Abstract
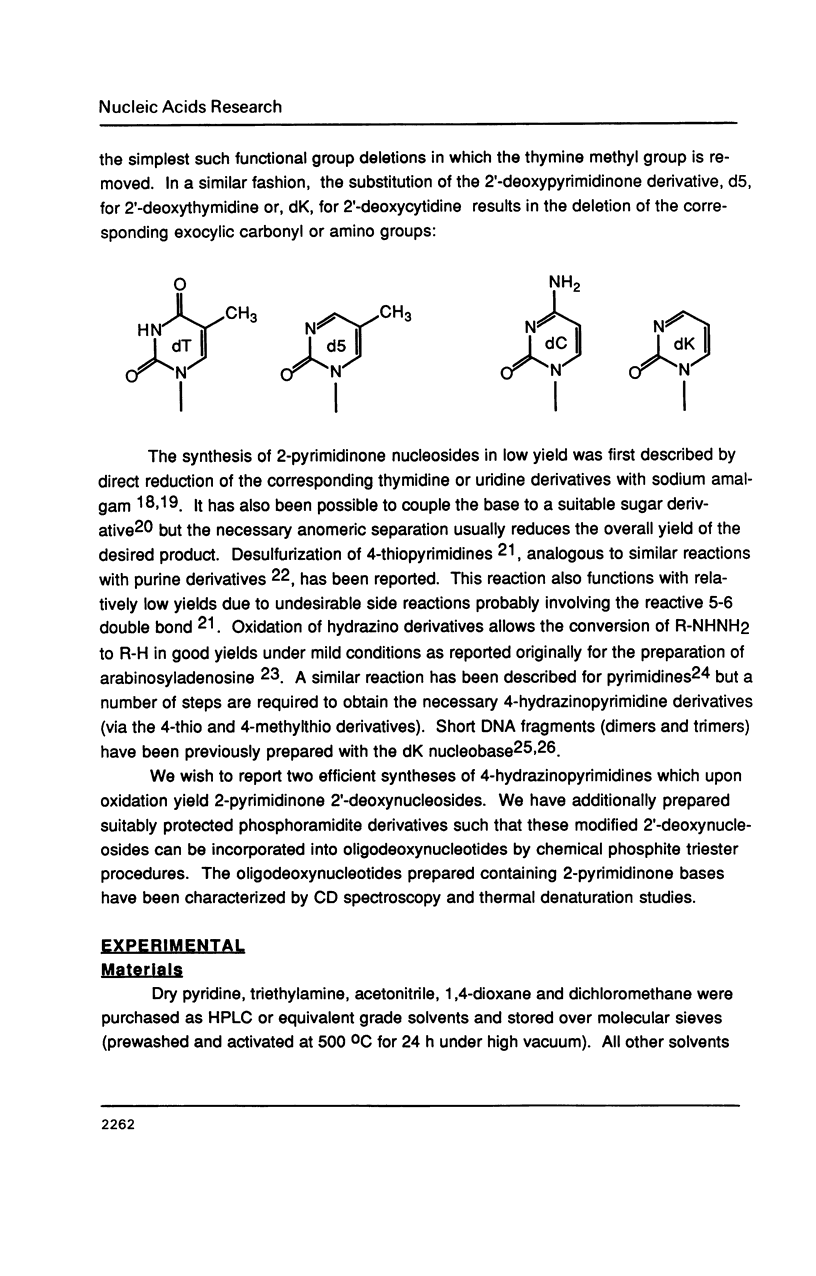
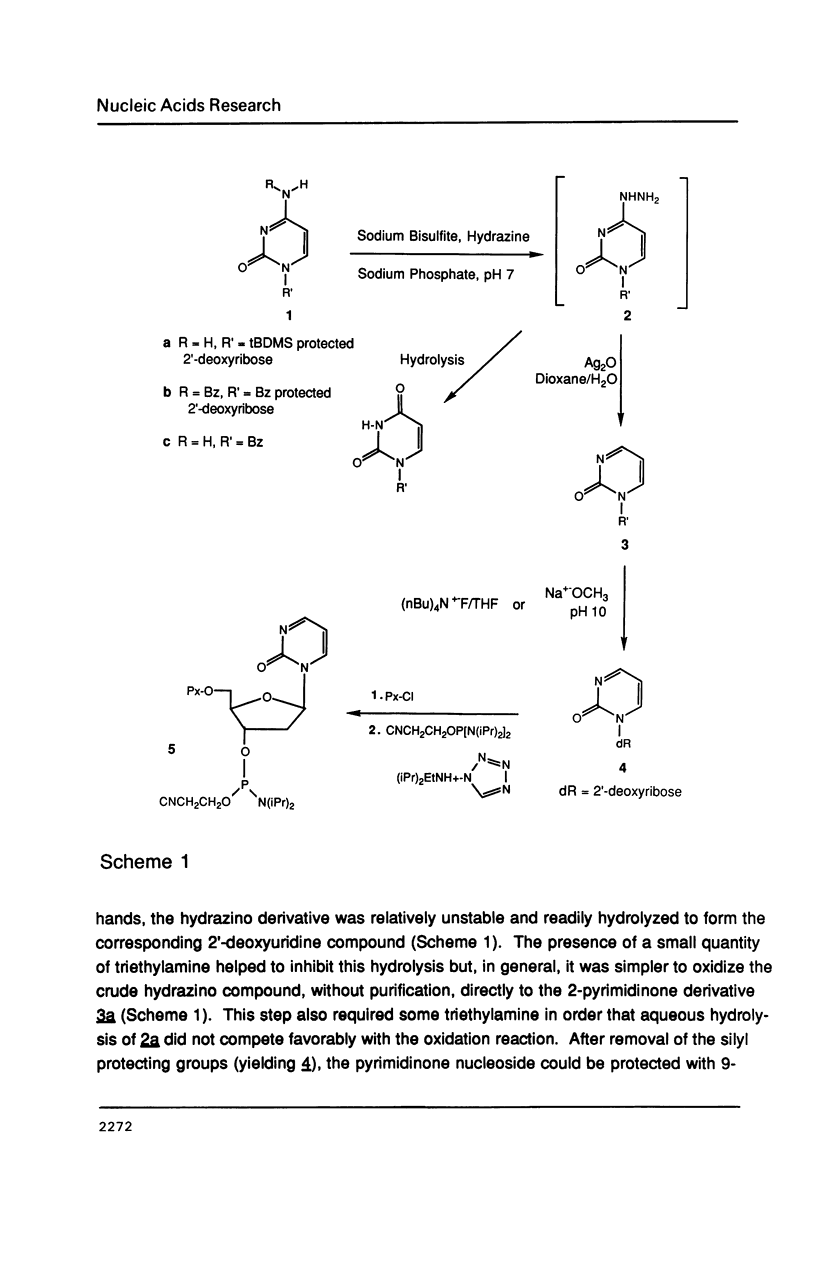
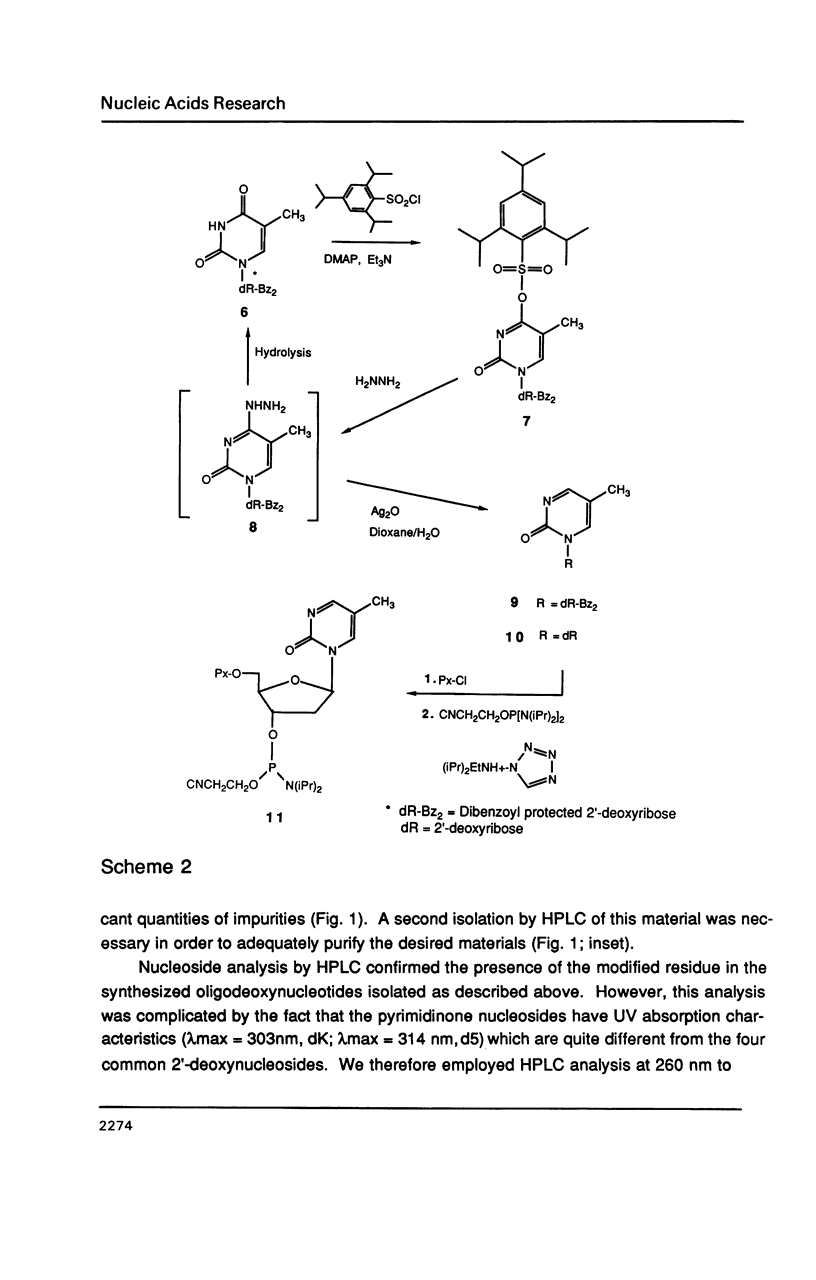
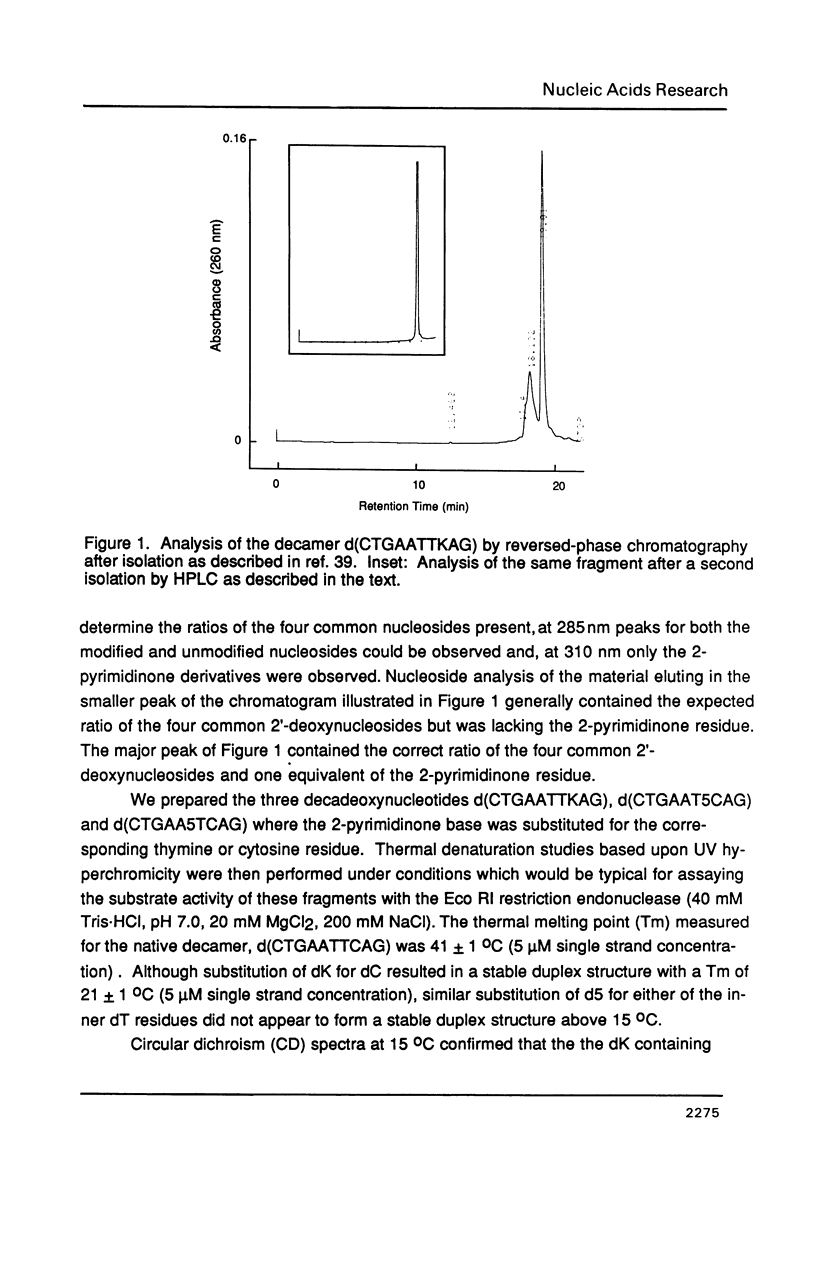
The synthesis of 1-(beta-D-2'-deoxyribosyl)-2-pyrimidinone (dK) and its 5-methyl derivative (d5) from 2'-deoxycytidine or 2'-deoxythymidine, respectively, via silver oxide oxidation of 4-hydrazinopyrimidines is described. The necessary hydrazine substituted pyrimidine nucleosides have been prepared by transamination of a protected cytidine derivative or by addition/elimination reactions to an O4-sulfonated thymidine derivative. Oxidation of the 4-hydrazino pyrimidines was complicated by a competing hydrolytic reaction which generated 2'-deoxyuridine or 2'-deoxythymidine. However, in the presence of an organic base such as triethylamine, oxidation became the predominant reaction. After suitable protection and formation of the 3'-phosphoramidite derivatives, these modified nucleosides were incorporated into seven self-complementary oligodeoxynucleotides by chemical synthesis using phosphite triester methodology. Oligodeoxynucleotides were prepared such that dA-dT and dG-dC base pairs were substituted by dA-d5 or dG-dK base pairs, respectively. Both circular dichroism spectra and thermal denaturation studies were used to characterize the modified oligodeoxynucleotides.
