Fas-dependent activation of both autophagy and apoptosis and the antagonist relationship between these two pathways represent a novel mechanism of controlling photoreceptor survival after retina–RPE separation.
Abstract
Purpose.
To examine the activation of autophagy and its relationship to Fas-mediated photoreceptor apoptosis during experimental retinal detachment.
Methods.
Retina–retinal pigment epithelium (RPE) separation was created in Brown-Norway rats by subretinal injection of 1% hyaluronic acid and the intraretinal levels of the autophagy proteins LC3 and Atg5, the time course of LC3-I to LC3-II conversion, and the activation of cathepsins B and D were assayed with Western blot analysis and immunohistochemistry. We measured the ability of a Fas-activating antibody to induce LC3-I to LC3-II conversion in 661W cells, and the in vivo effect of Met12, a small molecule inhibitor of the Fas receptor, on LC3-I to LC3-II conversion and Atg5 expression. Autophagy activation was inhibited using 3-methyladenine (3-MA) or siRNA knockdown of Atg5 and the effect on apoptosis was measured using a caspase 8 activity assay, caspase 8 immunoblots, and photoreceptor TUNEL staining.
Results.
Retina–RPE separation resulted in a Fas-dependent activation of autophagy, with increased Atg5 levels and intraphotoreceptor conversion of LC3-I to LC3-II. Detached retinas had increased levels of autophagosome-associated lysosomal proteases, cathepsins B and D. Inhibition of autophagy by 3-MA or siAtg5 accelerated the time course of caspase 8 activation and photoreceptor TUNEL staining.
Conclusions.
Autophagy activation occurs in the photoreceptors after retina–RPE separation. This appears to be, at least in part, dependent on Fas receptor activation, and plays a role in regulating the level of photoreceptor apoptosis.
Separation of the retina from the retinal pigment epithelium (RPE) is a common form of injury that may occur alone (retinal detachment) or as a result of other blinding disease processes such as age-related macular degeneration or diabetic retinopathy. Despite significant advances in the medical and surgical management of retina–RPE separation, patients often lose vision, primarily due to the death of photoreceptors.1,2 We previously showed that the main event causing photoreceptor death is the activation of the Fas receptor and the downstream cascade of caspases 8, 3, 7, and 9.3–5 Preventing Fas pathway activity provides significant protection against separation-induced death of the photoreceptors.
Although Fas-mediated apoptosis is activated rapidly after retina–RPE separation, a surprising number of photoreceptors survive for extended periods of time. This is best exemplified by the clinical observation that patients with retinal detachments affecting central vision generally recover near-normal vision if the detachment is repaired within 1 week.6,7 If repair is delayed beyond 1 week, visual outcomes become significantly poorer. This suggests that early in the course of the detachment, antiapoptotic pathways are activated within the retina to counteract the effect of proapoptotic signals and that they are responsible for the therapeutic window of opportunity for reattachment.8
Autophagy is a complex process that results in the autovacuolization of damaged or redundant cellular components. These vacuoles (i.e., autophagosomes) fuse with lysosomes and their contents are enzymatically degraded and released back into the cell.9 Autophagy activation typically occurs during periods of metabolic stress, usually serving as a cytoprotective process.10 Autophagy has been shown to occur in photoreceptors as part of basal processing of rod outer segments11 as well as in animal models of hereditary retinal degeneration and in models of oxidative stress.12–14 The role of autophagy in these settings is not well defined.
We examined the role of autophagy and its control of photoreceptor apoptosis using a well-established rodent model of experimental retina–RPE separation. Our findings demonstrate that retinal detachment results in the activation of autophagy in photoreceptors. Similar to apoptosis, detachment-induced autophagy activity is Fas dependent. In detached retinas, autophagy acts as a prosurvival pathway through negative inhibition of apoptosis. Our data suggest a novel mechanism by which autophagy can be activated and regulate photoreceptor apoptosis.
Methods
Experimental Model of Retinal Detachment
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines established by the University Committee on Use and Care of Animals of the University of Michigan. Detachments were created in adult male Brown-Norway rats (300–400 g; Charles River Laboratories, Wilmington, MA) as described previously.3 Briefly, rodents were anesthetized with a 50:50 mix of ketamine/xylazine, and pupils were dilated with topical phenylephrine (2.5%) and tropicamide (1%). A 20-gauge microvitreoretinal blade was used to create a sclerotomy 2 mm posterior to the limbus, carefully avoiding lens damage. A subretinal injector (Glaser, 32-gauge tip; BD Ophthalmic Systems, Franklin Lakes, NJ) was introduced through the sclerotomy into the vitreous cavity and then through a peripheral retinotomy into the subretinal space. Sodium hyaluronate (10 mg/mL, Healon OVD; Abbott Medical Optics, Uppsala, Sweden) was slowly injected to detach the neurosensory retina from the underlying RPE. In all experiments, approximately one third to one half of the neurosensory retina was detached. Detachments were created in the left eye. The right eye served as the control, with all the steps of the procedure performed except for introduction of the subretinal injector and injection of the sodium hyaluronate. In some experimental eyes, subretinal injection of either 3-methyladenine (3-MA, 50 μg; Sigma-Aldrich, St. Louis, MO), bafilomycin A (Baf A, 50 μg; Enzo Life Sciences, Plymouth Meeting, PA), Met YLGA 12-mer (HHIYLGAVNYIY, Met, 50 μg), a mutant Met12-mer (HHGSDHERNYIY, mMet, 50 μg), or vehicle (DMSO) was performed at the time of retina–RPE separation. In these experiments, the subretinal sodium hyaluronate was injected as described earlier, immediately followed by additional subretinal injection of the specific agent delivered in a 10 μL volume.
