This article reports the novel finding that VLA-1 directly mediates lymphangiogenesis. Anti–VLA-1 treatment is effective in suppressing corneal inflammatory lymphangiogenesis in vivo and several lymphatic endothelial cell functions in vitro.
Abstract
Purpose.
To investigate the specific role of very late antigen-1 (VLA-1; also known as integrin α1β1) in corneal inflammatory lymphangiogenesis in vivo and lymphatic endothelial cell functions in vitro.
Methods.
A standard suture-induced corneal inflammatory lymphangiogenesis model was used in normal adult BALB/c mice to test the effect of systemic administration of VLA-1–neutralizing antibody on lymphatic formation and macrophage infiltration in vivo. Additionally, a human lymphatic endothelial cell culture system was used to examine the effect of VLA-1 gene depletion on lymphatic endothelial cell functions in vitro using small interfering RNAs.
Results.
These data demonstrated, for the first time, that VLA-1 blockade significantly suppressed corneal lymphangiogenesis and macrophage infiltration during inflammation. Moreover, VLA-1 gene depletion led to a marked inhibition of lymphatic endothelial cell processes of adhesion, proliferation, and capillary tube formation.
Conclusions.
These novel findings together indicate that VLA-1 is critically involved in the processes of lymphangiogenesis. Further investigation on this factor may provide novel therapies for corneal inflammation, transplant rejection, and other lymphatic-related disorders in the body.
Lymphatic research denotes a field of new discovery in recent years largely because of the advancement of technology and the discovery of several lymphatic-specific molecules including lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1).1–8 In parallel to the blood circulatory system, the lymphatic network penetrates most tissues in the body and plays critical roles in a broad spectrum of functions, which include but are not limited to immune responses, fat and vitamin absorption, and body fluid regulation. Numerous diseases and conditions are therefore associated with lymphatic dysfunctions, such as cancer metastasis, inflammation, transplant rejection, hypertension, obesity, and lymphedema.1,3–5,9–12 To date, there is no effective treatment for lymphatic disorders and a continuing and increasing demand for new therapeutic protocols.
Because of its accessible location and transparent nature, the cornea provides an optimal site for lymphatic research. The normal cornea does not have lymphatic vessels; however, lymphangiogenesis (LG; the development of new lymphatic vessels) can be induced in this tissue after inflammatory, traumatic, chemical, or infectious damage.3,13,14 LG also constitutes the afferent arm of the immune reflex arc of corneal transplantation immunity,3,14,15 and, most recently, it has been demonstrated that LG is a primary mediator of corneal transplant rejection.16
LG is a complex process involving lymphatic endothelial cell (LEC) proliferation, migration, adhesion, and interaction with the extracellular matrix. Integrins are a diverse family of heterodimeric cell surface transmembrane glycoproteins that mediate cell-cell and cell-matrix interactions.17,18 The specific roles of integrins in LG are still largely unknown. VLA-1 (very late antigen-1), also known as integrin α1β1, is a receptor for collagen and laminin. Its expression in adults is primarily in mesenchymal cells, such as fibroblasts, microvascular endothelium, and some activated cells of the immune system.19–21 Previously, we reported an observation that transplantation-associated LG is suppressed in VLA-1 knockout mice.22 However, the molecular and cellular mechanisms underlying this phenomenon are yet to be determined. In this article, we report the new findings that VLA-1 is critically involved in LG processes. Its expression is upregulated during corneal inflammation, and its blockade suppresses corneal inflammatory LG and macrophage infiltration in vivo and several LEC functions in vitro.
Methods
Animals
Six- to 8-week-old normal adult male BALB/c mice were purchased from Taconic Farms (Germantown, NY). All mice were treated according to ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal Care and Use Committee of the University of California at Berkeley. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50 mg, 10 mg, and 1 mg/kg body weight, respectively) for each surgical procedure.
