Calpain inhibition attenuates the expression of proapoptotic proteases and inflammatory factors and enhances the survival of retinal ganglion cells in experimental optic neuritis.
Abstract
Purpose.
Optic neuritis (ON), inflammation of the optic nerve, is strongly associated with the pathogenesis of multiple sclerosis (MS) and is initiated by the attack of autoreactive T cells against self-myelin antigens, resulting in demyelination, degeneration of retinal ganglion cells (RGCs), and cumulative visual impairment.
Methods.
Experimental autoimmune encephalomyelitis (EAE) was induced in Lewis rats on day 0, and animals received daily intraperitoneal injections of calpain inhibitor (calpeptin) or vehicle from day 1 until killed. Retinal cell death was analyzed by DNA fragmentation, and surviving ganglion cells were quantified after double labeling of retinal tissue with TUNEL and Brn3a. The expression of apoptotic and inflammatory proteins was determined by Western blotting.
Results.
It was demonstrated that calpain inhibition downregulates expression of proapoptotic proteins and the proinflammatory molecule nuclear factor-kappa B (NF-κB) in the retina of Lewis rats with acute EAE. Immunofluorescent labeling revealed that apoptotic cells in the RGC layer of vehicle-treated EAE animals were Brn3a positive, and a moderate dose of calpeptin dramatically reduced the frequency of apoptotic RGCs.
Conclusions.
These results suggest that calpain inhibition might be a useful supplement to immunomodulatory therapies such as corticosteroids in ON, due to its neuroprotective effect on RGCs.
Multiple sclerosis (MS) is an autoimmune, demyelinating disease of the central nervous system (CNS), with clinical signs including fatigue, paralysis, and visual dysfunction.1 Optic neuritis (ON), inflammation of the optic nerve, is associated with MS. Manifestations of ON include decreased sensation of light brightness, changes in color vision, and reduced visual acuity, which are the initial signs of MS in 15%–20% of patients. Moreover, 38%–50% of MS patients eventually develop ON during subsequent relapses.2 MS and ON are thought to result from inflammatory attacks on the myelin sheath by autoreactive T cells and other immune cells, resulting in demyelination, axonal damage, and cell death.3 Throughout disease progression, neurodegenerative events may occur even in the absence of an acute immune attack.4 Postmortem and magnetic resonance imaging (MRI) studies have demonstrated optic nerve atrophy after the onset of ON and MS.5–8 Moreover, both experimental and clinical optical coherence tomography (OCT) studies revealed thinning of the retinal nerve fiber layer (RNFL) in the diseased retina.9–11
Experimental autoimmune encephalomyelitis (EAE) is an animal model commonly used for studying the pathophysiology of ON (EAE–ON) and MS. EAE is induced in rodents by administering spinal cord homogenate and myelin proteins, such as myelin basic protein (MBP), myelin oligodendrocyte protein (MOG), and proteolipid protein, or by adoptive transfer of MBP or MOG-specific T cells to naïve rodents. Experiments with EAE animals12,13 and examination of CNS lesions of MS patients14 have identified the calcium (Ca2+)-dependent neutral protease calpain as an integral player in the damaging events of ON and MS. Both ubiquitous and tissue-specific calpains have been recognized, which play roles in cell proliferation and differentiation, T-cell activation, cell migration, signal transduction, necrosis, and apoptosis.15 Moreover, increased expression of μ-calpain and m-calpain, requiring μM and mM Ca2+ concentrations for activation, respectively, was detected in EAE–ON optic nerve before the onset of clinical signs of paralysis.12,13
Calpain inhibition has shown neuroprotective efficacy in animal models of ischemia, cataracts,16 traumatic brain injury,17 photoreceptor degeneration,18 and glutamate excitotoxicity,19 indicating that the inhibitors cross the blood–brain barrier under pathologic conditions and are cell-permeable. Calpeptin attenuated apoptosis in a retinal explant culture after optic nerve injury.20,21 Furthermore, calpain inhibition can reduce the development of acute and chronic inflammation in vivo.22,23 The effects of calpain inhibitors in ON are unknown, although a recent in vitro study demonstrated that calpeptin provides functional neuroprotection to retinal ganglion cells (RGCs) in response to IFNγ exposure.20 Thus, we undertook a study to investigate the efficacy of this cell-permeable calpain inhibitor to reduce inflammation and attenuate apoptosis in the retina of animals after induction of acute EAE–ON.
We have previously established that increased calpain activity is detected in EAE optic nerves before onset of clinical signs of paralysis. In the present study, we observed increased expression and activity of calpain in the retina during acute EAE–ON. Furthermore, proapoptotic proteases and both calpain- and caspase-3–specific spectrin breakdown products (SBDPs) were upregulated in the retina during the disease peak. Our investigation showed that daily treatment with the calpain inhibitor calpeptin attenuated the expression of proapoptotic proteases and inflammatory molecules in the retina, ultimately leading to an increase in surviving RGCs.
