Acute foveal blue-light–induced damage was greater in monkeys fed xanthophyll-free diets and lacking macular pigment than in normal monkeys, but decreased to normal after supplementation with lutein or zeaxanthin. Monkeys that were also deficient in n-3 fatty acids had greater damage in the parafovea. These findings support both nutrients' protective role in macular disease.
Abstract
Purpose.
Blue-light photooxidative damage has been implicated in the etiology of age-related macular degeneration (AMD). The macular pigment xanthophylls lutein (L) and zeaxanthin (Z) and n–3 fatty acids may reduce this damage and lower the risk of AMD. This study investigated the effects of the lifelong absence of xanthophylls followed by L or Z supplementation, combined with the effects of n–3 fatty acid deficiency, on acute blue-light photochemical damage.
Methods.
Subjects included eight rhesus monkeys with no lifelong intake of xanthophylls and no detectable macular pigment. Of these, four had low n–3 fatty acid intake and four had adequate intakes. Control subjects had typical L, Z, and n–3 fatty acid intake. Retinas received 150-μm-diameter exposures of low-power 476-nm laser light at 0.5 mm (∼2°) eccentricity, which is adjacent to the macular pigment peak, and parafoveally at 1.5 mm (∼6°). Exposures of xanthophyll-free animals were repeated after supplementation with pure L or Z for 22 to 28 weeks. Ophthalmoscopically visible lesion areas were plotted as a function of exposure energy, with greater slopes of the regression lines indicating greater sensitivity to damage.
Results.
In control animals, the fovea was less sensitive to blue-light–induced damage than the parafovea. Foveal protection was absent in xanthophyll-free animals but was evident after supplementation. In the parafovea, animals low in n–3 fatty acids showed greater sensitivity to damage than animals with adequate levels.
Conclusions.
After long-term xanthophyll deficiency, L or Z supplementation protected the fovea from blue light–induced damage, whereas adequate n–3 fatty acid levels reduced the damage in the parafovea.
Photooxidative damage due to free radicals and singlet oxygen can occur in the retina and retinal pigment epithelium (RPE) when light interacts with photosensitizers in the presence of oxygen.1–4 Understanding the factors affecting photooxidative damage has become an area of substantial research interest because of the potential role of oxidative damage in the etiology of age-related macular degeneration (AMD).5–7 One focus of attention has been the potential for dietary antioxidants, including the retinal xanthophylls lutein and zeaxanthin, to reduce the risk of AMD.8,9 Support for this idea has been provided by the Age-Related Eye Disease Study (AREDS),10 which showed that dietary supplements containing the antioxidants vitamin C, vitamin E, and β-carotene in combination with zinc reduced the rate of progression to advanced AMD. The AREDS study also found evidence that lutein and zeaxanthin may retard progression of AMD,11 and these nutrients have been included in the follow-up clinical trial, AREDS2.
Lutein (L) and zeaxanthin (Z) are present throughout the retina,12 but are found in especially high concentrations in the fiber layers of the fovea,13 where they form the macular pigment. In addition to absorbing blue light, these compounds are effective antioxidants and may protect against photooxidative insult.14–16 Oxidative stress can damage the retina by several mechanisms, including the generation of oxidation products of retinal fatty acids that can trigger an inflammatory response promoting the initiation and progression of AMD.7
Studies have shown that macular pigment optical density (MPOD) is dependent on dietary intake17–20 and varies markedly among individuals,18,21 even monozygotic twins.22 MPOD is reduced in obese persons,23 smokers,24,25 and other persons at risk of AMD.26,27 The low values of MPOD in some individuals may combine with the age-related decline in the retina and RPE of other natural biochemical defenses, such as catalase activity, that decrease resistance to oxidative stress, which is considered a risk factor for AMD.15,16,28,29 AMD risk may be further increased by exposure to blue (short-wavelength) light, although the results of epidemiologic studies are conflicting. The study of most relevance in our present work30 reported that estimated lifetime blue-light exposure increases the risk of neovascular AMD, specifically in individuals with low plasma levels of antioxidants, including zeaxanthin. Experimental tests of the relationship of L and Z to light-induced damage and macular disease have been few, due to a lack of appropriate animal models. Only higher primates have a macula lutea, with its high foveal concentration of macular pigment. However, some cone-dominated bird retinas selectively concentrate L and Z as oil droplets in cone inner segments. In a quail model of acute white-light–induced damage, dietary Z supplementation correlated with reduced apoptotic photoreceptor death.31
There is inconsistent evidence from human studies that the potential protective actions of macular pigment translate into lower risk of AMD, but some of the largest epidemiologic studies found a reduced prevalence or risk of progression to advanced AMD in individuals with higher intakes or higher plasma levels of xanthophylls.8,11 Furthermore, a randomized trial of supplementation with an L/Z mixture in patients with atrophic AMD found increased MPOD and improvements in visual function, particularly in patients with low baseline MPOD.32
In this study, we used a nonhuman primate model to examine the effects of the macular pigment xanthophylls on vulnerability to blue-light exposure. Blue light is transmitted to the retinal surface, is absorbed by photosensitizing chromophores,1–4,14 and causes both acute and chronic photooxidation in the retina and RPE.1,33–35 Macular pigment can reduce these potentially pathologic effects in two ways. First, its diffuse presence in photoreceptor outer segments,13,36,37 coupled with its ability to quench reactive oxygen species,4,9,16 provides a mechanism for protection against oxidative damage to photoreceptors.38 Second, the spectral absorption of the macular pigment (λ max = 460 nm) and its high density within Henle's fiber layer make it an ideal prereceptoral absorber of blue light2,14 in the wavelength range (400–550 nm) that is known to produce severe damage to photoreceptors and the RPE.1,33,34,39 The selective absorption of these damaging wavelengths is thought to be the basis of macular sparing during excessive exposure to surgical and ophthalmic light sources.40,41 It is also implicated in the macular sparing seen in some degenerative retinopathies.42
A second factor that we examined in this study is the effect of n–3 fatty acids, which are also known to modulate susceptibility to light-induced damage.43,44 The n–3 fatty acid, docosahexaenoic acid (DHA, 22:6n–3) has six double bonds that are vulnerable to oxidative damage, and DHA is present at exceptionally high levels in the retina, particularly in photoreceptor outer segment membranes.45 However, DHA serves as the precursor for a series of neuroprotective factors including neuroprotectin D1, which is synthesized by RPE cells in response to oxidative stress and reduces the resulting apoptotic cell death.46 In addition, DHA counters inflammation by blocking conversion of the n–6 fatty acid, arachidonic acid, to inflammatory eicosanoids and by activating anti-inflammatory nuclear hormone receptors (reviewed in Ref. 47). Consistent with these biological properties, people with lower intakes of DHA or its precursor, eicosapentaenoic acid (EPA, 20:5n–3), or of fish (their principal dietary source) have been reported in several studies to have higher risk of advanced AMD.48–50 We showed in the present study that low DHA levels increase the sensitivity to light-induced damage in the parafovea, but not in the fovea.