Full text
PDF










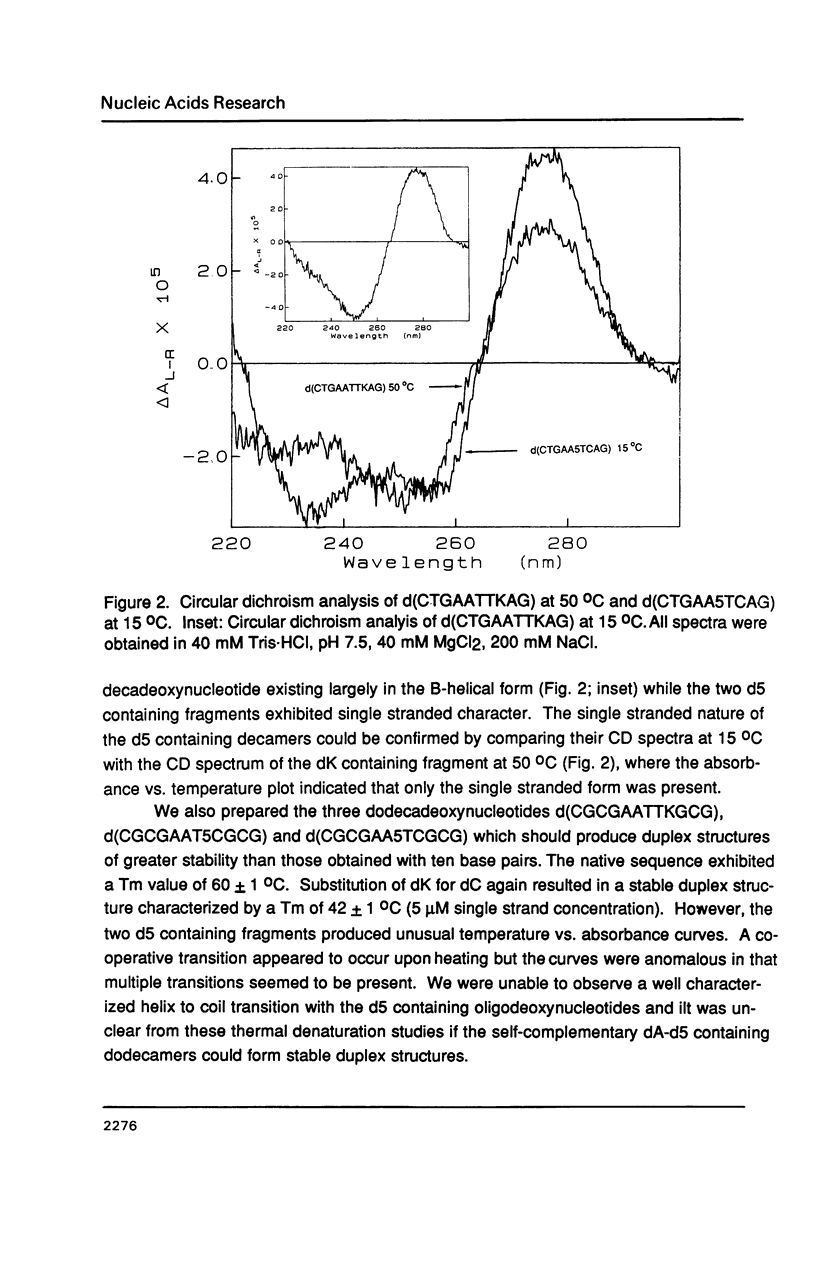
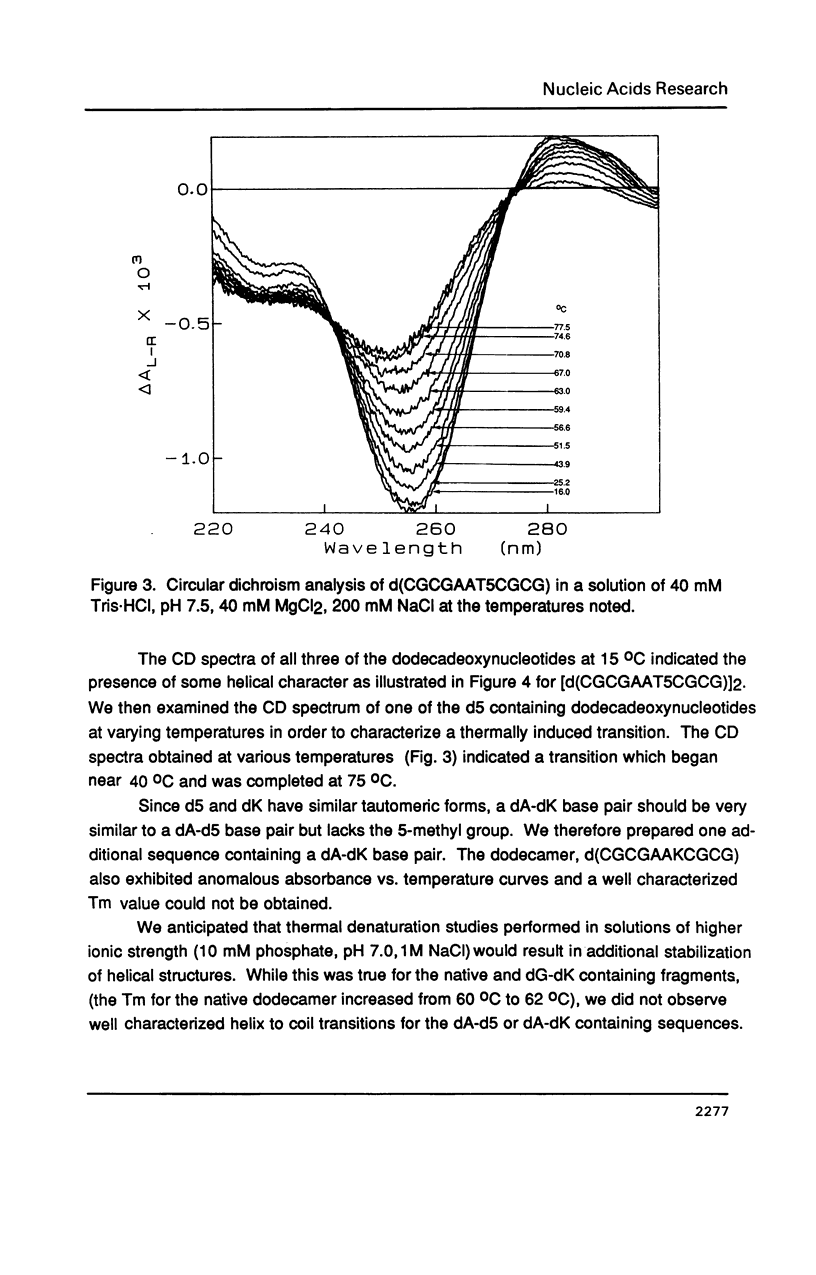

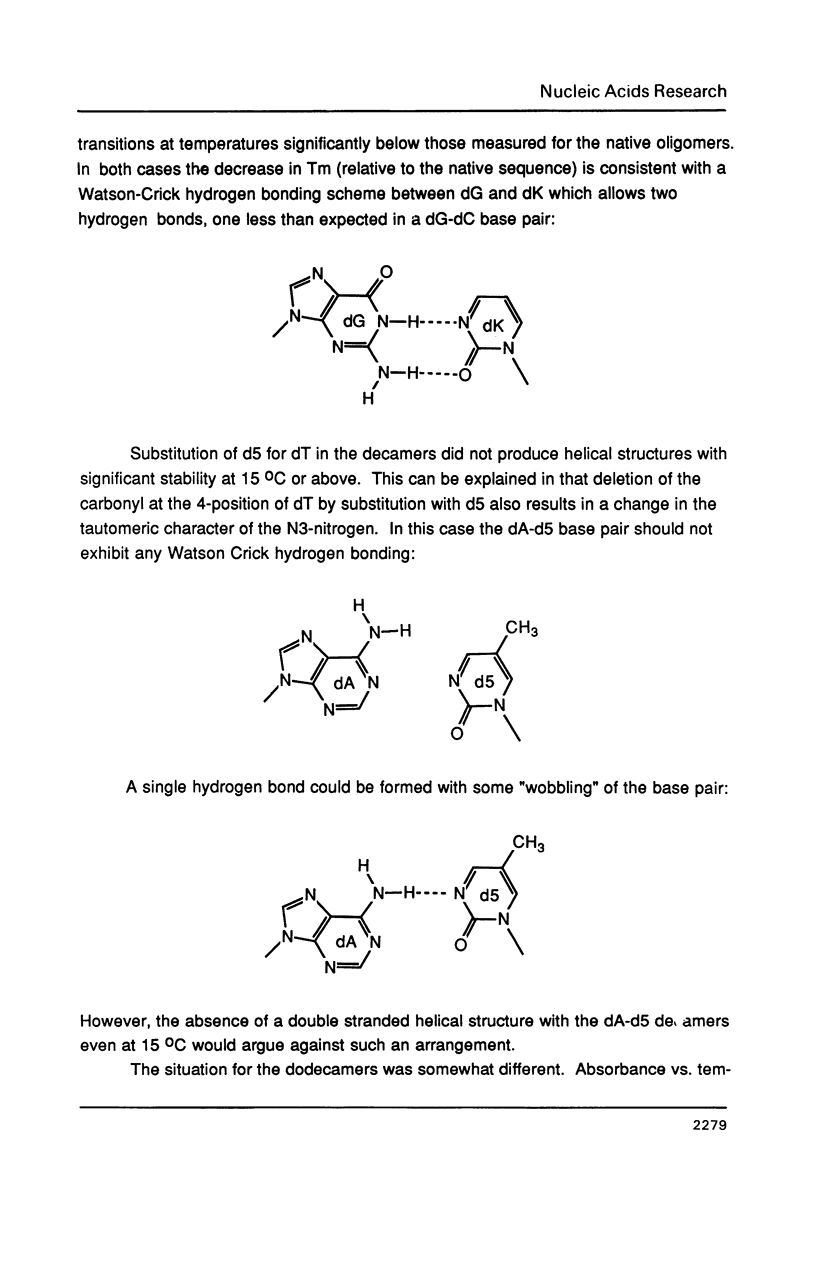


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barone A. D., Tang J. Y., Caruthers M. H. In situ activation of bis-dialkylaminophosphines--a new method for synthesizing deoxyoligonucleotides on polymer supports. Nucleic Acids Res. 1984 May 25;12(10):4051–4061. doi: 10.1093/nar/12.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Van Cleve M. D., Gumport R. I. The effects of base analogue substitutions on the cleavage by the EcoRI restriction endonuclease of octadeoxyribonucleotides containing modified EcoRI recognition sequences. J Biol Chem. 1986 Jun 5;261(16):7270–7278. [PubMed] [Google Scholar]
- Brennan C. A., Van Cleve M. D., Gumport R. I. The effects of base analogue substitutions on the cleavage by the EcoRI restriction endonuclease of octadeoxyribonucleotides containing modified EcoRI recognition sequences. J Biol Chem. 1986 Jun 5;261(16):7270–7278. [PubMed] [Google Scholar]