Materials and Methods
Induction of EAE and Calpeptin Therapy
The Lewis rat model is an acute model of EAE in which animals develop one inflammatory episode and then fully recover with no further relapse. Male Lewis rats (6 weeks) were obtained from a commercial vendor (Charles River Laboratories, Wilmington, MA) and provided with unrestricted access to water and food pellets. Animals were immunized subcutaneously with 0.2 mL of purified guinea pig MBP (200 μg/mL) and guinea pig spinal cord homogenate (200 μg/mL) in saline, emulsified with an equal volume of complete Freund's adjuvant (CFA) containing Mycobacterium tuberculosis H37Ra (10 mg/mL; Difco, Detroit, MI). Controls were injected with saline/CFA only. All rats received an intraperitoneal (IP) injection of 0.125 mL of pertussis toxin (12.5 μg/mL) 2 hours after immunization. On days 1 to 10 post-EAE induction, control, and EAE rats received twice-daily IP injections of either vehicle (0.1% dimethyl sulfoxide [DMSO] in saline) or the cell-permeable calpain inhibitor calpeptin (50–250 μg/kg; EMD Chemicals, Gibbstown, NJ). All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Medical University of South Carolina Animal Care and Use Committee.
Protein Analysis by Western Blotting
Procedures for protein analyses have been previously described.17–19 Briefly, optic nerves were homogenized in 50 mM Tris buffer (pH 7.4) containing 5 mM EGTA and 1 mM phenylmethylsulfonyl fluoride. After determination of protein concentration, known amounts of all samples were separated by 4%–20% linear gradient SDS-PAGE. After SDS-PAGE, proteins were transferred onto nitrocellulose membranes, which were probed with primary antibodies then incubated with horseradish peroxidase–conjugated secondary antibodies. Specific protein bands were detected using a charge-coupled device detection system (Fluorchem FC2 Chemiluminescent CCD Detection System; Alpha Innotech, San Leandro, CA). Western blot data were analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index/html) to determine optical density (OD) of the bands. The OD reading was normalized to β-actin to account for variations in loading, and the mean of each treatment group was determined. Differences between groups were analyzed by the Kruskal–Wallis test followed by the Mann–Whitney U test for individual comparisons. The results were expressed as mean ± SEM of independent experiments (n ≥ 3). The null hypothesis for each comparison was rejected at P < 0.05.
Analysis of Genomic DNA Fragmentation
EAE–ON animals were killed and whole retinas were digested in a homogenization solution (10 mM Tris–HCl, pH 8.0, 50 mM NaCl, 10 mM EDTA, 0.5% SDS, 250 ng/μL proteinase K) at 37°C for 24 hours. Digests were extracted twice with a 1:1 (v/v) mixture of phenol and chloroform, and once with chloroform alone. Total genomic DNA was precipitated, dried in air, and dissolved in TE (10 mM Tris–HCl, pH 8.0, 1 mM EDTA) buffer during a 1-hour incubation at 37°C. Equal amounts of sample were loaded onto 1.6% agarose gels and electrophoresed in 1× TAE (40 mM Tris–acetate, pH 8.3, 1 mM EDTA) buffer. The gels were stained with ethidium bromide (1 mg/mL) and the DNA profile was observed on a UV (303 nm) transilluminator.
TUNEL Assay and Immunohistochemistry
Enucleated eyes were fixed in 4% methanol-free formaldehyde in PBS (pH 7.4) for 4 hours before retinal dissection. The tissue was then saturated in 30% sucrose, embedded in OCT, and cryosectioned (14 μm). The TUNEL assay was performed according to a previously described protocol.24 Before primary antibody staining, nonspecific binding sites were blocked with the same serum as the secondary antibody for 1 hour at room temperature then incubated with Brn3a antibody (1:200; Santa Cruz Biotechnologies, Santa Cruz, CA) overnight at 4°C. Colabeled Brn3a sections were incubated with a (1:100) secondary antibody conjugated with a fluorescent dye (Texas Red; Vector Laboratories, Burlingame, CA) for 30 minutes in the dark. The slides were mounted with a single drop of mounting medium (Vectashield; Vector Laboratories) and coverslipped. The sections were viewed under a fluorescence microscope at magnification ×200 (Carl Zeiss Meditec, Dublin, CA).
Cell Counting
Brn3a-positive RGCs were counted within four fields (approximately 400 μm in length) in two identical areas of each central retina and two areas of each peripheral retina (one eye each from three to five animals, four sections per eye). The fields were placed at a maximum of 500 μm from the optic disc. Brn3a-positive cells were counted in each field, excluding cells that were also TUNEL-positive, the values were averaged, and the SE was calculated. The percentage difference from the control-vehicle counts, set at 100%, was calculated for each treatment group. One-way ANOVA followed by Tukey's Honestly Significant Difference test were used to calculate P-values between treatment groups. A significant difference was defined as a value of P < 0.05.