This study was part of a larger project that examined the effects of L or Z supplementation in xanthophyll-free rhesus monkeys reared with adequate or low dietary intakes of n–3 fatty acids.51–54 Earlier papers from this project demonstrated that L or Z supplementation, at 3.9 micromoles/kg/d (2.2 mg/kg/d) resulted in serum levels of total xanthophylls several times those in control animals fed a stock laboratory diet. During supplementation, MPOD showed a progressive increase over the 22 to 28 weeks that preceded the final blue-light exposures.51 The present study tested the photoprotective effects of L and Z in vivo by measuring the ophthalmoscopically observable effects of acute blue-light exposure before and after supplementation, and comparing the results to data from control animals with typical levels of L and Z in the diet and in the retina.
Materials and Methods
All procedures were approved by the Institutional Animal Care and Use Committees of the Oregon National Primate Research Center at Oregon Health and Science University and the Schepens Eye Research Institute and conformed to NIH guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Xanthophyll-Free Animals
The experimental subjects included eight rhesus monkeys (Macaca mulatta), 9 to 16 years of age, reared on one of two semipurified diets. The two diets differed only in their fatty acid composition, with one diet having very low amounts (0.3% of total fatty acids) of α-linolenic acid (18:3n–3), the primary dietary n–3 fatty acid, and the other providing adequate levels (8% of total fatty acids). Both diets provided adequate levels of all essential nutrients, including vitamin A and α-tocopherol, but contained no detectable carotenoids—in particular, no L or Z. As described in detail in another paper,55 these diets were fed to the mothers of the subjects throughout pregnancy and to the subjects from the day of birth until the end of the study. The low n–3 fatty acid diet was shown to reduce retinal levels of DHA by 80% compared with the adequate diet or a standard stock diet. Table 1 lists the characteristics of the subjects, including their diet group, sex, age, and weight. All animals also received small amounts of low-carotenoid or carotenoid-free foods, such as cereals, fruits (e.g., pineapple and banana) and sweetened gelatin. The subjects were fed three times per day, had fresh water continuously available, and were maintained on a 12:12-hour light–dark cycle with an ambient light level of 50 to 90 lux produced by full-spectrum fluorescent lamps (F32-T8-TL850; Philips, Eindhoven, The Netherlands). Because they had never received dietary xanthophylls, these animals had no detectable serum xanthophylls and no detectable macular pigment51,53 and are referred to as xanthophyll-free throughout this paper.
Table 1.
Subject Characteristics and Composition of Experimental Groups
| Animal ID | Sex | Age at Start (y) | Body Weight (kg)* | Duration of Supplementation (wk)† | n–3 Fatty Acid Status | Total Serum Xanthophylls at 24 wk (nanomoles/L) | Integrated MPOD at 24 wk (OD · mm)‡ |
|---|---|---|---|---|---|---|---|
| Zeaxanthin Supplemented | |||||||
| 586 | M | 11.0 | 12.3 | 22 (15, 7) | Low | 631.8 | 0.044 |
| 398 | M | 14.8 | 11.1 | 22 (15, 7) | Adequate | 841.3 | 0.030 |
| 224 | F | 16.4 | 8.2 | 28 | Low | 1995.8 | 0.060 |
| 217 | F | 16.5 | 6.5 | 28 | Adequate | 536.8 | 0.038 |
| Mean | 14.7 | 9.5 | 1001.4 | 0.043 | |||
| SEM | 1.3 | 1.3 | 337.5 | 0.006 | |||
| Lutein Supplemented | |||||||
| 636 | M | 9.7 | 11.5 | 22 (15, 7) | Low | 860.6 | 0.040 |
| 463 | M | 13.3 | 12.0 | 22 (15, 7) | Adequate | 387.2 | 0.056 |
| 362 | F | 13.8 | 6.4 | 28 | Low | 809.6 | 0.079 |
| 397 | M | 13.4 | 8.6 | 28 | Adequate | 851.8 | 0.054 |
| Mean | 12.6 | 9.6 | 727.3 | 0.057 | |||
| SEM | 1.0 | 1.3 | 113.9 | 0.008 | |||
| Stock Diet Controls | |||||||
| 410 | F | 14.9 | 7.4 | 0 | Adequate | 73.9 | 0.060 |
| 415 | F | 14.9 | 6.3 | 0 | Adequate | 68.6 | 0.102 |
| 546 | F | 11.4 | 5.5 | 0 | Adequate | 109.1 | 0.118 |
| 402 | F | 13.8 | 5.1 | 0 | Adequate | 105.6 | 0.102 |
| Mean | 13.8 | 6.1 | 89.3 | 0.095 | |||
| SEM | 0.8 | 0.5 | 10.5 | 0.012 | |||
Body weight at time of blue light exposures.
Weeks of xanthophyll supplementation prior to blue light exposures. Values in parentheses indicate number of weeks of daily supplementation, followed by number of weeks of supplementation 4 days/week. These differences in schedules of supplementation were due to limitations in supply of purified Z.53 MPOD levels were stable after 8 weeks.51
Please note that the integrated values listed in this paper are much lower than those previously published51 because a scaling factor of 0.02 was inadvertently omitted from the integrated values in the earlier paper.