Cell Culture
The 661W photoreceptor cell line was generously provided by Dr. Muayyad Al-Ubaidi (Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK). The 661W cell line was maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 300 mg/L glutamine, 32 mg/L putrescine, 40 μL/L β-mercaptoethanol, and 40 μg/L of both hydrocortisone 21-hemisuccinate and progesterone. The media also contained penicillin (90 units/mL) and streptomycin (0.09 mg/mL). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Western Blot Analysis
Retinas from experimental eyes with detachments and control eyes without detachments were dissected from the RPE–choroid, homogenized, and lysed in buffer containing 10 mM HEPES (pH 7.6), 0.5% IgEPal, 42 mM KCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 5 mM MgCl2, and one tablet of protease inhibitors per 10 mL buffer (Complete Mini; Roche Diagnostics GmbH, Madison, WI). Retinas from at least three animals were pooled per condition at each time point examined. Assays were repeated in triplicate on independently collected samples. 661W cells were plated at 20,000 cells per well in six-well culture dishes in growth media. After 24 hours, the cells were washed one time with warmed PBS solution and then placed in growth media without fetal bovine serum. After a 24-hour incubation, the media was removed and new media without fetal bovine serum containing 500 ng/mL Fas-activating antibody was added. The cells were incubated for various lengths of time and then lysed and homogenized in lysis buffer (as above). The homogenates were incubated on ice and centrifuged at 22,000g at 4°C for 60 minutes. The protein concentration of the supernatant was then determined (DC Protein Assay kit; Bio-Rad Laboratories, Hercules, CA). The protein samples were separated on SDS-polyacrylamide gels (Tris-HCl Ready Gels; Bio-Rad Laboratories). After electrophoretic separation, the proteins were transferred onto polyvinylidene fluoride membranes (Immobilon-P; Millipore, Billerica, MA). Protein bands were visualized with Ponceau S staining, and the lanes were assessed for equal loading by densitometry of entire lanes. Membranes were then immunoblotted with antibodies according to the manufacturer's instructions. The following antibodies were used: LC3 (Novus Biologicals, Littleton, CO), cathepsin B and cathepsin D (Santa Cruz Biotechnology, Santa Cruz, CA), receptor interacting protein kinase 1 (RIPK-1; BD Biosciences, Franklin Lakes, NJ), Atg5 (Abgent, San Diego, CA), and caspase 8 (Cell Signaling, Danvers, MA). Densitometry measurements were performed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Immunohistochemistry
The eyes were enucleated and placed in 4% paraformaldehyde overnight at 4°C. Whole eyes were then placed in a tissue processor (Tissue-Tek II; Sakura, Tokyo, Japan) for standard paraffin embedding. Eyes were then sectioned at a width of 6 μm on a standard paraffin microtome. Immunohistochemistry was performed on sections obtained from paraffin-embedded retinas. Epitope unmasking was accomplished by incubating the sections in boiling 10 mM citrate buffer (pH 6.0) for 10 minutes. Slides were then washed with PBS with 0.5%Tween 20, incubated for 10 minutes in 3% hydrogen peroxide at room temperature, and followed by another PBS with a 0.5% Tween 20 wash. The 661W cells were fixed 30 minutes in 4% paraformaldehyde at 4°C, followed by PBS wash three times. The incubation with primary and secondary antibodies was performed in accordance with the manufacturer's instructions. The number of autophagasomes in 661W cells treated with the Fas-activating antibody (Fas-AAb) or vehicle control was quantified by counting punctate spots in cells stained with LC3 antibody. Data are represented as mean ± SE (n = 9 per group).
Caspase Assay
Caspase 8 activity was measured by a commercially available luminescent tetrapeptide cleavage assay kit (Promega, Madison, WI). The 661W cells were seeded in 96-well plates (Nunc, Rochester, NY) at 1500 cells/well for 24 hours before treatment. Cells were pretreated with 10 nM 3-MA for 30 minutes before treatment with 500 ng/mL Fas-agonistic Jo2 monoclonal antibody (BD Biosciences). Caspase 8 activity was measured at various time points by incubating the cells with the proluminescent substrate in 96-well plates according to the manufacturer's instructions. Controls included untreated cells and wells with no cells. Luminescence was measured in a plate reader luminometer (Turner Biosystems, Sunnyvale, CA).