- Dubendorff J. W., deHaseth P. L., Rosendahl M. S., Caruthers M. H. DNA functional groups required for formation of open complexes between Escherichia coli RNA polymerase and the lambda PR promoter. Identification via base analog substitutions. J Biol Chem. 1987 Jan 15;262(2):892–898. [PubMed] [Google Scholar]
- Dwyer-Hallquist P., Kézdy F. J., Agarwal K. L. Interaction of the HpaI endonuclease with synthetic oligonucleotides. Biochemistry. 1982 Sep 14;21(19):4693–4700. doi: 10.1021/bi00262a027. [DOI] [PubMed] [Google Scholar]
- Fisher E. F., Caruthers M. H. Studies on gene control regions XII. The functional significance of a lac operator constitutive mutation. Nucleic Acids Res. 1979 Sep 25;7(2):401–416. doi: 10.1093/nar/7.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliess A., Wolfes H., Rosenthal A., Schwellnus K., Blöcker H., Frank R., Pingoud A. Role of thymidine residues in DNA recognition by the EcoRI and EcoRV restriction endonucleases. Nucleic Acids Res. 1986 Apr 25;14(8):3463–3474. doi: 10.1093/nar/14.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. Studies on gene control regions. VI. The 5- methyl of thymine, a lac repressor recognition site. Nucleic Acids Res. 1977 Sep;4(9):3039–3054. doi: 10.1093/nar/4.9.