Results
Attenuation of DNA Fragmentation and Preservation of Retinal Ganglion Cell Survival in the Retina of EAE–ON Animals Treated with Calpeptin
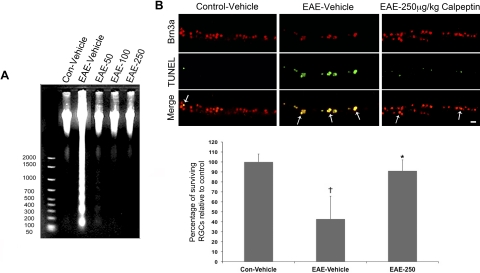
We first sought to determine whether cell death was occurring in the retina of acute EAE–ON animals after challenge with myelin antigens (Fig. 1). Analysis of internucleosomal DNA fragmentation indicated a substantial increase in retinal cell death 10 days after EAE induction (Fig. 1A). At all doses tested, calpeptin attenuated internucleosomal DNA fragmentation in EAE–ON retinas, such that apoptotic cell death levels were similar to those found present in control retinas. Because apoptotic cell death was increased in acute EAE–ON retina and calpain inhibition was protective, we expected that RGCs were dying, possibly after retrograde degeneration of the optic nerve axons. Brn3a is an excellent marker for rat RGCs, and it has been shown to label as many RGCs as a fluorescent retrograde axonal tracer (Fluoro-Gold). Moreover, Brn3a expression is still present in injured cells.25,26 The TUNEL+ cells identified were also positive for Brn3a, and calpeptin treatment decreased the frequency of TUNEL+ Brn3a cells in the RGC layer, leading to an overall increase in the frequency of viable RGCs (Fig. 1B).
Figure 1.
Calpain inhibition attenuated DNA fragmentation and improved RGC survival in the retina of acute EAE–ON animals. After induction of EAE, Lewis rats received twice-daily IP doses of calpeptin or vehicle (0.1% DMSO in saline) and were killed on day 10 post-induction. Treatment groups included control-vehicle, EAE-vehicle, and EAE treated with 50, 100, or 250 μg/kg calpeptin (EAE-50, EAE-100, or EAE-250). Retinas were dissected and processed for (A) analysis of genomic DNA fragmentation and (B) costaining of Brn3a (red) and TUNEL (green). Images were taken at magnification ×200. Scale bar, 50 μm. n = 3–5.
Effect of Calpeptin on Calpain and Calpastatin Expression in EAE–ON Retina
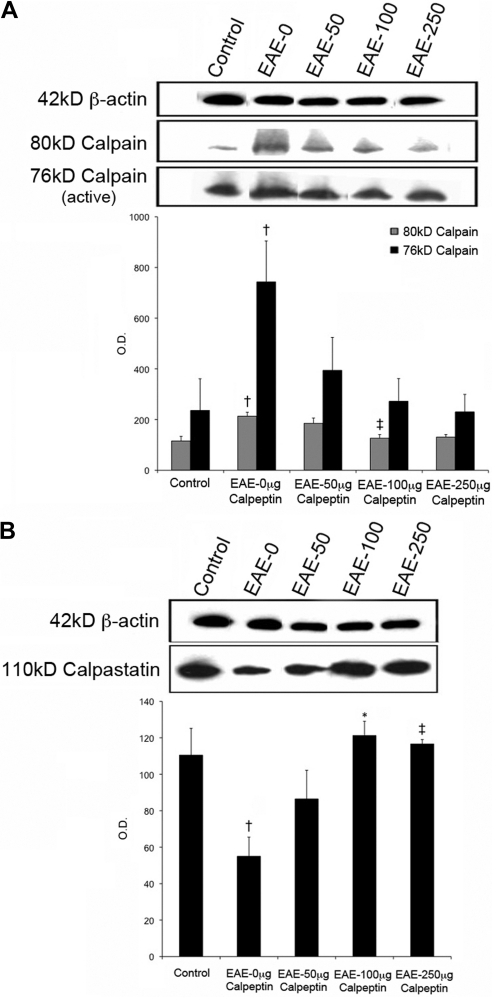
Since calpeptin limited RGC death in EAE–ON animals, we next examined the changes in expression of calpain and its endogenous inhibitor, calpastatin, in the retina of untreated and treated EAE–ON animals (Fig. 2). Expression of the inactive 80-kDa calpain subunit in retina was increased in EAE–ON animals and significantly decreased in animals treated daily with 100 μg/kg calpeptin (Fig. 2A). Although retinal expression of the active 76-kDa calpain subunit was upregulated in EAE–ON animals, it was not significantly reduced by any dose of calpeptin tested, due to the variability in expression between samples (Fig. 2A). Inversely, calpastatin was downregulated in retina of EAE–ON animals, but treatment with 100 or 250 μg/kg calpeptin restored calpastatin expression to levels comparable to those of control animals (Fig. 2B).
Figure 2.
Calpeptin decreased calpain expression and increased calpastatin in the retina of EAE–ON animals. After induction of EAE, Lewis rats received twice-daily IP doses of calpeptin or vehicle (0.1% DMSO in saline) and were killed on day 10 post-induction for tissue analysis. Representative blots and densometric analysis of (A) calpain and (B) calpastatin expression are shown. β-actin was used as a loading control. Treatment groups included Control-Vehicle, EAE-Vehicle, and EAE treated with 50, 100, or 250 μg/kg calpeptin (EAE-50, EAE-100, or EAE-250). †P < 0.05 vs. Control-Vehicle; ‡P < 0.05 vs. EAE-Vehicle;*P < 0.01 vs. EAE-Vehicle. n = 3–5.