TUNEL Staining
At 24 hours after creation of the detachment, the animals were euthanatized and the eyes were enucleated. For TUNEL staining, whole eyes were fixed overnight at 4°C in PBS with 4% paraformaldehyde (pH 7.4). The specimens were embedded in paraffin and sectioned at a thickness of 6 μm. TUNEL staining was performed on the sections with an apoptosis detection kit (ApopTag Fluorescein In Situ Kit; Millipore, Billerica, MA), according to the manufacturer's instructions. TUNEL-positive cells in the outer nuclear layer were counted in a masked fashion.
siRNA Treatment
To inhibit autophagosome formation, we injected small inhibitory RNA against Atg5 (siAtg5; Invitrogen Life Technologies, Carlsbad, CA) to prevent the separation-induced increase in the transcription of Atg5 gene. siAtg5 was injected at a dose of 0.5 × 10−9 mol into the subretinal space of the detached retina in a volume of 10 μL PBS. Injection of siAtg5 was also performed using a syringe (Hamilton Co., Reno, NV) that passed through the same sclerotomy and retinotomy used to create the retina–RPE separation. Control animals were injected with an inactive scrambled siRNA construct in a similar fashion. Real-time quantitative polymerase chain reactions were performed as previously described5 to confirm the decrease in Atg5 transcript.
Data Analysis
Statistical analysis comparing groups was performed using two-tailed Student's t-tests assuming equal variance. Differences were considered significant at P ≤ 0.05.
Results
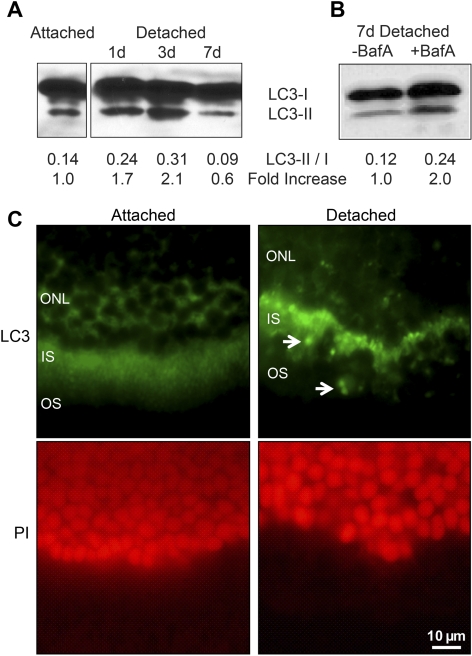
We first showed that autophagy is activated during retinal detachment. The primary biochemical marker of autophagy activation and autophagosome formation is the protein LC3, an essential component of the autophagosome complex.15 Endogenous LC3 migrates as two bands on polyacrylamide gel electrophoresis. The upper band represents LC3-I, which is cytosolic, and the lower, faster-migrating band represents LC3-II. LC3-II is conjugated with phosphatidylethanolamine and is present on autophagosomes. In our in vivo model, there was a time-dependent increase in the levels of LC3-II in detached retinas (Fig. 1A), which peaked 3 days postdetachment and returned to baseline levels by day 7. LC3-II, however, can itself be a target of autophagy-induced lysosomal degradation. Baf A can prevent autophagosome–lysosome fusion as well as lysosomal acidification required for content degradation,16 and is an accepted method for measuring autophagic flux.17 When we reexamined the levels of retinal LC3 in the presence of Baf A after 7 days of detachment, there was a persistently increased level of LC3-II (Fig. 1B). This suggested that the decreased level of LC3-II seen 7 days postdetachment, without Baf A treatment, was secondary to increased degradation of the LC3 by autophagy and not to a decrease in autophagy itself.
Figure 1.
(A) Western blot of LC3 showing increased levels of LC3-II as a function of time after retina–RPE separation. The ratio of LC3-II to LC3-I appeared to peak at 3 days postdetachment, with an approximately twofold increase compared with baseline (attached). An equal amount of protein was loaded in each lane, as confirmed by Ponceau S staining. (B) LC3-II levels are increased in the presence of an inhibitor of autophagosome–lysosome fusion, suggesting that the decreased level of LC3-II seen at day 7 in (A) is due to increased degradation of the LC3 protein by autophagy. Rat retinas were detached in the presence of bafilomycin A (BafA) or vehicle (DMSO), and protein was isolated 7 days after retina–RPE separation. (C) LC3 immunohistochemistry (green) in attached versus detached retinas shows a shift to a more punctate staining pattern (arrowheads), consistent with the formation of autophagosomes. The red staining is propidium iodide (PI), highlighting the outer nuclear layer. Magnification, ×40.