Supplementation of Xanthophyll-Free Animals with Xanthophylls
After an initial series of blue-light exposures (described later), four of the xanthophyll-free animals were supplemented with 3.9 micromoles/kg/d (2.2 mg/kg/d) of pure L (n = 4), and four were supplemented with pure Z (n = 4). The two supplemented groups were matched as far as possible with regard to their n–3 fatty acid status, sex, age, and body weight. L for this study was purified by HPLC in a noncommercial process, and Z (Optisharp) was synthesized by DSM Nutritional Products, Ltd. (Kaiseraugst, Switzerland; formerly Roche Vitamins, Ltd.). Each xanthophyll was formulated into gelatin beadlets (Actilease; DSM Nutritional Products., Ltd.). Reversed-phase HPLC analysis confirmed that the beadlets contained 4% to 9% of the purified xanthophylls and that the L beadlets contained only all-trans-L and no detectable Z.51 In the Z beadlets, approximately 90% of the Z was in the all-trans form and 10% was present as cis-Z, with no detectable L. The limit of detection for both xanthophylls was 0.2 picomoles. The cis-Z isomer was tentatively identified as 13-cis-Z by comparing absorption spectra and HPLC retention time with a known standard. Daily doses of beadlets were measured for each animal based on body weight and were inserted into food treats, such as marshmallows or small pieces of fruit that were fed just before the animals' midday meal of semipurified diet. Beadlets and individual supplement doses were stored in the dark at 4°C. The efficacy of supplementation was assessed by measuring serum levels of xanthophylls and MPOD, as described previously.51 After 4 weeks of supplementation, serum levels of total xanthophylls in both supplement groups rose to levels 10 to 20 times higher than those in animals fed stock diets.51 MPOD, measured over the central 1 mm by in vivo two-wavelength reflectometry, rose gradually over the first 24 weeks, and at 24 weeks ranged from 0.030 to 0.079 OD · mm (integrated OD at 460 nm · mm, with the baseline subtracted), which corresponded to MPOD peak values of approximately 0.1 to 0.2. (Please note that the integrated values listed in this article are much lower than those that have been published,51 because a scaling factor of 0.02 was inadvertently omitted from the integrated values in the earlier paper. The authors apologize for this oversight.) The concentration of xanthophylls in the central retina (a 4-mm disc centered on the fovea) was determined by HPLC at the end of the study after 24 to 103 weeks of supplementation53 and reached concentrations as high as or higher than those in control animals.
Control Animals
The xanthophyll-free animals were compared to a control group of four normal age-matched rhesus monkeys that were fed a stock laboratory diet (5047 High Protein Monkey Chow; Ralston Purina, Richmond, IN) plus fruits and vegetables (primarily apples and carrots) and that were housed under the same general conditions as the experimental groups. The stock diet contained 7 to 10 nanomoles/g (4–6 μg/g) of L and 7 to 9 nanomoles/g (4–5 μg/g) of all-trans-Z, providing an estimated daily intake per kg of 0.26 micromoles (150 μg) of L and 0.24 micromoles (135 μg) of Z. As described previously,51 the control animals had measurable macular pigment with a mean integrated optical density of 0.095, corresponding to a peak optical density of 0.2 to 0.3 at 460 nm, as estimated by reflectometry.
Blue-Light Exposure
The foveal and parafoveal regions of one retina of each animal was exposed to small spots of coherent blue light according to the general approach described by Ham et al.1 in 1984. Xanthophyll-free animals underwent blue-light exposures in the right eye; the left eye was reserved for quantitative morphologic analysis, the results of which have been reported elsewhere.52,54 One hemiretina received exposures before supplementation and the opposite hemiretina after supplementation. Location was counterbalanced by delivering the initial exposures to the nasal location for one half of the animals and the temporal location for the other half. Control animals received one or two sets of exposures in each eye at the same time. Since control animals were also used in initial experiments to establish the range of exposure energies needed to bracket damage thresholds, some of them had a wider range of exposures than did the xanthophyll-free animals.
The animals were prepared for blue-light exposure by initial sedation with ketamine (10–15 mg/kg IM) and dilation of the pupils with 2 drops each of 1% tropicamide and 2.5% phenylephrine. The animals were intubated and anesthetized with isoflurane vaporized in 100% oxygen and placed prone on an adjustable table. The head was positioned vertically, facing the laser system, with the jaw resting on an adjustable pad. A thoroughly cleaned and polished gas-permeable rigid contact lens was placed on the cornea with only its back surface wetted with contact lens solution. The contact lens prevented corneal desiccation and provided stable clear optics throughout the laser exposures. Fundus imaging and delivery of the laser beam was accomplished by use of a slit lamp biomicroscope and a colorless +90-D condensing lens (Volk, Mentor, OH) mounted in front of the eye by a lens holder (Steady Mount; Volk).
Blue-light exposures were made with an argon laser (MF-2000; MIRA, Boston, MA) adjusted to deliver only the 476.5-nm line. The delivery beam was set for a nominal 150-μm spot size and was operated at a very low total power level of 2–7 mW. The system was allowed to stabilize before each exposure session, and output power was monitored during exposures by intrabeam radiometric measurement (IL-1700 Radiometer; International Light, Boston, MA) to ensure that the power did not change during the exposure. The exact power used for the exposure (which varied between 2 and 7 mW) was recorded and multiplied by the duration of that exposure (5–120 seconds) to obtain the total energy of the exposure. The range of exposure energies was chosen based on preliminary studies. Each test series was composed of four or five exposures of increasing duration designed to deliver energies (J) above and below the threshold for producing visible photochemical lesions. For reference, delivery of 0.025 J (at the low-to-middle portion of the exposure range) to a 150-μm-diameter area is equivalent to an exposure of ∼140 J/cm2.