Lymphatic Endothelial Cells, Reagents, and Antibodies
Human microdermal LECs were purchased from Lonza (Walkersville, MD) and maintained in EGM-2 MV medium (Lonza) according to manufacturer's instructions. Passages 4 to 8 were used in experiments. Basement membrane matrix (Matrigel), collagen type I, and calcein AM, were purchased from BD Biosciences (San Jose, CA). Donkey serum was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). The following antibodies were used: rabbit–anti-mouse polyclonal LYVE-1 antibody, rat–anti-mouse monoclonal F4/80 antibody (Abcam, Cambridge, MA), mouse–anti-human monoclonal VLA-1 antibody (Millipore, Billerica, MA), rat–anti-mouse CD16/CD32 Fc block (BD PharMingen, San Jose, CA), Cy3 conjugated donkey–anti-rabbit secondary antibody, 488-conjugated donkey–anti-rat secondary antibody (DyLight; Jackson ImmunoResearch, West Grove, PA), and FITC conjugated goat–anti-mouse secondary antibody (Millipore, Billerica, MA).
Corneal Inflammatory Lymphangiogenesis and Pharmaceutical Interventions
The suture-induced inflammatory LG model was used to induce the growth of new lymphatic vessels into the corneas, as described previously.23–26 Briefly, three 11–0 nylon sutures (AROSurgical, Newport Beach, CA) were placed into the stroma of central corneas without penetrating the anterior chamber. Mice were randomly selected to receive intraperitoneal injection of either 200 μg VLA-1 blocking antibodies or its isotype controls (kindly provided by Covella Pharmaceuticals, Inc., and Biogen Idec, Inc.) twice a week on day 0 of the suture placement and thereafter for 1 week for macrophage infiltration studies. Experiments were repeated twice with a total of six mice in each group. For LG studies, similar treatments were given for 2 weeks after the suture placement. Experiments were repeated three times with a total of 15 mice in each group.
Corneal Immunofluorescence Microscopic Assay and Quantification
The experiments were performed according to our standard protocol.25–27 Briefly, whole-mount corneas were sampled at day 7 or 14 and were fixed in acetone for immunofluorescence staining with purified rabbit–anti-mouse LYVE-1 antibody or purified rat–anti-mouse F4/80 antibody. Samples were visualized by Cy3-conjugated donkey–anti-rabbit or 488-conjugated donkey–anti-rat secondary antibody (DyLight) and were covered with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA). Digital images were taken with an epifluorescence microscope (AxioImager M1; Carl Zeiss AG, Göttingen, Germany) and software (AxioVision 4.8; Carl Zeiss AG). Corneal lymphatic vessels were analyzed as described previously using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) to evaluate their coverage area and clockwise extent.25–28 Macrophage infiltration was also evaluated as previously described.29,30 Briefly, 10 areas (eight from the periphery, two from the center) of each sample were randomly picked and examined under the epifluorescence microscope, and total numbers of F4/80-positive cells were counted throughout the whole thickness of the picked area.
Corneal Macrophage Isolation
Corneal macrophages were isolated as described previously.31 Briefly, 20 corneal buttons were harvested and cultured in RPMI-1640 with 10% FBS, 10 mM HEPES, 0.1 mM nonessential amino acid, 1 × 10−5 M 2-mercaptoethanol, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Invitrogen, Carlsbad, CA) at 37°C for 1 week. Nonadherent cells were discarded, and adherent cells of the macrophage population were collected for further experiments.
Reverse Transcription, PCR, and Real-time PCR
Total RNA was extracted and purified from LECs, corneal stroma, or macrophages with a mini-kit (RNeasy; Qiagen, Valencia, CA). Reverse transcription was performed using a cDNA synthesis kit from Invitrogen (SuperScript VILO; Carlsbad, CA). PCR was performed with the PCR mix from Promega (Master Mix; Madison, WI). Primer sequences were as follows: human VLA-1, forward 5′-GAATGCAGCACTCAACTGGA-3′, reverse 5′-GCCTCCTTTCTTGCTGTGTC-3′; human GAPDH, forward 5′-CCACAGTCCATGCCATCAC-3′, reverse 5′-TCCACCACCCTGTTGCTGT-3′; mouse VLA-1, forward 5′-TCACTGTAGCCTGCATCTGG-3′, reverse 5′-GGCCAATGTCCATCCTCTTA-3′; and mouse GAPDH, forward 5′-ACCACAGTCCATGCCATCAC-3′, reverse 5′-TCCACCACCCTGTTGCTGTA3′. For semiquantitative PCR analysis of VLA-1 expression in corneal stroma, ImageJ software was used for normalization and analysis of band intensity. For real-time PCR of VLA-1 expression in LECs, supermix was used with the CFX96 sequence detection system (SsoFast EvaGreen; Bio-Rad Laboratories, Inc., Hercules, CA). Relative expression of the VLA-1 gene was calculated from the δ-Ct (threshold cycle) of the targeted gene normalized to the δ-Ct of GAPDH.