Increased LC3-I to LC3-II conversion was confirmed by examining the postdetachment staining pattern of LC3 on immunohistochemistry. Under normal conditions the LC3 staining is diffuse (Fig. 1C). The pattern of LC3 staining changed to a punctate pattern after retina–RPE separation, consistent with the formation of autophagosomes induced by the conversion of LC3-I to LC3-II. In the attached retina, LC3 was found primarily in the inner segments of photoreceptors, consistent with previous reports.11 After detachment, the punctate LC3 signal also appeared to be primarily localized in the inner segments. Outer-segment shortening is a well-described morphologic change that occurs in detached photoreceptors,18 and perhaps these structures are included in the autophagosomes for recycling, similar to their role in normal processing of shortening outer segments that occurs as part of normal photoreceptor homeostasis.11
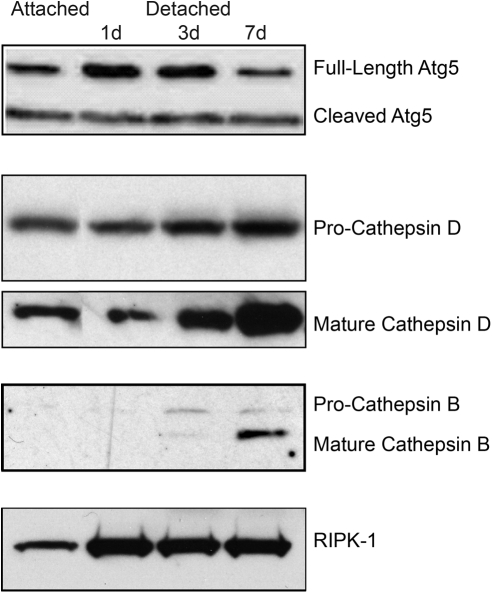
Autophagy activation is regulated by several Atg proteins, including Atg5. The Atg5 protein exists in two forms, full-length and cleaved. The full-length Atg5 is critical for the formation of autophagosome. When Atg5 levels were examined in attached and detached retinas, there was an increase in full-length Atg5 after 24 and 72 hours of retinal detachment, which returned to baseline levels by 7 days (Fig. 2).
Figure 2.
Western blot analysis showing increased levels of Atg5, mature cathepsins D and B, and the autophagy-regulating protein RIPK-1 as a function of time after retina–RPE separation. Equal amounts of protein were loaded in each lane.
Additional evidence for the activation of autophagy was demonstrated by an increase in the levels of activated cathepsins D and B (Fig. 2). Cathepsins are lysosomal proteases that are required for the breakdown of the recycled cellular components sequestered by the autophagosomes.19 The increase in the levels of these proteases after retina–RPE separation is consistent with autophagosome–lysosome fusion and activation of autophagy. We were also able to detect a significant increase in the level of RIPK-1 after retina–RPE separation (Fig. 2). RIPK-1 interacts with activated Fas receptor, and serves to modulate levels of autophagy activation.20 Finally, we evaluated the levels of Beclin-1, another autophagy regulator, after retinal detachment.21 Levels of Beclin-1 were unchanged in detached retinas (data not shown).
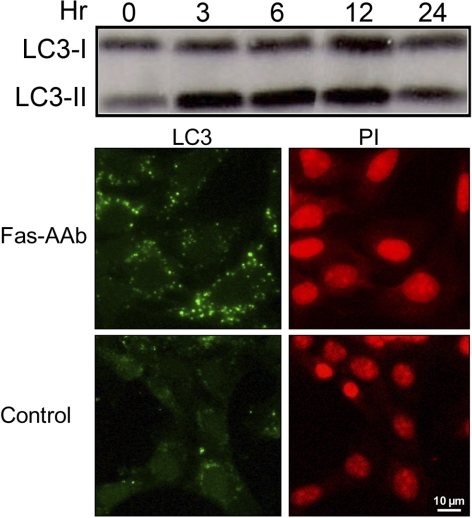
We next examined the role of Fas signaling in the activation of autophagy using 661W cells, a cone-like photoreceptor line that has been immortalized by the expression of SV40-T antigen under control of the human interphotoreceptor retinol-binding protein (IRBP) promoter.22 We recently reported that treatment of 661W cells with Fas-AAb leads to caspase 8 activation, indicating that the Fas receptor signaling pathway is intact in these cells and the 661W cell line is a functional in vitro model of Fas-mediated photoreceptor apoptosis.23 Treatment of the 661W cells with Fas-AAb resulted in increased levels of LC3-II formation (Fig. 3). There was also a resultant increase in the diffuse-to-punctate conversion of LC3 staining, with Fas-AAb–treated cells having an average of 38.2 ± 3.1 punctate autophagosomes compared with the 2.8 ± 1.4 seen in control cells (Fig. 3).
Figure 3.
Top: 661W cells treated with 500 ng/mL Fas-activating antibody (Fas-AAb) show increased levels of LC3-II, indicating Fas-dependent activation of autophagy. Cells were treated from 3 to 24 hours, lysed, and then analyzed via immunoblotting. Control cells, represented by the zero time point, were not treated with Fas-AAb. Bottom: Fas-AAb treatment induces autophagosome formation in 661W cells with the LC3 staining pattern changing from diffuse to punctate. Fas-AAb–treated cells have a 13-fold increase in the number of punctate figures per cell compared with control (P < 0.001). PI (red) indicates the nuclei. Magnification, ×40.