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Winston C., Caruthers M. H. Studies on gene control regions. VII. Effect of 5-bromuracil-substituted lac operators on the lac operator-lac repressor interaction. J Mol Biol. 1978 Aug 25;123(4):661–687. doi: 10.1016/0022-2836(78)90211-5. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., Hilbers C. W., van der Marel G. A., van Boom J. H., Singh U. C., Pattabiraman N., Kollman P. A. On loop folding in nucleic acid hairpin-type structures. J Biomol Struct Dyn. 1986 Apr;3(5):843–857. doi: 10.1080/07391102.1986.10508468. [DOI] [PubMed] [Google Scholar]
- Hayatsu H. Reaction of cytidine with semicarbazide in the presence of bisulfite. A rapid modification specific for single-stranded polynucleotide. Biochemistry. 1976 Jun 15;15(12):2677–2682. doi: 10.1021/bi00657a030. [DOI] [PubMed] [Google Scholar]
- Helgeland L., Laland S. The synthesis, characterization and biological properties of a new substance, 5-fluoropyrimidine-2-one. Biochim Biophys Acta. 1964 Jun 22;87(2):353–355. doi: 10.1016/0926-6550(64)90236-1. [DOI] [PubMed] [Google Scholar]
- Ikuta S., Chattopadhyaya R., Ito H., Dickerson R. E., Kearns D. R. NMR study of a synthetic DNA hairpin. Biochemistry. 1986 Aug 26;25(17):4840–4849. doi: 10.1021/bi00365a018. [DOI] [PubMed] [Google Scholar]
- Jiricny J., Wood S. G., Martin D., Ubasawa A. Oligonucleotide duplexes containing inosine, 7-deazainosine, tubercidin, nebularine and 7-deazanebularine as substrates for restriction endonucleases HindII, SalI and TaqI. Nucleic Acids Res. 1986 Aug 26;14(16):6579–6590. doi: 10.1093/nar/14.16.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland S. G., Serck-Hanssen G. Synthesis of pyrimidin-2-one deoxyribosides and their ability to support the growth of the deoxyriboside-requiring organism Lactobacillus acidophilus R 26. Biochem J. 1964 Jan;90(1):76–81. doi: 10.1042/bj0900076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. F., Reese C. B., Swann P. F. Synthesis and characterization of oligodeoxynucleotides containing 4-O-methylthymine. Biochemistry. 1987 Feb 24;26(4):1086–1093. doi: 10.1021/bi00378a015. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. W., Benseler F., Graeser E., Piel N., Scholtissek S. Effects of functional group changes in the EcoRI recognition site on the cleavage reaction catalyzed by the endonuclease. Biochemistry. 