However, the IRF4 induction was not further influenced by NOD2 genotype, which would not support a mechanism involving IRF4 in the eye. IRF4-independent suppressive functions of NOD2 in intestinal epithelium have recently been reported,33 suggesting a far more complicated mechanism. Strain, types of host cells, or the specific tissue exposed to pathogens may also influence how NOD2 regulates inflammation. For example, NOD2 KO mice are susceptible to infection with Listeria only after oral challenge but not intravenous administration,15 further supporting the tissue-specific role for NOD2 within the intestine for host defense against this pathogen. Our own studies in a murine model of inflammatory arthritis have demonstrated that NOD2 cooperates with TLR2 activation by PGN to promote joint inflammation rather than suppress inflammation as we find in the eye.34 Within the eye, collectively our studies would suggest that NOD2 exerts dual and paradoxical effects wherein direct activation of NOD2 by MDP promotes inflammation while NOD2 suppresses PGN-triggered inflammation. Such differential roles for NOD2 have also been reported within osteoblasts.35 A recently described role for PGN in priming systemic innate immune function36 underscores the importance of NOD1 and NOD2 as homeostatic regulators. This may mean that in the absence of NOD2, protective host responses are lacking, and the eye becomes more susceptible to infection. Interestingly, that we observed differential roles for NOD2 and NOD1 in regulating ocular inflammation would be consistent with what we and others have observed in inflammatory arthritis models.37 The difference in function may also reflect their ability to distinguish PGN motifs. PGN from S. aureus used here should lack the meso-DAP motif that stimulates NOD1. Future investigation as to how these 2 NLRs may counterbalance ocular inflammatory responses would be interesting.
Clearly, several questions remain regarding how exactly PGN is sensed and the role of NOD2 in negatively or positively promoting inflammation within the eye. The mutations associated with Blau syndrome are widely regarded as gain of function mutations, but this anti-inflammatory role for NOD2 in the eye fits poorly with this conceptualization of the impact of Blau-associated mutations. Our studies provide the first insight into a negative regulatory function for NOD2 in the eye's responsiveness to PGN, which may pertain to other situations wherein the eye would be exposed to PGN. We suggest that NOD2 serves differential roles in the eye to promote inflammatory responses to MDP while tempering inflammatory responses to PGN.
Acknowledgments
The authors thank Jordan Allensworth and Charles Kintzley for their technical contributions, Shizuo Akira (Osaka University, Japan) for the provision of the MyD88 KO mice, and Steve Planck and Tammy Martin for their critical discussions.
Footnotes
Supported by NEI Grant EY019020 along with Research to Prevent Blindness awards (HLR and JTR) and awards from the Fight for Sight Foundation; William and Mary Bauman Foundation; Stan and Madelle Rosenfeld Family Foundation; and William C. Kuzell Foundation.
Disclosure: H.L. Rosenzweig, None; K. Galster, None; E.E. Vance, None; J. Ensign-Lewis, None; G. Nunez, None; M.P. Davey, None; J.T. Rosenbaum, None
References
- 1. Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCormack WJ, Parker AE, O'Neill LA. Toll-like receptors and NOD-like receptors in rheumatic diseases. Arthritis Res Ther. 2009;11:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen G, Shaw MH, Kim YG, Nunez G. Nod-like receptors: role in innate immunity and inflammatory disease. Ann Rev Pathol. 2009;4:365–398 [DOI] [PubMed] [Google Scholar]
- 4. Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20 [DOI] [PubMed] [Google Scholar]
- 5. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603 [DOI] [PubMed] [Google Scholar]
- 6. Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606 [DOI] [PubMed] [Google Scholar]
- 7. Girardin SE, Travassos LH, Herve M, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708 [DOI] [PubMed] [Google Scholar]
- 8. Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707 [DOI] [PubMed] [Google Scholar]
- 9. Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512 [DOI] [PubMed] [Google Scholar]