Calpeptin Attenuated Activities of Calpain and Caspase-3 in EAE–ON Retina
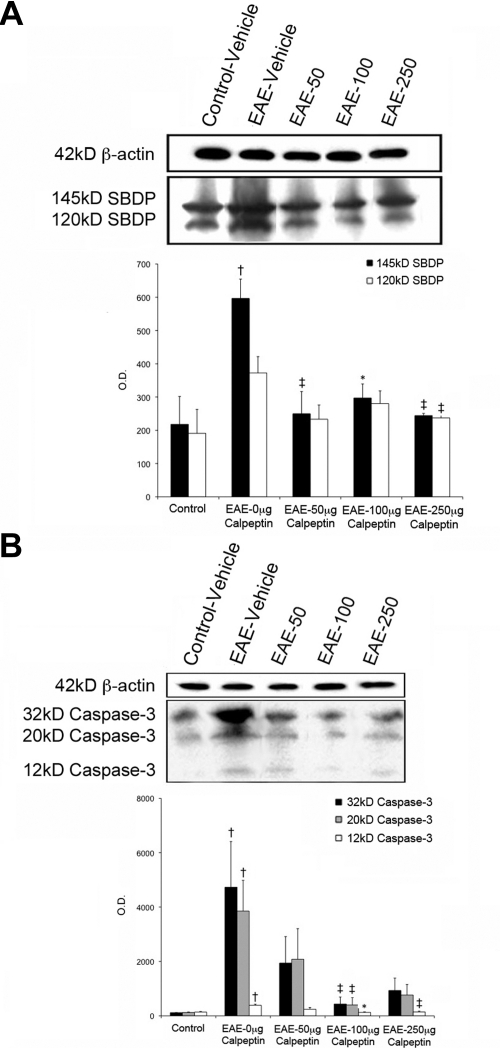
We determined the activities of both calpain and caspase-3 in retina of EAE–ON animals before and after treatment with calpeptin (Fig. 3). Because calpain directly cleaves α-spectrin into a 145-kDa SBDP, we have determined the level of SBDP as a surrogate of calpain activity. Expression of 145-kDa SBDP was increased by nearly threefold in the retina of acute EAE–ON animals and restored to control levels after treatment with all doses of calpeptin (Fig. 3A). Caspase-3 is a common effector caspase of both the intrinsic and extrinsic pathways of apoptosis. All three isoforms of caspase-3 were upregulated in EAE–ON, and levels of all isoforms were significantly reduced in retinas of rats treated with 100 μg/kg calpeptin (Fig. 3B). The 120-kDa SBDP, a surrogate of caspase-3 activity, was not significantly increased in retina of EAE–ON animals (Fig. 3A), although its activity was decreased with the highest dose of calpeptin. These results suggest a predominant role for calpain activity in the pathogenesis of EAE–ON.
Figure 3.
Activities of calpain and caspase-3 were attenuated in calpeptin-treated retinas. After induction of EAE, Lewis rats received twice-daily IP doses of calpeptin or vehicle (0.1% DMSO in saline) and were killed on day 10 post-induction for tissue analysis. Representative blots and densometric analysis of (A) SBDP (145- and 120-kDa) and (B) caspase-3 isoforms are shown. β-actin was used as a loading control. Treatment groups included Control-Vehicle, EAE-Vehicle, and EAE treated with 50, 100, or 250 μg/kg calpeptin (EAE-50, EAE-100, or EAE-250). †P < 0.05 vs. Control-Vehicle; ‡P < 0.05 vs. EAE-Vehicle;*P < 0.01 vs. EAE-Vehicle. n = 3–5.
Calpain Inhibition Decreased Expression of Caspase-8, tBID, and the Bax/Bcl-2 Ratio in EAE–ON Retina
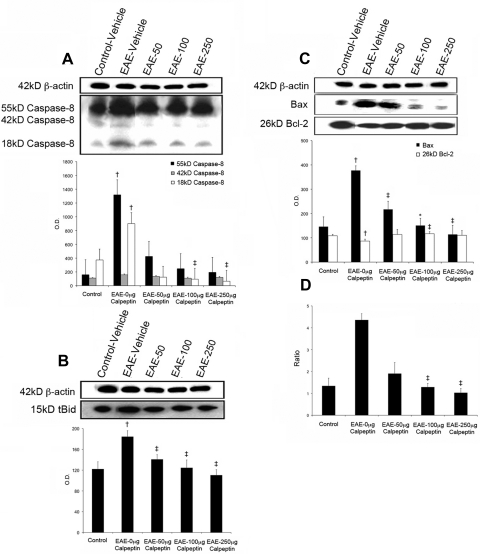
Because cross-talk exists between calpain and caspase-8, an extrinsic initiator caspase in pathways of cell migration27,28 and apoptosis,29 we examined whether calpain inhibition can affect processing of pro-caspase-8 to active caspase-8 as a mechanism for inducing retinal degeneration. With substrates that include apoptosis-related effector caspases and proapoptotic Bcl-2 family members, active caspase-8 is capable of initiating cascades of cellular events that result in apoptosis.30 Expression levels of the 55-kDa pro-caspase-8 and active 18-kDa caspase-8 were significantly increased in the retina of acute EAE–ON animals (Fig. 4A), and 100 or 250 μg/kg calpeptin significantly decreased retinal expression of the active isoform (Fig. 4A). Active caspase-8 cleaves Bid to truncated Bid (tBid), which then translocates to the mitochondria to activate Bax.31,32 Moreover, calpain cleaves Bax into a 21-kDa Bax fragment that is a more potent inducer of apoptosis than uncleaved Bax. At all doses tested, calpain inhibition attenuated levels of tBid (Fig. 4B) and Bax (Fig. 4C). Bcl-2 is an antiapoptotic protein and a negative regulator of cytochrome c release from the mitochondria; however, Bcl-2 can be truncated by calpain, producing fragments that promote cell death rather than survival. Bcl-2 protein expression was downregulated in the retina of EAE–ON animals, but treatment with 100 μg/kg calpeptin significantly increased expression of the Bcl-2 protein (Fig. 4C). Overall, the Bax/Bcl-2 ratio was increased nearly fourfold in EAE–ON retina, a trend largely driven by increased Bax expression, and it was effectively restored to the control level in retinas of EAE–ON animals treated with the two highest doses of calpeptin (Fig. 4D).