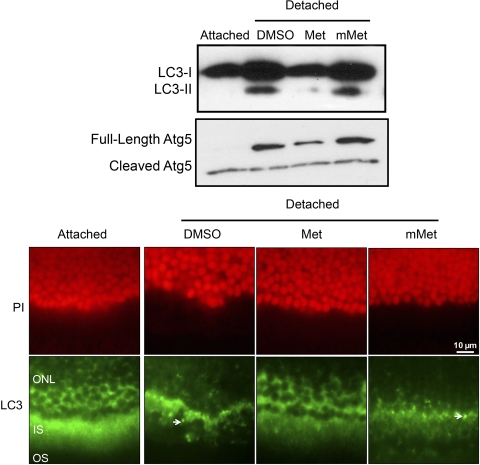
We next tested the in vivo effect of Fas-receptor inhibition using a small molecule antagonist of the Fas receptor called Met12 (Met).23 When the levels of LC3 in detached retinas were examined in the presence of Met or the inactive mutant Met (mMet), LC3-I to LC3-II conversion was significantly inhibited only in the presence of Met (Fig. 4), indicating that Fas-signaling plays a crucial role in autophagosome formation. This conclusion was supported by the observation that the LC3 staining pattern remained diffuse when retinas were treated with Met during retina–RPE separation (Fig. 4). Similar to LC3-II, the increase in Atg5 levels in detached retinas was partially Fas dependent because inhibition of the Fas-signaling pathway with Met reduced full-length Atg5 levels after retina–RPE separation (Fig. 4). In contrast, the detachment-induced increase in RIPK-1 expression was unchanged in the presence of Met (data not shown), suggesting that the expression of the autophagy regulator RIPK-1 is independent of Fas.
Figure 4.
Top: Western blot analysis showing the decreased conversion of LC3-I to LC3-II, reduced Atg5 synthesis, and persistent elevation of RIPK-1, when retina–RPE separation is induced in the presence of the Fas receptor antagonist Met, but not in the presence of a mutant, nonfunctioning form of the antagonist (mMet) or solvent alone (DMSO). Bottom: LC3 staining (green) remains diffuse when retina–RPE separation is induced in the presence of Met (left) but shifts to a punctate pattern when detachment occurs in the presence of the mMet (right, arrowhead). PI (red) is shown to highlight the outer nuclear layer. For all experiments, retina–RPE separation was performed in the presence of Met (50 μg), mMet (50 μg), or vehicle (DMSO) and harvested at 3 days postdetachment. Magnification, ×40.
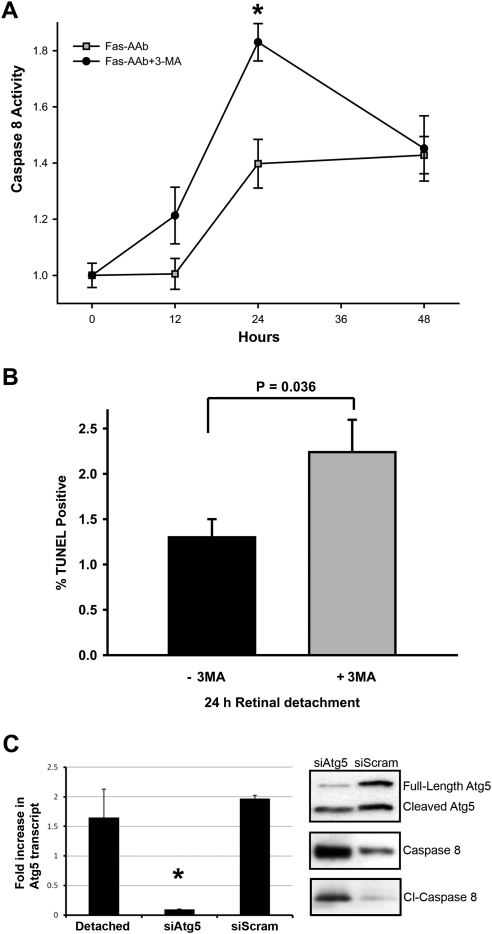
As expected, caspase 8 activity increased in 661W cells after treatment with Fas-AAb with peak levels achieved at 24 hours (Fig. 5A). However, when cells were also treated with 3-MA, an inhibitor of autophagy, caspase 8 activity increased at an earlier time point (12 hours) and peaked at a significantly higher level by 24 hours. This strongly suggested that autophagy can regulate Fas-mediated apoptosis. When 3-MA was administered in vivo at the time of retina–RPE separation, significantly more photoreceptors became TUNEL positive by 24 hours (Fig. 5B). This finding was confirmed by inhibiting autophagy activation in an independent manner. As shown in Figure 2, experimental retinal detachment leads to increased Atg5 levels. We examined the effect of reducing retinal Atg5 levels on photoreceptor apoptosis after retina–RPE separation. To decrease Atg5 production, an siRNA against the Atg5 transcript was used (siAtg5). A single dose of siAtg5 injected into the subretinal space at the time of retina–RPE separation resulted in significant reduction in the levels of Atg5 protein at 72 hours and a marked increase in full-length and cleaved caspase 8 levels (Fig. 5C). These data strongly support the hypothesis that inhibiting autophagosome formation by pharmacologic methods (3-MA) or molecular manipulation at the gene level (siAtg5) increased apoptosis of detached photoreceptors.