1987 Nov 17;26(23):7238–7245. doi: 10.1021/bi00397a007. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. W., Leong T., Benseler F., Piel N. A new approach to the synthesis of a protected 2-aminopurine derivative and its incorporation into oligodeoxynucleotides containing the Eco RI and Bam HI recognition sites. Nucleic Acids Res. 1988 Jun 24;16(12):5631–5644. doi: 10.1093/nar/16.12.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K., Harada C., Ohara Y., Oohara K., Nitta N., Hayatsu H. N4-aminocytidine, a nucleoside analog that has an exceptionally high mutagenic activity. Nucleic Acids Res. 1983 Aug 11;11(15):5223–5233. doi: 10.1093/nar/11.15.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A., Sato M., Ohtani Y., Ueda T. Synthesis of deoxyoligonucleotides containing 7-deazaadenine: recognition and cleavage by restriction endonuclease Bgl II and Sau 3AI (nucleosides and nucleotides Part 55). Nucleic Acids Res. 1984 Dec 11;12(23):8939–8949. doi: 10.1093/nar/12.23.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik P., Kanhouwa N., Kan L. S. Hairpin and duplex formation in DNA fragments CCAATTTTGG, CCAATTTTTTGG, and CCATTTTTGG: a proton NMR study. Biochemistry. 1988 Apr 19;27(8):3024–3031. doi: 10.1021/bi00408a054. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Ubasawa A. Nature of side-reactions in oligonucleotide synthesis involving arenesulphonyl derivatives of 3-nitro-1,2,4-triazole and related condensing agents. Nucleic Acids Symp Ser. 1980;(7):5–21. [PubMed] [Google Scholar]
- Seela F., Driller H. Palindromic oligonucleotides containing 7-deaza-2'-deoxyguanosine: solid-phase synthesis of d[(p)GG*AATTCC] octamers and recognition by the endodeoxyribonuclease EcoRI. Nucleic Acids Res. 1986 Mar 11;14(5):2319–2332. doi: 10.1093/nar/14.5.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Weisgras J. M. Bisulfite-catalyzed transamination of cytosine and cytidine. Biochem Biophys Res Commun. 1970 Aug 24;40(4):839–843. doi: 10.1016/0006-291x(70)90979-4. [DOI] [PubMed] [Google Scholar]
- Summers M. F., Byrd R. A., Gallo K. A., Samson C. J., Zon G., Egan W. Nuclear magnetic resonance and circular dichroism studies of a duplex--single-stranded hairpin loop equilibrium for the oligodeoxyribonucleotide sequence d(CGCGATTCGCG). Nucleic Acids Res. 1985 Sep 11;13(17):6375–6386. doi: 10.1093/nar/13.17.