The position of the exposure beam on the retina was visually monitored continuously during each exposure. If any movement occurred during the exposure, that exposure was immediately terminated and data from that location were excluded from the analyses. Exposure of each retina proceeded in an arc pattern, with one test series in the fovea and a second in the parafovea (Fig. 1). Foveal exposures were made at approximately 0.5 mm (∼2°) from the center of the fovea, just inside the foveal rim where the foveal depression slopes toward the foveola. At this eccentricity, the typical spatial distribution of xanthophyll as determined by photographic densitometry is asymptotic and changes little with eccentricity.51 This eccentricity was chosen so that small errors in placement would not confound the results. Parafoveal exposures were applied at approximately 1.5 mm (∼6°) from the foveal center, with careful reference to vascular landmarks in previously obtained fundus photographs. The location of each foveal and parafoveal exposure was drawn on an 8 × 10-in. black-and-white enlargement of a photograph of the posterior pole of each experimental animal. The magnification of the fundus photographs was calibrated in another monkey by comparing retinal landmarks in fundus photographs taken in vivo with the same landmarks visible in a histologic whole mount of the same retina prepared after death.56
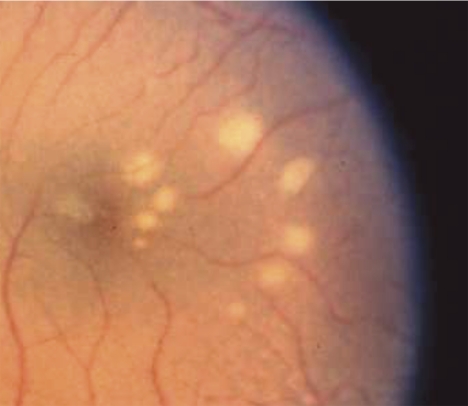
Figure 1.
Color photograph of the ocular fundus showing lesions (white spots) induced by blue-light exposures. There were two series of increasing energy with five exposures in each series. One series formed an inner arc of spots in the fovea at the edge of the macular pigment peak, whereas the outer arc of spots were in the parafovea in an area where macular pigment was optically undetectable. Lesions in the inner arc were smaller and there was no lesion at the location where the lowest energy was delivered. This image is from a control monkey with typical macular pigment.
In no case were any retinal lesions visible immediately after the exposures were made. At 24 to 48 hours after exposure, above-threshold laser lesions were seen as whitish spots of various diameters ranging from 100 to 1500 μm, and they remained stable thereafter. Color fundus photographs were obtained with a fundus camera (Carl Zeiss Meditec, Inc., Dublin, CA) on 35-mm slide film (Ektachrome 100 ASA; Eastman Kodak, Rochester, NY) (Fig. 1). The slide transparencies taken at 48 hours after exposure were read with a magnifier with a reticle, to determine the diameter in millimeters of any visible lesions. The experimenter performing the measurements was unaware of the treatment group to which each animal was assigned. If the lesions were slightly elliptical instead of circular, the major and minor axes of the ellipse were measured separately and averaged to calculate an equivalent diameter for the most similar circle. The area of the lesion was calculated, assuming a circle with this equivalent diameter. This procedure slightly overestimates the true area, but even for an ellipse with a major axis as much as twice the minor axis, the error is only 11%.
Statistical Analysis
The relationship between the area of the blue-light lesions and exposure energy was analyzed by means of a linear analysis of covariance (ANCOVA) model with generalized estimating equations (GEEs).57 The GEE is an extension of generalized linear models that can evaluate independent and correlated data at the same time, and thus allows analysis of the data of all animals simultaneously in a global and comprehensive approach. In contrast to repeated-measures ANOVA, GEE can deal with unequal group sizes and missing data and therefore optimizes the amount of data that can be used for the evaluation. The independent (between-subjects) factors in the analysis were long-term xanthophyll status (xanthophyll-free or stock diet control), the type of xanthophyll supplementation (L or Z), and n–3 fatty acid diet group (low or adequate). The correlated (within-subjects) factors were those involving data collected within the same monkey, including measures obtained before and after supplementation, exposure location (fovea and parafovea), and different levels of exposure energy. The GEE method was used to calculate P-values for comparisons of interest, and differences were considered significant at P < 0.05. All analyses were performed using the GenMod Procedure in SAS (ver. 9.1.3 for Windows; SAS Institute Inc., Cary, NC).
Results
The retinas of 12 monkeys were exposed to a total of 399 separate blue-light exposures. Four animals were normal controls; eight monkeys were xanthophyll-free at the time of the first set of exposures and received a second set of exposures after 22 or 28 weeks of L or Z supplementation. In 84 cases, eye movements or other energy delivery factors caused the exposures to be unreliable, and these were excluded from the data set. The remaining 315 exposures that resulted from stable energy delivery were divided into two groups: (1) 82 subthreshold exposures, in which no change was seen in the ophthalmoscopic appearance of the fundus at the site of energy delivery 48 hours after exposure, and (2) 233 suprathreshold exposures, in which higher exposure energy produced increasing areas of whitening centered at the point of exposure (Fig. 1). Only the suprathreshold exposures were used for the analyses.
Evaluation of the Relationship between Lesion Area and Exposure Energy
Initial ANCOVA models included comparisons of the L- and Z-supplemented groups, but found no significant differences (P = 0.33 for foveal exposures and P = 0.80 for parafoveal exposures). The two supplement groups were therefore combined in subsequent models. In addition, no interaction was found between xanthophyll status and n–3 fatty acid group (P = 0.66), and so this interaction was also excluded from the model, and effects of xanthophyll status were evaluated separately within each fatty acid group. In the final linear model, the areas of blue-light lesions were modeled by regressor variables corresponding to xanthophyll status (stock diet control, xanthophyll-free [before supplementation] or supplemented), n–3 fatty acid status (adequate or low), exposure location, and exposure energy. In addition to main effects, interactions of regressor variables were included in the model equation, with the exception noted earlier. Thus, the slope of the relationship between exposure energy and lesion area was allowed to take different values for each combination of the factors: xanthophyll status, n–3 fatty acid status, and exposure location. Inspection of the residual plots indicated that the degree of homogeneity of variance was acceptable.