Figure 5.
(A) Inhibiting autophagy increases Fas-dependent activation of caspase 8 in vitro. 661W cells were treated with 500 ng/mL Fas-AAb ± 10 mM 3-MA and caspase 8 activity was then measured. Data were normalized to untreated controls; *P = 0.001, t-test, n = 8, mean ± SE. (B) Autophagy inhibitor 3-MA increases the number of TUNEL-positive photoreceptors after 24 hours of retinal detachment; mean ± SE, n = 6–7, P = 0.036, t-test. (C) Subretinal injection of small inhibitory RNA against the Atg5 transcript (siAtg5) resulted in reduced Atg5 protein levels and increased caspase 8 and cleaved (Cl−) caspase 8 levels after retina–RPE separation. Injection of a scrambled siRNA served as a control, and did not induce the same changes.
Discussion
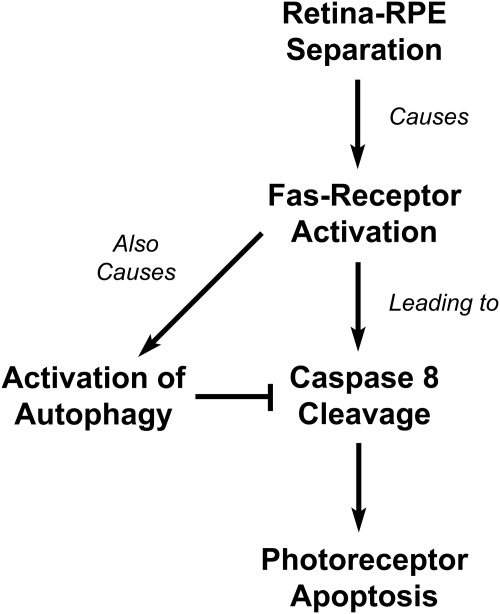
In this study, we describe our initial characterization of autophagy pathway activation in the retina after separation of photoreceptors from the RPE. Our results show that retinal detachment induces autophagosome formation in photoreceptors. Upstream signaling events that regulate autophagy activity are controlled, in part, by Fas-receptor activation. Thus, we propose that the simultaneous activation of autophagy and apoptosis by the Fas-signaling pathway appears to serve as a regulatory checkpoint controlling photoreceptor survival after retinal detachment (Fig. 6).
Figure 6.
Model of Fas-mediated activation of autophagy and apoptosis in photoreceptors after retina–RPE separation.
We have previously shown that the main event causing photoreceptor death after the separation of the neurosensory retina from the underlying RPE is the activation of the Fas receptor, which regulates several members of the caspase family of serine proteases.3–5 Interventions that prevent Fas-receptor activation or transcription of a new Fas receptor provide significant protection against the separation-induced death of the photoreceptors. Although the biochemical markers of Fas activation are detected shortly after retina–RPE separation, significant numbers of photoreceptors can survive for extended periods of time.
Our results demonstrate that in addition to activating apoptosis, Fas signaling is also critical for inducing photoreceptor autophagy. Fas-mediated autophagy activation has been reported in cell line systems including SH-Sy5Y neuroblastoma cells and HeLa cells.24–26 We show that Fas signaling simultaneously activates autophagic and apoptotic cascades in an animal model of retinal detachment. This concurrent induction of autophagy and apoptosis by the same stress signal facilitates fine-tuning of committing detached photoreceptors to the irreversible path of apoptosis. This type of checkpoint may have evolved to ensure that cells do not commit to rapid self-destruction during periods of transient stress. Our findings are different from those of Kunchithapautham and Rohrer,12 who reported that oxidative stress causes both autophagy and apoptosis in photoreceptors, with the former being induced by the latter. The different results obtained may be due to differences in the type of injury induced, RPE separation versus oxidative stress, and require more investigation.
Activation of autophagy after photoreceptor–RPE separation is not unexpected. The choroid–RPE complex is the primary source of nutritional and metabolic support for photoreceptors and healthy RPE is critical for photoreceptor homeostasis. Retina–RPE separation leads to the disruption of this homeostasis, causing photoreceptor hypoxia and nutritional deprivation.27,28 Metabolic stress is a known activator of prosurvival pathways, including autophagy. The protective role of autophagy has been demonstrated experimentally in many different systems.29 In a rat model of neonatal central nervous system hypoxia, autophagy was activated within the ischemic areas and reduced the cellular loss induced by the lack of oxygen.30 Interventions that increased autophagy resulted in decreased cell death, and interventions that reduced autophagy resulted in increased cell death. Similarly, in animal models of chronic, neurodegenerative diseases, activation of autophagy is correlated with increased cell survival.31,32 Our findings suggest that autophagy may play a protective role in the injured photoreceptor.