Figure 4.
Calpeptin decreased expression of caspase-8, tBid, and reduced the Bax/Bcl-2 ratio in retina of EAE–ON animals. After induction of EAE, Lewis rats received twice-daily IP doses of calpeptin or vehicle (0.1% DMSO in saline) and were killed on day 10 post-induction for tissue analysis. Representative blots and densometric analysis of (A) caspase-8 isoforms, (B) tBid), and (C) the expression and (D) ratio of Bax and Bcl-2 are shown. The Bax antibody recognized both 24- and 21-kDa isoforms of Bax. β-actin was used as a loading control. Treatment groups included Control-Vehicle, EAE-Vehicle, and EAE treated with 50, 100, or 250 μg/kg calpeptin (EAE-50, EAE-100, or EAE-250). †P < 0.05 vs. Control-Vehicle; ‡P < 0.05 vs. EAE-Vehicle;*P < 0.01 vs. EAE-Vehicle. n = 3–5.
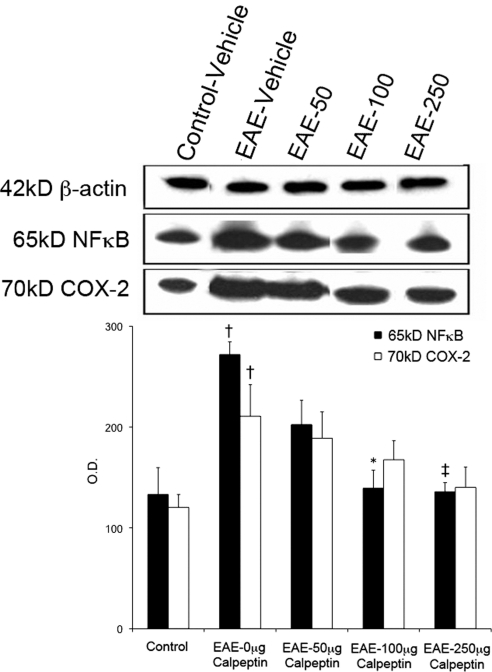
Effect of Calpeptin on Expression of NF-κB and COX-2 in EAE–ON Retina
Since retinal astrocytes, Müller cells, and microglia are sensitive to changes in the microenvironment due to stress or injury,33–35 we hypothesized that these cells might produce proinflammatory factors in response to apoptosis of RGCs in the retina of EAE–ON animals. Retinal glia are capable of producing a host of proinflammatory molecules after such insults as retinal ganglion cell axotomy.36 Furthermore, significant activation of the transcription factor NF-κB has been detected in glial cells after CNS injury and disease.37 Since activated calpain mediates degradation of IκBα, an endogenous inhibitor of NF-κB, independently of caspases,38 we investigated the effect of calpain inhibition on NF-κB expression. We found that NF-κB is significantly upregulated in the retina of EAE–ON animals, and treatment with the two highest doses of calpeptin decreased expression of NF-κB nearly to the control level (Fig. 5). Inducible isoforms of COX-2 are transcribed by NF-κB; thus, expression of this molecule might contribute to localized inflammation. Expression of COX-2 was increased in retinas of EAE–ON animals and, although there was a trend toward decreased COX-2 expression in calpeptin-treated animals, this difference was not statistically significant.
Figure 5.
Calpeptin treatment decreased expression of the proinflammatory molecule NF-κB in retina of EAE–ON animals. After induction of EAE, Lewis rats received twice-daily IP doses of calpeptin or vehicle (0.1% DMSO in saline) and were killed on day 10 post-induction for tissue analysis. Representative blots and densometric analysis of NF-κB and COX-2 are shown. β-actin was used as a loading control. Treatment groups included Control-Vehicle, EAE-Vehicle, and EAE treated with 50, 100, or 250 μg/kg calpeptin (EAE-50, EAE-100, or EAE-250). †P < 0.05 vs. Control-Vehicle; ‡P < 0.05 vs. EAE-Vehicle;*P < 0.01 vs. EAE-Vehicle. n = 3–5.
Discussion
The current goal of therapy for ON and MS is to prevent cumulative neurologic disability. In ON, low-contrast visual acuity is strongly correlated with thickness of the RNFL, indicating that preservation of optic nerve axons with subsequent salvaging of the ganglion cell bodies that give rise to them may protect vision.39 The present study revealed that treatment of animals with calpain inhibitor was sufficient to decrease the expression of proapoptotic and proinflammatory proteins that are known to interact with calpain. Moreover, we found that calpain inhibition significantly improved the survival of RGCs in the retina of EAE–ON rats. We have previously demonstrated increased expression of active calpain in microglia, macrophages, and astrocytes located in inflammatory foci of EAE optic nerves.12 In models of optic nerve crush and transection, the calpain 1 signal became overexpressed within hours of injury, and was specifically colabeled with RGCs.40 An experimental glaucoma study revealed that calpain cleavage of upstream proapoptotic substrates (e.g., calcineurin) was upregulated in RGCs during conditions of elevated intraocular pressure.41 The acute elevation of calpain activity in response to damage of RGCs makes it an ideal target for the attenuation of both inflammatory and neurodegenerative events in ocular pathologies including optic neuritis.