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer D. E., Chou S. H., Hare D. R., Reid B. R. Duplex-hairpin transitions in DNA: NMR studies on CGCGTATACGCG. Nucleic Acids Res. 1985 May 24;13(10):3755–3772. doi: 10.1093/nar/13.10.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yansura D. G., Goeddel D. V., Cribbs D. L., Caruthers M. H. Studies of gene control regions. III. Binding of synthetic and modified synthetic lac operator DNAs to lactose repressor. Nucleic Acids Res. 1977 Mar;4(3):723–737. doi: 10.1093/nar/4.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yansura D. G., Goeddel D. V., Kundu A., Caruthers M. H. Studied on gene control regions IX. The effect of hypoxanthine-substituted lac operators on the lac operator--lac repressor interaction. J Mol Biol. 1979 Sep 5;133(1):117–135. doi: 10.1016/0022-2836(79)90253-5. [DOI] [PubMed] [Google Scholar]
- Yolov A. A., Vinogradova M. N., Gromova E. S., Rosenthal A., Cech D., Veiko V. P., Metelev V. G., Kosykh V. G., Buryanov Y. I., Bayev A. A. Interaction of EcoRII restriction and modification enzymes with synthetic DNA fragments. VI. The binding and cleavage of substrates containing nucleotide analogs. Nucleic Acids Res. 1985 Dec 20;13(24):8983–8998. doi: 10.1093/nar/13.24.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]