Immunocytofluorescence Microscopic Assay
LECs were grown on coverslips and fixed with 4% paraformaldehyde for staining with the primary antibody overnight at 4°C. Secondary antibody incubations were performed at room temperature for 1 hour. Samples were mounted with mounting medium with DAPI (Vectashield; Vector Laboratories), and images were taken with an epifluorescence microscope (Axioplan 2; Carl Zeiss AG).
siRNA Transfection
Custom-designed siRNA duplexes were synthesized by Qiagen. siRNA sequences were designed against the human VLA-1 mRNA (5′-TCACAGAAGTAAAGGAGAAA-3′). A scrambled siRNA control was purchased from Ambion (Austin, TX). Transfections were carried out with transfection reagent (RNAiMax; Invitrogen) and reduced serum medium (Opti-MEM; Invitrogen) overnight at 37°C in a 5% CO2 humidified air incubator according to the manufacturer's instructions.
Adhesion Assay
LECs were seeded in six-well plates and transfected with siRNA, as described. Assay plates were first prepared by coating with collagen type I for 1 hour at room temperature and then blocked with 1% bovine serum albumin for 1 hour at 37°C. Forty-eight hours after the transfection, 100 μL cells (4 × 105 cells/mL) were detached and added to the collagen-coated 96-well plates in Hanks' balanced salt solution (HBSS) for 1 hour at 37°C. The plates were then washed several times and incubated with calcein (1 μg/mL) in HBSS for 30 minutes at room temperature. Plates were washed once with PBS, and fluorescence intensity was measured with a microplate reader (Spectramax M5e; Molecular Devices, Sunnyvale, CA). Assays were performed in triplicate and were repeated at least three times.
Proliferation Assay
LECs were seeded on collagen I-coated 96-well plates. Forty-eight hours after siRNA transfection, the cells were subjected to a proliferation assay (MTS; Promega (Madison, WI) according to the manufacturer's protocol. Assays were performed in duplicate and were repeated at least three times.
Three-Dimensional Culture and Tube Formation Assay
Forty-eight hours after siRNA transfection, LECs were reseeded (30,000 cells/well) onto 48-well plates containing solidified basement membrane matrix (Matrigel; BD Biosciences) and were monitored for 24 hours under an inverted microscope (Axio Observer A1; Carl Zeiss AG). Phase images of tubes were taken, and total tube lengths were analyzed (Qcapture; Qimaging, Surrey, BC, Canada). Assays were performed in triplicate and repeated at least three times.
Statistical Analysis
Mean differences in cell adhesion, proliferation, and tube formation and semiquantitative PCR were analyzed by Student's t-test. The difference in LG and macrophage infiltration was analyzed with the Mann-Whitney U test. Scientific graphing software (Prism; GraphPad, La Jolla, CA) was used; P < 0.05 was considered significant.
Results
Effect of VLA-1 Blockade on Corneal Inflammatory LG
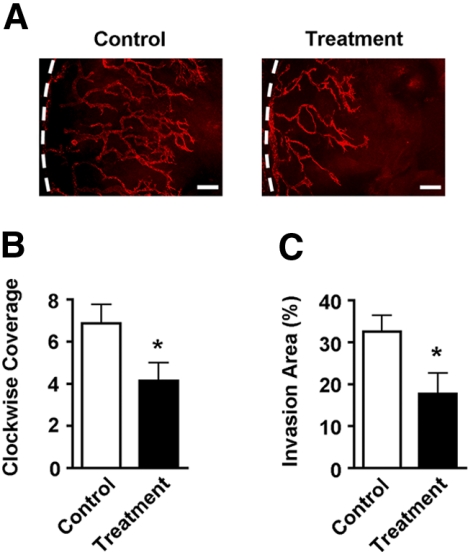
A standard suture placement model was used to induce LG, as described previously.23–26 This procedure also upregulated VLA-1 expression in the corneal stroma (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.10-6580/-/DCSupplemental). Mice were randomized to receive systemic injections of either VLA-1 neutralizing antibody or its isotype control twice a week for 2 weeks, when whole-mount corneas were sampled and examined by immunofluorescent microscopic assays using the specific antibody against LYVE-1, as described previously.25–27 Results from these studies clearly demonstrated that VLA-1–neutralizing antibody treatment led to a significant suppression of LG during corneal inflammation. Both the circumstantial (clockwise) extent and the coverage area of the newly formed lymphatic vessels were inhibited in the treatment groups compared with the control condition (Figs. 1A–C).