Because the range of exposure energies varied among groups, most notably for the control group, we repeated the analyses with a restricted exposure range of 0 to 0.05 J, the smallest range used for any group. The resulting slopes and P-values confirmed all the findings obtained with the full dataset, including differences between the fovea and parafovea and effects of xanthophyll supplementation; furthermore, the size of the differences (absolute difference in slopes) remained similar. Therefore, the data presented include all the data points collected.
Comparisons among Experimental Conditions and Exposure Locations
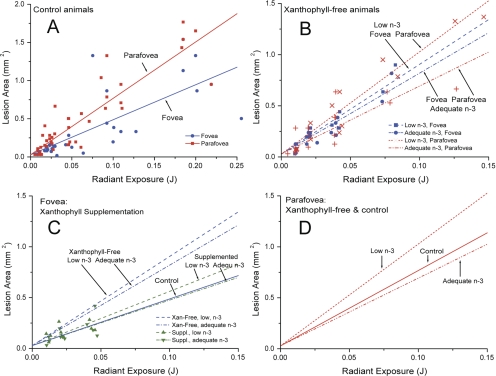
Scatterplots of the raw data are shown in Figure 2, together with the regression lines fit by the GEE analysis. The slopes of the regression lines reflect the sensitivity to blue-light–induced damage for each condition. Numerical values for slopes of the lines and P-values for comparisons are summarized in Tables 2 and 3. Several strong relationships emerged from the data, and the P-values for all differences considered to be significant were equal to or less (typically much less) than 0.0013. For control animals with normal macular pigment, the fovea was less sensitive to blue-light–induced damage than the parafovea (Fig. 2A). However, the foveas of the xanthophyll-free monkeys lacking macular pigment did not have this relative advantage. Instead, the fovea and parafovea had similar vulnerability as shown within each fatty acid group (Fig. 2B). Note that the slopes of the regression lines for the foveas in the two fatty-acid groups were not significantly different, but the parafoveal slopes differed between the two groups as described later.
Figure 2.
Lesion area as a function of exposure energy. Each data point is the area of a single lesion measured on the fundus photographs. Regression lines were computed from the GEE analyses. Slopes of the lines and P-values for comparisons are summarized in Tables 2 and 3. (A) Foveal and parafoveal lesion areas of control animals with typical laboratory diets and typical macular pigment densities. The fovea is less sensitive to blue-light exposure than the parafovea. (B) Foveal and parafoveal lesion areas of xanthophyll-free animals, with points for low-n–3 and adequate-n–3 animals plotted separately. Unlike the controls, the sensitivity of the fovea and parafovea were not significantly different within each fatty acid group. (C) Foveal lesion areas of xanthophyll-free animals before and after supplementation with lutein or zeaxanthin, compared with foveal lesions of control animals. Before supplementation, foveas of the xanthophyll-free animals were more sensitive to blue-light exposure than were the controls, but after supplementation they were not different from the controls. Regression lines for the other groups are repeated from (A) and (B), and therefore only data points for the supplemented animals are shown. Supplementation had no effect on the parafovea (data not shown). (D) Regression lines from (A) and (B) for parafoveal lesion areas of xanthophyll-free animals and controls plotted together for comparison. Again, the individual data points are shown in (A) and (B) and are not repeated here. Animals fed diets with adequate amounts of n–3 fatty acids were not different from the controls, but the parafoveal regions of animals fed low amounts of n–3 fatty acids were more sensitive to blue-light exposure than were the controls.
Table 2.
Differences between the Fovea and Parafovea in Regression Line Slopes for Lesion Size versus Exposure Energy
| Diet Group | FA Status | Slope Fovea (F) | Slope Parafovea (P) | Difference (P − F) | Direction of Difference | P |
|---|---|---|---|---|---|---|
| Control | Adequate | 4.59 | 7.39 | 2.80 | P>F | <0.0001 |
| Xanthophyll-free | Low | 8.77 | 9.99 | 1.22 | P>F | 0.2750 |
| Xanthophyll-free | Adequate | 7.91 | 6.65 | −1.26 | F>P | 0.2512 |
| Supplemented | Low | 5.33 | 12.36 | 7.03 | P>F | 0.0002 |
| Supplemented | Adequate | 4.47 | 9.02 | 4.55 | P>F | 0.0013 |
Data are in square millimeters per joule. Statistically significant comparisons are indicated in bold.
Table 3.
Effects of Xanthophyll Supplementation and n–3 Fatty Acid Status on Regression Line Slopes for Lesion Size versus Exposure Energy in the Fovea and Parafovea
| Group Comparison* |
P |
|
|---|---|---|
| Fovea | Parafovea | |
| Xanthophyll Effects in Adequate n–3 Fatty Acid Groups | ||
| Control vs. xanthophyll-free | 0.0001 | 0.5294 |
| Control vs. supplemented | 0.8900 | 0.3474 |
| Xanthophyll-free versus supplemented | <0.0001 | 0.1693 |
| Xanthophyll Effect in Low n–3 Fatty Acid Groups | ||
| Xanthophyll-free versus supplemented | <0.0001 | 0.1693 |
| Low versus Adequate n–3 Fatty Acid Status | ||
| In xanthophyll-free groups | 0.1936 | 0.0031 |
| In supplemented groups | 0.1936 | 0.0031 |
The slope values for each group are shown in Table 2. Statistical significance is indicated in bold.
L or Z supplementation of xanthophyll-free monkeys and the resulting accumulation of macular pigment decreased their foveal sensitivity to blue-light–induced damage (Fig. 2C). After supplementation, the foveal sensitivity of the previously xanthophyll-free monkeys did not differ from that of the controls. Furthermore, as in the controls, the fovea was now less sensitive to damage than the parafovea (Table 2), because the parafoveal location did not benefit from supplementation and the slope of its regression line did not change (Table 3).