Several questions remain to be answered concerning molecular control of autophagy in the detached retina. The signaling pathways that are critical for the activation of autophagy downstream of Fas receptor are unknown. Previous studies have shown that the c-Jun N-terminal kinase (JNK) family of stress kinases can lead to increased LC3-II levels in mammalian cells.25 We have preliminary data showing activation of JNK signaling after retina–RPE separation in vivo or after direct activation of Fas signaling in vitro (Chinskey ND, et al. IOVS 2010;51:ARVO E-Abstract 6082). One hypothesis is that photoreceptor JNK signaling facilitates the conversion of LC3-I to LC3-II and increases autophagosome formation in detached photoreceptors.
Photoreceptor autophagy may also be regulated by JNK signaling at the level of Beclin 1.33 Our experiments showed no detectable increase in Beclin 1 levels after retinal detachment. Perhaps Beclin 1 function is regulated by JNK-mediated post-translational processes. Stress-kinase signaling may also regulate the function of the other autophagy-related proteins. In addition to the JNK family, PI-3 kinase (PI3K) signaling may also be important for the regulation of autophagy in photoreceptors. Our future studies will examine the effects of JNK and PI3K inhibition on the activation of autophagy in detached photoreceptors as well as in cell culture after direct Fas-receptor activation.
Hypoxia after retina–RPE separation may be a trigger for autophagy activation in the photoreceptors. One of the mediators of hypoxia signaling, hypoxia-inducible factor (HIF), is a known activator of autophagy.34,35 HIF has three major isoforms (1α, 2α, and 3α), and is constitutively expressed in most cells.36 Under normal oxygen tension, HIF is targeted for rapid degradation by other constitutively expressed proteins such as prolyl hydroxylase domain proteins (PHDs) and factor-inhibiting HIF (FIH). Hypoxia results in the inactivation of PHDs and FIH, resulting in increased levels of HIF. Our future studies will examine the effect of retina–RPE separation on the levels of HIF, PHD, and FIH, and determine whether these proteins are involved in the activation of autophagy.
A major question that remains to be answered is the mechanism of autophagy-mediated inhibition of the photoreceptor apoptosis. Autophagy may have several targets at various levels of apoptotic signaling. One is the inhibition of Fas-mediated caspase 8 activation. Our results showed that inhibiting autophagosome formation resulted in more rapid and increased levels of caspase 8 activation in 661W cells as well as increased caspase 8 levels in detached retinas. This is consistent with a recent report that demonstrated that tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL)–mediated autophagy inhibits TRAIL-mediated apoptosis by increased sequestration of caspase 8 in autophagosomes and lysosomal degradation of cleaved caspase 8.37 A separate mechanism by which autophagy reduces TUNEL counts may be secondary to the inhibition of caspase 8–dependent activation of the downstream mitochondrial apoptosis cascade. One such mechanism may be the indirect inhibition of the proapoptoptic members of the Bcl-2 family such as Bax. Beclin 1 has a BH3 domain, which interacts with members of the Bcl-2 family, including antiapoptotic Bcl-2 and Bcl-XL. When Beclin 1 is recruited away from Bcl-2/Beclin 1 complex during autophagosome formation, the free Bcl-2 may then become available to bind and sequester proapoptotic Bcl-2 homologs and prevent progression of photoreceptor apoptosis. The binding of Bcl-2 to Beclin 1 or the other Bcl-2 homologs may be regulated by JNK-mediated phosphorylation of Bcl-2.38 The formation of Beclin 1/Bcl-2 complex may be one of the mechanisms of regulating the balance between photoreceptor apoptosis and autophagy.
Although autophagy appears to reduce photoreceptor apoptosis, if the retina remains detached from the RPE for a long enough period, then the photoreceptors will ultimately continue down the apoptosis pathway. The mechanisms by which apoptosis eventually proceeds despite the initial activation of autophagy remain unclear and require further investigation. One potential area of investigation is the control of Atg5 levels. We noted that the level of full-length Atg5 was increased after retinal detachment, but appeared to return to its baseline level by day 7. The reason for this is unclear. Atg5 is important in autophagosome formation, but unlike LC3, it is not necessarily degraded by the autophagy process itself.39 Cleavage of Atg5 by non–autophagy- mediated pathways is suggested as one mechanism by which the cytoprotective function of autophagy is shifted toward a cytodestructive process, or at least the shift from autophagy to apoptosis is made.40 Whether this occurs in the photoreceptor is not known, and we are currently investigating this possibility.
Our findings demonstrate that activation of autophagy after retina–RPE separation is critical for regulating photoreceptor apoptosis. The detachment induces Fas-receptor activation that results in both autophagy and apoptosis, with the former serving as a negative regulator of the latter. This represents a novel mechanism of autophagy–apoptosis interaction, and provides a potential new avenue for modulating photoreceptor cell death after injury.
Footnotes
Supported in part by Michigan Eye Bank and Transplantation Center (DNZ); and National Eye Institute (NEI) Grants R01-020823 (DNZ) and NEI-EY-07003 from the Departmental Core Center for Vision Research.