- 10. Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenzweig HL, Martin TM, Jann MM, et al. NOD2, the gene responsible for familial granulomatous uveitis, is essential in a mouse model of muramyl dipeptide-induced uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenzweig HL, Kawaguchi T, Martin TM, Planck SR, Davey MP, Rosenbaum JT. Nucleotide oligomerization domain-2 (NOD2)-induced uveitis: dependence on IFN-gamma. Invest Ophthalmol Vis Sci. 2009;50:1739–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenzweig HL, Martin TM, Planck SR, et al. Activation of NOD2 in vivo induces IL-1 beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fritz JH, Girardin SE, Fitting C, et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol. 2005;35:2459–2470 [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734 [DOI] [PubMed] [Google Scholar]
- 16. van Heel DA, Ghosh S, Butler M, et al. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005;35:2471–2476 [DOI] [PubMed] [Google Scholar]
- 17. Uehara A, Yang S, Fujimoto Y, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with a chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocyte cells in culture. Cell Microbiol. 2005;7:53–61 [DOI] [PubMed] [Google Scholar]
- 18. Uehara A, Takada H. Synergism between TLRs and Nod1/2 in oral epithelial cells. J Dent Res. 2008;87:682–686 [DOI] [PubMed] [Google Scholar]
- 19. Shikama Y, Kuroishi T, Nagai Y, et al. Muramyldipeptide augments the actions of lipopolysaccharide in mice by stimulating macropahges to produce pro-IL-1B and by down-regulation of suppressor of cytokine signaling 1 (SOCS1). Innate Immun. 2011;17(1):3–15 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–485 [DOI] [PubMed] [Google Scholar]
- 21. Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808 [DOI] [PubMed] [Google Scholar]
- 22. Watanabe T, Asano N, Murray PJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenzweig H, Galster K, Planck S, Rosenbaum J. NOD1 is expressed in the eye and functionally contributes to IL-1{beta} dependent ocular inflammation in mice. Invest Ophthalmol. 2009;50:1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Netea MG, Ferwerda G, de Jong DJ, Girardin SE, Kullberg BJ, van der Meer JW. NOD2 3020insC mutation and the pathogenesis of Crohn's disease: impaired IL-1beta production points to a loss-of-function phenotype. Neth J Med. 2005;63:305–308 [PubMed] [Google Scholar]
- 25. Van Heel DA, Hunt KA, King E, et al. Detection of muramyl dipeptide-sensing pathway defects in patients with Crohn's disease. Inflam Bowel Dis. 2006;12:598–605 [DOI] [PubMed] [Google Scholar]
- 26. Takada H, Uehara A. Enhancement of TLR-mediate innate immune responses by peptidoglycans through NOD signaling. Curr Pharm Des. 2006;12:4163–4172 [DOI] [PubMed] [Google Scholar]
- 27. Williams KL, Lich JD, Duncan JA, et al. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280:39914–39924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conti BJ, Davis BK, Zhang J, O'Connor WJ, Williams KL, Ting JP. CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J Biol Chem. 2005;280:18375–18385 [DOI] [PubMed] [Google Scholar]
- 29. Bruey JM, Bruey-Sedano N, Newman R, Chandler S, Stehlik C, Reed JC. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J Biol Chem. 2004;279:51897–51907 [DOI] [PubMed] [Google Scholar]
- 30. Yang Z, Fuss IJ, Watanabe T, et al. NOD2 transgenic mice exhibit enhanced MDP-mediated down-regulation of TLR2 responses and resistance to colitis induction. Gastroenterology. 2007;133:1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:1069–1079 [DOI] [PubMed] [Google Scholar]
- 32. Negishi H, Ohba Y, Yanai H, et al. Negative regulation of Toll-like-receptor signaling IRF-4. Proc Natl Acad Sci USA. 2005;102:15989–15994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson WM, Sodhi CP, Russo A, et al. Nucleotide-binding oligomerization domain-2 inhibits Toll like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139(3):904–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenzweig HL, Jann MJ, Vance EE, Planck SR, Rosenbaum JT, Davey MP. Nucleotide-binding oligomerization domain 2 and Toll-like receptor 2 function independently in a murine model of arthritis triggered by intraarticular peptidoglycan. Arthritis Rheum. 2010;62:1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chauhan VS, Marriott I. Differential roles for NOD2 in osteoblast inflammatory immune responses to bacterial pathogens of bone tissue. J Med Microbiol. 2010;59:755–762 [DOI] [PubMed] [Google Scholar]
- 36. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joosten LA, Heinhuis B, Abdollahi-Roodsaz S, et al. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci USA. 2008;105:9017–9022 [DOI] [PMC free article] [PubMed] [Google Scholar]