In contrast, the parafovea, but not the fovea, showed an effect of n–3 fatty acid status both before and after xanthophyll supplementation. Animals fed the low n–3 fatty acid diet were more sensitive to light-induced damage in the parafovea than the adequate n–3 fatty acid group, whereas xanthophyll-free animals with adequate n–3 fatty acid intake were not different from controls (Fig. 2D, Table 3). Note that the effect of n–3 fatty acid status, and the resulting P-values, were identical in the xanthophyll-free and supplemented conditions (Table 3)—a necessary result of the statistical model, given the lack of interaction between xanthophyll and n–3 fatty acid status, as noted.
Discussion
This study used a unique set of rhesus monkeys that were reared from conception on xanthophyll-free diets. In these animals no macular pigment was detected in vivo by reflectometry,51 and the absence of xanthophylls in the serum and retina was confirmed biochemically.53 The availability of these animals allowed us to test the effects of the absence of macular pigment on sensitivity to acute blue-light–induced damage. We also demonstrated the effects of subsequent introduction of xanthophylls by dietary supplementation with pure L or Z.
Rhesus monkeys fed a commercial stock diet containing xanthophylls and having typical MPOD levels (control animals) showed significant protection against short-wavelength photochemical damage in the fovea when compared with the parafovea. Reduced foveal damage was indicated by a shallower slope of the relationship between lesion sizes and exposure energy, so that a given energy exposure consistently produced smaller lesions in the fovea than in the parafovea (Fig. 2A). The protective effect was evident even though the exposures were not located at the peak of the macular pigment profile, but adjacent to it.51 The higher MPOD in the very center of the fovea would presumably have provided even greater photoprotection.
In contrast, monkeys with no lifetime intake of xanthophylls and no measurable macular pigment showed no difference between the fovea and parafovea in sensitivity to blue-light–induced damage (Fig. 2B), a result that strongly supports the hypothesis that foveal photoprotection is due to the presence of macular pigment. This hypothesis was further confirmed by the results of L or Z supplementation, which enhanced foveal protection so that sensitivity to damage was no longer significantly different from the stock diet group (Fig. 2C). Biochemical analysis of the central retinas of the supplemented animals confirmed that xanthophyll levels resembled those of the stock diet controls.53
Lifelong n–3 fatty acid deficiency also affected the relationship between blue-light exposure and lesion size, but unlike xanthophyll deficiency, the effect was seen, not in the fovea but in the parafovea (Fig. 2D), a locus of particular susceptibility to age-related rod loss.58 This protective effect of n–3 fatty acids must be qualified as occurring on a background of life-long absence of xanthophylls, since our experiment did not include a group deficient in n–3 fatty acids while having long-term normal xanthophyll levels. However, the effect was seen in both the xanthophyll-free and supplemented conditions. Unlike L and Z, DHA is a major structural component of outer segment membranes throughout the retina and appears to comprise a slightly higher proportion of total fatty acids in the peripheral retina than in the macula.59 At first glance, our results appear inconsistent with those in previous studies of acute light-induced damage in n–3 fatty acid–deficient rodents, which showed an opposite, protective effect of low DHA concentrations that was ascribed to the high vulnerability of DHA to oxidative damage.43,44 However, these studies involved different light damage mechanisms, as they used diffuse light exposures at middle wavelengths designed to maximally activate rhodopsin.60 Other evidence of potentially negative effects of n–3 fatty acids comes from studies showing that oxidative products of DHA form protein adducts that are elevated in AMD patients' plasma and RPE.61 On the other hand, several recent studies provide rationales for antioxidative, anti-inflammatory, and neuroprotective actions of DHA and its metabolites within the retina. For example, DHA is the precursor of neuroprotectin D1, a lipid mediator that promotes RPE cell survival by multiple mechanisms under conditions of oxidative stress (e.g., Ref. 62) and it also is the precursor of related anti-inflammatory compounds termed resolvins.63 Furthermore, in cultures of retinal neurons and RPE cells, DHA or its derivatives block oxidative damage-induced apoptosis.64,65 These considerations suggest that DHA probably has both positive and negative influences on neuronal sensitivity to light damage and that the final outcome depends on the balance among them, which could be dependent on the animal species and the type of light exposure.
Most studies of retinal light-induced damage have measured the damage threshold. This method was not feasible in our study, because reliable threshold estimates require multiple exposures at each of several power levels, which could not be administered in a narrow annulus at 0.5 mm eccentricity—particularly given the need to compare the retinal response to exposures before and after supplementation in the same animals. We found that the linear function relating lesion size to radiant exposure provided a more robust measure that used all the exposure data. It is unlikely that the increase in lesion size was due to movement of the beam, because exposures were closely monitored for stability and were rejected a priori if any motion was detected. Furthermore, lesions were almost entirely circular, whereas motion would have caused irregular lesion shapes. In any case, if there were inflation of lesion size due to motion, it would not bias the group comparisons because all dietary groups would have been equally affected, particularly since the exposures were performed by an investigator masked to diet. The mechanisms causing larger lesions with higher exposure energies cannot be determined from our experiments. It is likely that the reactive species produced by the blue light diffused farther beyond the borders of the exposed area when larger amounts were generated by higher energies. Whatever the mechanism, the result was that a minimally suprathreshold lesion approximated the size of the delivery beam, while higher energy exposures produced progressively larger lesion areas.
The foveal photoprotection provided by macular pigment could occur both by absorption of blue light and by photochemical mechanisms such as quenching of reactive species. However, our in vivo macular pigment densitometry data,51 as well as two-wavelength densitometry of excised retinas (Snodderly DM. IOVS 2008;49:ARVO E-Abstract 4966), show that in rhesus monkeys MPOD declines to asymptotically low levels at the 0.5-mm eccentricity foveal exposure site used in this study. The low levels of absorption indicated by these data suggest that photochemical mechanisms contribute more strongly than blue light filtering to the protection we have documented. This suggestion is consistent with our biochemical measurements of retinal L and Z concentrations in a 4-mm diameter punch centered on the foveas of these same retinas.53 We found 12.6 to 37.7 picomoles of total xanthophylls per foveal punch for these animals. As a rough estimate, we calculated (according to the method of Handelman et al.66) an upper limit of the optical density that would result from these amounts of pigment, if the pigment were uniformly distributed over the area of the punch, to be approximately 0.03 at the laser wavelength. Since the pigment is fairly uniform in density beyond 0.5 mm eccentricity, but is higher in the central peak, this measurement should be an overestimate of the density at 0.5 mm eccentricity and beyond, which implies relatively weak absorption of the damaging light at all but the most central retinal loci.