Disclosure: C.G. Besirli, None; N.D. Chinskey, None; Q.-D. Zheng, None; D.N. Zacks, None
References
- 1. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139(1):87–99 [DOI] [PubMed] [Google Scholar]
- 2. Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982;80:475–497 [PMC free article] [PubMed] [Google Scholar]
- 3. Zacks DN, Hänninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003;44(3):1262–1267 [DOI] [PubMed] [Google Scholar]
- 4. Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2004;45(12):4563–4569 [DOI] [PubMed] [Google Scholar]
- 5. Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the Fas-signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol. 2007;125(10):1389–1395 [DOI] [PubMed] [Google Scholar]
- 6. Ross WH, Stockl FA. Visual recovery after retinal detachment. Curr Opin Ophthalmol. 2000;11:191–194 [DOI] [PubMed] [Google Scholar]
- 7. Ross WH. Visual recovery after macula-off retinal detachment. Eye. 2002;16:440–446 [DOI] [PubMed] [Google Scholar]
- 8. Chong DY, Boehlke CS, Zheng QD, Zhang L, Han Y, Zacks DN. Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2008;49(7):3193–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–1109 [DOI] [PubMed] [Google Scholar]
- 10. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reme CE, Sulser M. Diurnal variation of autophagy in rod visual cells in the rat. Graefes Arch Clin Exp Ophthalmol. 1977;203:261–270 [DOI] [PubMed] [Google Scholar]
- 12. Kunchithapautham K, Rohrer B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007;3(5):433–441 [DOI] [PubMed] [Google Scholar]
- 13. Kunchithapautham K, Rohrer B. Autophagy is one of the multiple mechanisms active in photoreceptor degeneration. Autophagy. 2007;3(1):65–66 [DOI] [PubMed] [Google Scholar]
- 14. Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ichimura Y, Kirisako T, Takao T, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492 [DOI] [PubMed] [Google Scholar]
- 16. Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–850 [DOI] [PubMed] [Google Scholar]
- 17. Rubinsztein DC, Cuervo AM, Ravikumar B, et al. In search of an “autophagomometer.” Autophagy. 2009;5:585–589 [DOI] [PubMed] [Google Scholar]
- 18. Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Ret Eye Res. 2005;24(3):395–431 [DOI] [PubMed] [Google Scholar]
- 19. Uchiyama Y. Autophagic cell death and its execution by lysosomal cathepsins. Arch Histol Cytol. 2001;64(3):233–246 [DOI] [PubMed] [Google Scholar]
- 20. Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281(28):19179–19187 [DOI] [PubMed] [Google Scholar]
- 21. Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(suppl 1):S137–S148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45(3):764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Besirli CG, Chinskey ND, Zheng Q-D, Zacks DN. Inhibition of retinal detachment–induced apoptosis in photoreceptors by a small peptide inhibitor of the Fas receptor. Invest Ophthalmol Vis Sci. 2010;51(4):2177–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Giorgio R, Volta U, Stanghellini V, et al. Neurogenic chronic intestinal pseudo-obstruction: antineuronal antibody-mediated activation of autophagy via Fas. Gastroenterology. 2008;135(2):601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Wu Y, Cheng Y, et al. Fas-mediated autophagy requires JNK activation in HeLa cells. Biochem Biophys Res Commun. 2008;377(4):1205–1210 [DOI] [PubMed] [Google Scholar]
- 26. Park MA, Reinehr R, Häussinger D, et al. Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol Cancer Ther. 2010;9(8):2220–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547–557 [DOI] [PubMed] [Google Scholar]
- 28. Sakai T, Lewis GP, Linberg KA, Fisher SK. The ability of hyperoxia to limit the effects of experimental detachment in cone-dominated retina. Invest Ophthalmol Vis Sci. 2001;42(13):3264–3273 [PubMed] [Google Scholar]
- 29. Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16(1):21–30 [DOI] [PubMed] [Google Scholar]
- 30. Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32(3):329–339 [DOI] [PubMed] [Google Scholar]
- 31. Cherra SJ, Chu CT. Autophagy in neuroprotection and neurodegeneration: a question of balance. Future Neurobiol. 2008;3(3):309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131(Pt 8):1969–1978 [DOI] [PubMed] [Google Scholar]
- 33. Li DD, Wang LL, Deng R, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28(6):886–898 [DOI] [PubMed] [Google Scholar]
- 34. Rouschop KM, Wouters BG. Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med. 2009;9(4):417–424 [DOI] [PubMed] [Google Scholar]
- 35. Bellot G, Garcia-Medina R, Gounon P, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29(10):2570–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122(Pt 8):1055–1057 [DOI] [PubMed] [Google Scholar]
- 37. Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy. 2010;6(7):891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei Y, Sinha S, Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4(7):949–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Codogno P, Meijer AJ. Atg5: more than an autophagy factor. Nat Cell Biol. 2006;8:1045–1047 [DOI] [PubMed] [Google Scholar]