Expression and activity of calpain were increased in the retina of EAE–ON animals but significantly reduced in animals treated with calpeptin. Conversely, expression of the endogenous calpain inhibitor calpastatin was decreased in the retina of EAE–ON animals, whereas expression was increased to control levels with calpeptin treatment. The attenuation of active caspase-3 expression by calpeptin may have prevented truncation of calpastatin by this protease. It was demonstrated that specific inhibition of caspase-3 rescued axotomized RGCs from secondary neuronal death in vivo.42 Moreover, optic nerve transection did not alter caspase-3 mRNA levels but resulted in a translational or post-translational upregulation of caspase-3 activity in axotomized RGCs, which supports the hypothesis that the proteolytic activity of calpain is inducing caspase-3 activity.
When caspase-8 is knocked out in the T-cell lineage of transgenic mice via Cre/loxP recombination (Lck/Cre), the mice are viable, but appear to have deficiencies in both T-cell expansion and activation.43 Moreover, a caspase-8–deficient Jurkat T-cell line manifested defects in activation responses that could be corrected by retroviral expression of the wild-type caspase-8 gene. Thus, agents that alter expression of this caspase are potentially useful for immunosuppressive therapy. We determined that calpeptin-treated animals manifested a decrease in expression of retinal caspase-8. Moreover, active caspase-8–mediated cleavage of Bid to tBid was abrogated in calpeptin-treated retinas. In neuron-like differentiated PC12 cells, calpain promotes formation of active caspase-8 from pro-caspase-8 via the Aβ and CD95 pathways, along with degradation of the pro-caspase-8 processing inhibitor caspase-8 (FLICE)–like inhibitory protein, short isoform (FLIPS). Inhibition of calpain prevents the cleavage of pro-caspase-8 to active caspase-8 and also inhibits FLIPS degradation in PC12 cells.44 This mechanism of calpain interaction with caspase-8 may be contributing to the neuronal damage we observed in EAE–ON.
Calpain induces an increase in the ratio of the proapoptotic protein Bax to the antiapoptotic protein Bcl-2 by directly cleaving Bax to an activated form through a mitochondrial pathway.45 Activated Bax forms multimers in the mitochondria, with subsequent cytochrome c release, which ultimately leads to apoptosis via formation of the apoptosome protein complex and activation of downstream caspases 3 and 7.46 In a model of diabetic retinopathy, there was a positive correlation between apoptotic cells and the Bax/Bcl-2 ratio in the retina.47 Furthermore, an N-methyl-d-aspartate receptor antagonist decreased the Bax/Bcl-2 ratio and the frequency of apoptotic RGC death after ischemia–reperfusion injury to the rat retina.48 Our finding of a four-fold increase in the Bax/Bcl-2 ratio in EAE–ON retina at disease peak provides further evidence that disruption of this ratio jeopardizes RGC survival. Moreover, calpain inhibition restores the Bax/Bcl-2 ratio to control levels in EAE–ON retina, indicating that treatment with calpeptin can attenuate a proapoptotic sequence at the mitochondrial level.
NF-κB is upregulated in the EAE–ON retina and higher doses of calpeptin reverse this effect. The contribution of NF-κB activation to EAE pathology has been demonstrated,49 and transgenic inhibition of NF-κB in astrocytes led to functional improvement after both spinal cord injury and EAE, achieved via sustained suppression of CNS inflammation.37,50 Although it is a less common MS-associated phenomenon than ON, there is evidence that autoreactive uveitis occurs in both EAE animals and patients with MS.35,51–54 This presentation of uveitis may occur as a result of IgG antibody reactivity against retinal tissue, which has been reported to increase throughout the disease course concurrent with significant microglial activation35 and glial fibrillary acidic protein immunoreactivity in the inner retinal layers.55 Calpain constitutively degrades IκBα, an endogenous inhibitor of NF-κB, in immune cells23; thus, calpeptin treatment may act via this mechanism to attenuate NF-κB expression by retinal inflammatory cells.
In conclusion, this is the first report to identify specific proapoptotic proteases that are upregulated in the retina during acute EAE–ON. In clinical ON, recent evidence suggests that a reduction in thickness of the RNFL correlates with poor visual function.39,56 Moreover, RNFL thinning is often accompanied by decreases in macular volume, indicating a secondary loss of neuronal cells.57,58 Our findings demonstrate that treatment with calpeptin significantly reduces the expression of proapoptotic proteases and enhances retinal ganglion cell survival in the retina of acute EAE–ON animals. We have also demonstrated that calpeptin reduces the severity of EAE by improving the clinical score of paralysis.24 A combination of anti-inflammatory compounds, together with calpain inhibitor to reduce neurodegeneration, may significantly augment the current treatment modality.
Footnotes
Supported, in part, by National Institutes of Health Grants NS31622, NS38146, NS41088, and EY019457.