Figure 1.
Corneal inflammatory lymphangiogenesis is inhibited by VLA-1 blockade. (A) Representative images of immunofluorescence microscopic assays showing significantly reduced lymphatic vessels in the inflamed corneas after VLA-1 antibody treatment. Red: LYVE-1. Dotted lines: Limbus. Scale bars, 200 μm. (B, C) Summarized data showing the difference on newly formed lymphatic vessels between the VLA-1 antibody and isotype control treatment groups in terms of vessel clockwise extent (B) and coverage area (C). *P < 0.05.
Effect of VLA-1 Blockade on Corneal Macrophage Infiltration
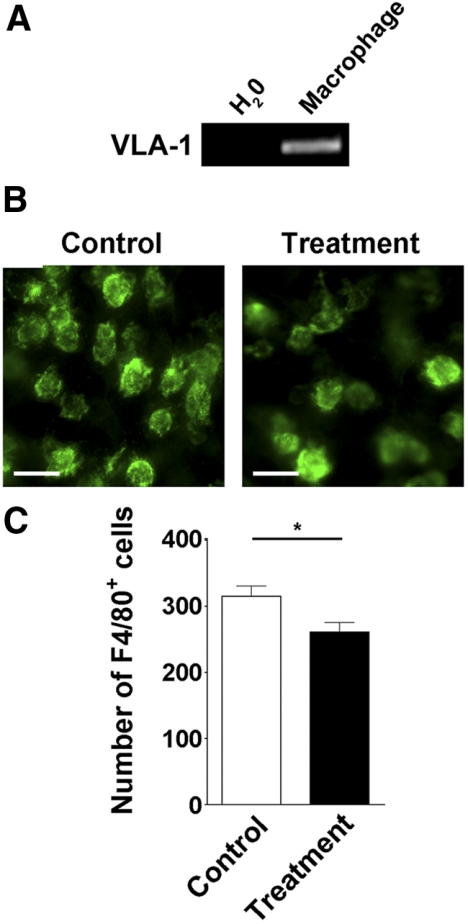
It is known that macrophages contribute to corneal LG,28,29 and their infiltration into corneal transplants was suppressed by VLA-1 inhibition.22 We next investigated whether corneal macrophage infiltration during suture-induced inflammation was also affected by the VLA-1 antibody treatment. To approach this, we first confirmed that VLA-1 was expressed in corneal macrophages, as shown in Figure 2A. Further analysis between the corneas of the VLA-1 antibody treatment and control groups also revealed that VLA-1 blockade led to a significant reduction of macrophage numbers in the corneas of the treatment group, as presented in Figures 2B and 2C.
Figure 2.
Corneal macrophage infiltration is inhibited by VLA-1 blockade. (A) RT-PCR analysis showing VLA-1 expression in corneal macrophages. (B) Representative images of immunofluorescence microscopic assays showing the number of F4/80-positive macrophages was significantly reduced in the cornea after VLA-1 antibody treatment. Green: F4/80. Scale bars, 25 μm. (C). Summarized data showing the difference of macrophage numbers between the VLA-1 antibody and isotype control treatment groups. *P < 0.05.