In this study, we have shown that L or Z, when provided in the diet and deposited as macular pigment, provided foveal protection from acute blue-light photochemical damage, whereas low n–3 fatty acid status increased sensitivity to damage in the parafovea. If chronic light exposure contributes similarly to RPE damage, as has been suggested,2,67 the same mechanisms may contribute to risk for AMD.9,16,30,68–70 While the decades-long chronic nature of such a process makes proof difficult, evidence is accumulating that the RPE is intrinsically vulnerable to blue-light–induced damage and that the damage can be minimized by key nutrients. For example, one important factor in the photooxidation process is A2E, the major fluorophore of RPE lipofuscin. When exposed to blue light, A2E generates singlet oxygen and oxidized byproducts, which damage DNA and cause apoptotic cell death of RPE cells.4 In vitro experiments by Kim et al.71 showed that L or Z substantially inhibited the blue-light–induced photooxidation of A2E and A2-PE (the immediate precursor of A2E); furthermore, L and Z efficiently quenched damaging singlet oxygen generated by these fluorophores without being consumed in the reaction. DHA-derived neuroprotectin D1 also blocked apoptosis triggered by A2E.65
Our study showed that the macular xanthophylls L and Z protect the fovea against acute blue-light–induced damage, and that n–3 fatty acids lessen damage in the parafovea. It therefore seems probable that these nutrients would also protect the macula against chronic blue-light–induced damage. Thus, our results offer hope that good nutrition, augmented by supplementation when appropriate, can contribute to reduction of risk for AMD, especially for persons with reduced macular xanthophyll levels due to retinal disease, poor diet, or genetic predisposition.
Acknowledgments
The authors thank Josephine Gold, Noelle Landauer, Dana Myers, Neal Young, and Audrey Trupp for care of the experimental animals and the preparation and feeding of supplements; Noelle Landauer for assistance with data analysis; Marita Sandstrom for assistance in developing the methodology; John Fanton for veterinary services; Regina Goralczyk (DSM Nutritional Products Ltd. [formerly Roche Vitamins Ltd.]) for providing logistical assistance; and Alfred Giger and colleagues (DSM Nutritional Products Ltd.) for providing specially purified zeaxanthin-free lutein.
Footnotes
Supported by DSM Nutritional Products Ltd., Kaiseraugst, Switzerland; Grant DK29930 from the Institute of Diabetes, Digestive and Kidney Diseases; Grant RR00163 from the National Center for Research Resources; Grant 581950-9-001 from the U.S. Department of Agriculture; Grant P30 EY03790 from the National Eye Institute; and a grant from The Foundation Fighting Blindness.
Disclosure: F.M. Barker, II, DSM Nutritional Products (F); D.M. Snodderly, DSM Nutritional Products (F); E.J. Johnson, DSM Nutritional Products (F); W. Schalch, DSM Nutritional Products (E); W. Koepcke, DSM Nutritional Products (C); J. Gerss, None; M. Neuringer, DSM Nutritional Products (F)
References
- 1. Ham WT, Mueller HA, Ruffolo JJ, et al. Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr Eye Res. 1984;3:165–174 [DOI] [PubMed] [Google Scholar]
- 2. Ham WT, Allen RG, Feeney-Burns L, Parver LM, Proctor PH, Wolbarscht ML. The involvement of the retinal pigment epithelium (RPE). In: Waxler M, Hitchins VM. eds. Optical Radiation and Visual Health. Boca Raton, FL: CRC Press; 1986:43–67 [Google Scholar]
- 3. Dayhaw-Barker P. Ocular photosensitization. Photochem Photobiol. 1987;46:1051–1055 [DOI] [PubMed] [Google Scholar]
- 4. Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606 [DOI] [PubMed] [Google Scholar]
- 5. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 6. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134 [DOI] [PubMed] [Google Scholar]
- 7. Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: The Proctor Lecture. Invest Ophthalmol Vis Sci. 2010;51:1276–1281 [DOI] [PubMed] [Google Scholar]
- 8. Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration: Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420 [PubMed] [Google Scholar]
- 9. Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–1461S [DOI] [PubMed] [Google Scholar]
- 10. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report no. 22. Arch Ophthalmol. 2007;125:1225–1232 [DOI] [PubMed] [Google Scholar]
- 12. Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849 [PubMed] [Google Scholar]
- 13. Snodderly DM, Auran JD, Delori FC. The macular pigment, II: spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685 [PubMed] [Google Scholar]
- 14. Schalch W. Carotenoids in the retina: a review of their possible role in preventing or limiting damage caused by light and oxygen. In: Emerit I, Chance B. eds. Free Radicals and Aging. Basel: Birkhauser Verlag; 1992:280–298 [DOI] [PubMed] [Google Scholar]
- 15. Landrum JT, Bone RA, Kilburn MD. The macular pigment: a possible role in protection from age-related macular degeneration. Adv Pharmacol. 1997;38:537–556 [DOI] [PubMed] [Google Scholar]
- 16. Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann Rev Nutr. 2003;23:171–201 [DOI] [PubMed] [Google Scholar]
- 17. Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci. 1980;19:857–863 [PubMed] [Google Scholar]
- 18. Hammond BR, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A. 1997;14:1187–1196 [DOI] [PubMed] [Google Scholar]
- 19. Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992–998 [DOI] [PubMed] [Google Scholar]
- 20. Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006;84:1107–1122 [DOI] [PubMed] [Google Scholar]
- 21. Hammond BR, Curran-Celentano J, Judd S, et al. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996;36:2001–2012 [DOI] [PubMed] [Google Scholar]
- 22. Hammond BR, Fuld K, Curran-Celentano J. Macular pigment density in monozygotic twins. Invest Ophthalmol Vis Sci. 1995;36:2531–2541 [PubMed] [Google Scholar]