Disclosure: A.W. Smith, None; A. Das, None; M.K. Guyton, None; S.K. Ray, None; B. Rohrer, None; N.L. Banik, None
References
- 1. Williams KC, Ulvestad E, Hickey WF. Immunology of multiple sclerosis. Clin Neurosci. 1994;2:229–245 [PubMed] [Google Scholar]
- 2. Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005;139:1101–1108 [DOI] [PubMed] [Google Scholar]
- 3. Compston A, Sadovnick AD. Epidemiology and genetics of multiple sclerosis. Curr Opin Neurol Neurosurg. 1992;5:175–181 [PubMed] [Google Scholar]
- 4. Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183 [DOI] [PubMed] [Google Scholar]
- 5. Gartner S. Optic neuropathy in multiple sclerosis. Optic neuritis. AMA Arch Ophthalmol. 1953;50:718–726 [DOI] [PubMed] [Google Scholar]
- 6. Hickman SJ, Toosy AT, Jones SJ, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127:2498–2505 [DOI] [PubMed] [Google Scholar]
- 7. Hickman SJ, Brex PA, Brierley CM, et al. Detection of optic nerve atrophy following a single episode of unilateral optic neuritis by MRI using a fat-saturated short-echo fast FLAIR sequence. Neuroradiology. 2001;43:123–128 [DOI] [PubMed] [Google Scholar]
- 8. Inglese M, Ghezzi A, Bianchi S, et al. Irreversible disability and tissue loss in multiple sclerosis: a conventional and magnetization transfer magnetic resonance imaging study of the optic nerves. Arch Neurol. 2002;59:250–255 [DOI] [PubMed] [Google Scholar]
- 9. Srinivasan VJ, Ko TH, Wojtkowski M, et al. Noninvasive volumetric imaging and morphometry of the rodent retina with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2006;47:5522–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814 [DOI] [PubMed] [Google Scholar]
- 11. Sergott RC, Frohman E, Glanzman R, Al-Sabbagh A. The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci. 2007;263:3–14 [DOI] [PubMed] [Google Scholar]
- 12. Shields DC, Tyor WR, Deibler GE, Banik NL. Increased calpain expression in experimental demyelinating optic neuritis: an immunocytochemical study. Brain Res. 1998;784:299–304 [DOI] [PubMed] [Google Scholar]
- 13. Guyton MK, Sribnick EA, Ray SK, Banik NL. A role for calpain in optic neuritis. Ann NY Acad Sci. 2005;1053:48–54 [DOI] [PubMed] [Google Scholar]
- 14. Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA. 1999;96:11486–11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu HY, Tomizawa K, Matsui H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007;61:123–137 [DOI] [PubMed] [Google Scholar]
- 16. Tamada Y, Fukiage C, Mizutani K, et al. Calpain inhibitor, SJA6017, reduces the rate of formation of selenite cataract in rats. Curr Eye Res. 2001;22:280–285 [DOI] [PubMed] [Google Scholar]
- 17. Kupina NC, Nath R, Bernath EE, et al. The novel calpain inhibitor SJA6017 improves functional outcome after delayed administration in a mouse model of diffuse brain injury. J Neurotrauma. 2001;18:1229–1240 [DOI] [PubMed] [Google Scholar]
- 18. Sharma AK, Rohrer B. Sustained elevation of intracellular cGMP causes oxidative stress triggering calpain-mediated apoptosis in photoreceptor degeneration. Curr Eye Res. 2007;32:259–269 [DOI] [PubMed] [Google Scholar]
- 19. Das A, Sribnick EA, Wingrave JM, et al. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562 [DOI] [PubMed] [Google Scholar]
- 20. Das A, Garner DP, Del Re AM, et al. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006;1084:146–157 [DOI] [PubMed] [Google Scholar]
- 21. McKernan DP, Guerin MB, O'Brien CJ, Cotter TG. A key role for calpains in retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2007;48:5420–5430 [DOI] [PubMed] [Google Scholar]
- 22. Cuzzocrea S, McDonald MC, Mazzon E, et al. Calpain inhibitor I reduces the development of acute and chronic inflammation. Am J Pathol. 2000;157:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem Res. 2004;29:1443–1451 [DOI] [PubMed] [Google Scholar]
- 24. Guyton MK, Das A, Samantaray S, et al. Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. J Neurosci Res. 2010;88:2398–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, et al. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868 [DOI] [PubMed] [Google Scholar]
- 26. Sánchez-Migallón MC, Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Exp Eye Res. 2011;92:260–267 [DOI] [PubMed] [Google Scholar]
- 27. Helfer B, Boswell BC, Finlay D, et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273–4278 [DOI] [PubMed] [Google Scholar]
- 28. Frisch SM. Caspase-8: fly or die. Cancer Res. 2008;68:4491–4493 [DOI] [PubMed] [Google Scholar]
- 29. Vaisid T, Barnoy S, Kosower NS. Calpain activates caspase-8 in neuron-like differentiated PC12 cells via the amyloid-beta-peptide and CD95 pathways. Int J Biochem Cell Biol. 2009;41:2450–2458 [DOI] [PubMed] [Google Scholar]
- 30. Lawen A. Apoptosis: an introduction. Bioessays. 2003;25:888–896 [DOI] [PubMed] [Google Scholar]
- 31. Gu Q, Wang JD, Xia HH, et al. Activation of the caspase-8/Bid and Bax pathways in aspirin-induced apoptosis in gastric cancer. Carcinogenesis. 2005;26:541–546 [DOI] [PubMed] [Google Scholar]
- 32. Roth W, Stenner-Liewen F, Pawlowski K, Godzik A, Reed JC. Identification and characterization of DEDD2, a death effector domain-containing protein. J Biol Chem. 2002;277:7501–7508 [DOI] [PubMed] [Google Scholar]
- 33. Drescher KM, Whittum-Hudson JA. Herpes simplex virus type 1 alters transcript levels of tumor necrosis factor-alpha and interleukin-6 in retinal glial cells. Invest Ophthalmol Vis Sci. 1996;37:2302–2312 [PubMed] [Google Scholar]
- 34. Roberge FG, Caspi RR, Chan CC, Nussenblatt RB. Inhibition of T lymphocyte proliferation by retinal glial Muller cells: reversal of inhibition by glucocorticoids. J Autoimmun. 1991;4:307–314 [DOI] [PubMed] [Google Scholar]
- 35. Gramlich OW, Joachim SC, Gottschling PF, et al. Ophthalmopathology in rats with MBP-induced experimental autoimmune encephalomyelitis. Graefes Arch Clin Exp Ophthalmol. In press [DOI] [PubMed] [Google Scholar]
- 36. Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol. 1999;158:366–381 [DOI] [PubMed] [Google Scholar]
- 37. Brambilla R, Bracchi-Ricard V, Hu WH, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li C, Chen S, Yue P, et al. Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IkappaB(alpha) degradation. J Biol Chem. 2010;285:16096–16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frohman EM, Dwyer MG, Frohman T, et al. Relationship of optic nerve and brain conventional and non-conventional MRI measures and retinal nerve fiber layer thickness, as assessed by OCT and GDx: a pilot study. J Neurol Sci. 2009;282:96–105 [DOI] [PubMed] [Google Scholar]
- 40. Agudo M, Perez-Marin MC, Sobrado-Calvo P, et al. Immediate upregulation of proteins belonging to different branches of the apoptotic cascade in the retina after optic nerve transection and optic nerve crush. Invest Ophthalmol Vis Sci. 2009;50:424–431 [DOI] [PubMed] [Google Scholar]
- 41. Huang W, Fileta J, Rawe I, Qu J, Grosskreutz CL. Calpain activation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3049–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kermer P, Klocker N, Labes M, Bahr M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci. 1998;18:4656–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salmena L, Lemmers B, Hakem A, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang TB, Ben-Moshe T, Varfolomeev EE, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984 [DOI] [PubMed] [Google Scholar]
- 45. Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res. 2000;256:375–382 [DOI] [PubMed] [Google Scholar]
- 46. Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489 [DOI] [PubMed] [Google Scholar]
- 47. Gao XY, Kuang HY, Zou W, Liu XM, Lin HB, Yang Y. The timing of re-institution of good blood glucose control affects apoptosis and expression of Bax and Bcl-2 in the retina of diabetic rats. Mol Biol Rep. 2009;36:1977–1982 [DOI] [PubMed] [Google Scholar]
- 48. Qiu W, Wei R, Zhang C, Leng W, Wang W. A glycine site-specific NMDA receptor antagonist protects retina ganglion cells from ischemic injury by modulating apoptotic cascades. J Cell Physiol. 2010;223:819–826 [DOI] [PubMed] [Google Scholar]
- 49. van Loo G, De Lorenzi R, Schmidt H, et al. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006;7:954–961 [DOI] [PubMed] [Google Scholar]
- 50. Brambilla R, Persaud T, Hu X, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Towler HM, Lightman S. Symptomatic intraocular inflammation in multiple sclerosis. Clin Exp Ophthalmol. 2000;28:97–102 [DOI] [PubMed] [Google Scholar]
- 52. Adamus G, Amundson D, Vainiene M, et al. Myelin basic protein specific T-helper cells induce experimental anterior uveitis. J Neurosci Res. 1996;44:513–518 [DOI] [PubMed] [Google Scholar]
- 53. Adamus G, Manczak M, Sugden B, Arendt A, Hargrave PA, Offner H. Epitope recognition and T cell receptors in recurrent autoimmune anterior uveitis in Lewis rats immunized with myelin basic protein. J Neuroimmunol. 2000;108:122–130 [DOI] [PubMed] [Google Scholar]
- 54. Adamus G, Sugden B, Arendt A, Hargrave PA. Importance of cryptic myelin basic protein epitopes in the pathogenicity of acute and recurrent anterior uveitis associated with EAE. J Neuroimmunol. 2001;113:212–219 [DOI] [PubMed] [Google Scholar]
- 55. Zheng X, Li PH, Song SF. Expression of glial fibrillary acidic protein in retina of rats in acute experimental autoimmune encephalomyelitis [in Chinese]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:719–722 [PubMed] [Google Scholar]
- 56. Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burkholder BM, Osborne B, Loguidice MJ, et al. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009;66:1366–1372 [DOI] [PubMed] [Google Scholar]
- 58. Salter AR, Conger A, Frohman TC, et al. Retinal architecture predicts pupillary reflex metrics in MS. Mult Scler. 2009;15:479–486 [DOI] [PubMed] [Google Scholar]