- 23. Hammond BR, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci. 2002;43:47–50 [PubMed] [Google Scholar]
- 24. Hammond BR, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res. 1996;36:3003–3009 [DOI] [PubMed] [Google Scholar]
- 25. Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A. 2001;18:1212–1230 [DOI] [PubMed] [Google Scholar]
- 26. Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001;42:439–446 [PubMed] [Google Scholar]
- 27. Nolan JM, Stack J, O' Donovan O, Loane E, Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007;84:61–74 [DOI] [PubMed] [Google Scholar]
- 28. Liles MR, Newsome DA, Oliver PD. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch Ophthalmol. 1991;109:1285–1288 [DOI] [PubMed] [Google Scholar]
- 29. Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fletcher AE, Bentham GC, Agnew M, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126:1396–1403 [DOI] [PubMed] [Google Scholar]
- 31. Thomson LR, Toyoda Y, Delori FC, et al. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp Eye Res. 2002;75:529–542 [DOI] [PubMed] [Google Scholar]
- 32. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004;75:216–230 [DOI] [PubMed] [Google Scholar]
- 33. Harwerth RS, Sperlng HG. Prolonged color blindness induced by intense spectral lights in rhesus monkeys. Science. 1971;174:520–523 [DOI] [PubMed] [Google Scholar]
- 34. Ham WT, Ruffolo JJ, Mueller HA, Clarke AM, Moon ME. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci. 1978;17:1029–1035 [PubMed] [Google Scholar]
- 35. Sperling HG, Johnson C, Harwerth RS. Differential spectral photic damage to primate cones. Vision Res. 1980;20:1117–1125 [DOI] [PubMed] [Google Scholar]
- 36. Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res. 1999;19:491–495 [DOI] [PubMed] [Google Scholar]
- 37. Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41:1200–1209 [PubMed] [Google Scholar]
- 38. Chucair AJ, Rotstein NP, SanGiovanni JP, During A, Chew EY, Politi LE. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:5168–5177 [DOI] [PubMed] [Google Scholar]
- 39. Harwerth RS, Sperling HG. Effects of intense visible radiation on the increment-threshold spectral sensitivity of the rhesus monkey eye. Vision Res. 1975;15:1193–1204 [DOI] [PubMed] [Google Scholar]
- 40. Hupp SL. Delayed, incomplete recovery of macular function after photic retinal damage associated with extracapsular cataract extraction and posterior lens insertion: case report. Arch Ophthalmol. 1987;105:1022–1023 [DOI] [PubMed] [Google Scholar]
- 41. Jaffe GJ, Wood IS. Retinal phototoxicity from the operating microscope: a protective effect by the fovea. Arch Ophthalmol. 1988;106:445–446 [DOI] [PubMed] [Google Scholar]
- 42. Weiter JJ, Delori F, Dorey CK. Central sparing in annular macular degeneration. Am J Ophthalmol. 1988;106:286–292 [DOI] [PubMed] [Google Scholar]
- 43. Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Visual Sci. 1996;37:2243–2257 [PubMed] [Google Scholar]
- 44. Bush RA, Reme CE, Malnoe A. Light damage in the rat retina: the effect of dietary deprivation of n-3 fatty acids on acute structural alterations. Exp Eye Res. 1991;53:741–752 [DOI] [PubMed] [Google Scholar]
- 45. Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131 [DOI] [PubMed] [Google Scholar]
- 46. Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104:13152–13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138 [DOI] [PubMed] [Google Scholar]
- 48. Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199 [DOI] [PubMed] [Google Scholar]
- 49. Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol. 2008;126:826–833 [DOI] [PubMed] [Google Scholar]
- 50. SanGiovanni JP, Chew EY, Agrón E, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243 [DOI] [PubMed] [Google Scholar]
- 52. Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45:3244–3256 [DOI] [PubMed] [Google Scholar]
- 53. Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702 [DOI] [PubMed] [Google Scholar]
- 54. Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, IV: effects of n-3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region. Exp Eye Res. 2005;81:513–529 [DOI] [PubMed] [Google Scholar]
- 55. Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega-3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986;83:4021–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci. 1992;12:1169–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130 [PubMed] [Google Scholar]
- 58. Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–2018 [PubMed] [Google Scholar]
- 59. van Kuijk FJGM, Buck P. Fatty acid composition of the human macula and peripheral retina. Invest Ophthalmol Vis Sci. 1992;33:3493–3496 [PubMed] [Google Scholar]
- 60. Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132 [DOI] [PubMed] [Google Scholar]
- 64. German OL, Insua MF, Gentili C, Rotstein NP, Politi LE. Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J Neurochem. 2006;98:1507–1520 [DOI] [PubMed] [Google Scholar]
- 65. Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: The Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–4881 [DOI] [PubMed] [Google Scholar]
- 66. Handelman GJ, Snodderly DM, Krinsky NI, Russett MD, Adler AJ. Biological control of primate macular pigment: biochemical and densitometric studies. Invest Ophthalmol Vis Sci. 1991;32:257–267 [PubMed] [Google Scholar]
- 67. Smith HE. Actinic macula retinal pigment degeneration. US Naval Medical Bulletin. 1944:675–680 [Google Scholar]
- 68. Margrain TH, Boulton M, Marshall J, Sliney DH. Do blue light filters confer protection against age-related macular degeneration? Prog Retin Eye Res. 2004;23:523–531 [DOI] [PubMed] [Google Scholar]
- 69. Mainster MA. Editorial: Light and macular aging. Light Lasers Ophthalmol. 1993;5:117–119 [Google Scholar]
- 70. Glazer-Hockstein C, Dunaief JL. Could blue light-blocking lenses decrease the risk of age-related macular degeneration? Retina. 2006;26:1–4 [DOI] [PubMed] [Google Scholar]
- 71. Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82:828–839 [DOI] [PubMed] [Google Scholar]