VLA-1 Expression and Depletion in LECs
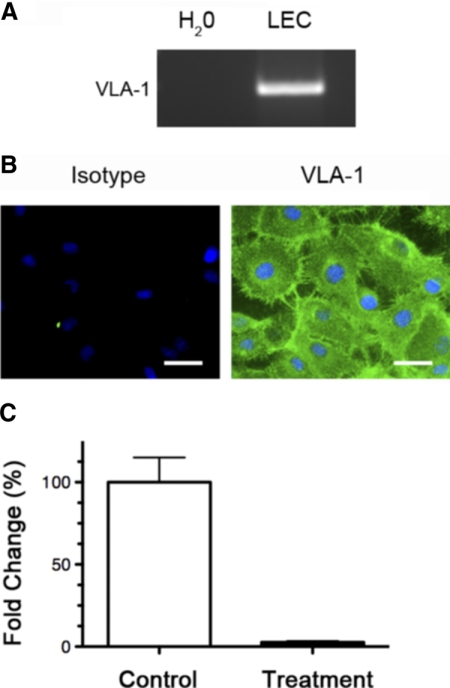
To further investigate the specific role of VLA-1 in LG processes, we used a human LEC culture system and first examined whether VLA-1 is expressed in these cells. As demonstrated in Figures 3A and 3B, our results from both RT-PCR and immunocytofluorescence microscopic assays showed that VLA-1 was highly expressed in LECs at both the mRNA and the protein levels. We next investigated whether VLA-1 expression in LECs can be downregulated by an siRNA-mediated gene silencing approach. LECs were transfected with either VLA-1 or scrambled siRNA, and the VLA-1 mRNA levels were measured by real-time quantitative PCR 48 hours after transfection. It was found that the siRNA approach was successful in reducing VLA-1 expression by approximately 95% (Fig. 3C), indicating that this method can be used to study functional roles of VLA-1 in LG processes in vitro.
Figure 3.
VLA-1 expression and depletion in human microdermal lymphatic endothelial cells. (A) RT-PCR analysis. (B) Immunocytofluorescence microscopic analysis of VLA-1 expression in LECs. Green: VLA-1. Blue: DAPI nuclear staining. Scale bars, 50 μm. (C) Real-time PCR analysis showing 95% downregulation of VLA-1 expression in LECs 48 hours after transfection with VLA-1 siRNA compared with scrambled siRNA.
Effect of VLA-1 Depletion on LEC Adhesion
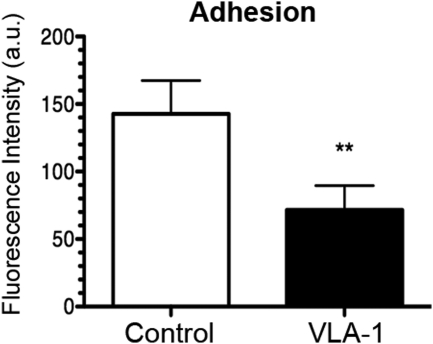
Integrins are known to have an essential role in the LG process by mediating cell interactions with the extracellular matrix.32 A role of VLA-1 in LEC cellular adhesion has yet to be defined. We, therefore, first tested whether VLA-1 is involved in LEC adhesion to collagen I. Forty-eight hours after the transfection with either VLA-1 or scrambled siRNA, LECs were detached and subjected to an adhesion assay. It was shown that VLA-1 depletion led to an approximately 50% reduction of cell adhesion to collagen I (Fig. 4), indicating that LECs may use VLA-1 to mediate interactions with the extracellular matrix.
Figure 4.
VLA-1 depletion inhibits lymphatic endothelial cell function of adhesion. Summarized data showing significant suppression of LEC adhesion to collagen type I after transfection with VLA-1 siRNA. **P < 0.01.
Effect of VLA-1 Depletion on LEC Proliferation
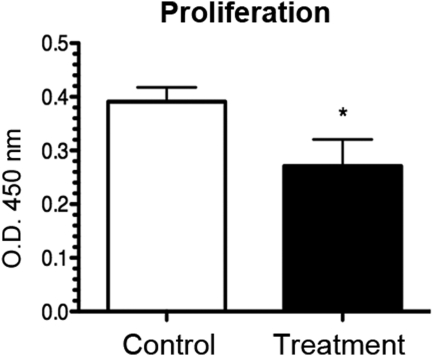
VLA-1 is also known to be involved in the collagen-dependent fibroblast proliferation pathway.33 To test its possible role in LECs, we next investigated the effect of the VLA-1 siRNA treatment on LEC proliferation. Forty-eight hours after siRNA transfection with either VLA-1 or scrambled siRNA, LECs were subjected to a proliferation assay (MTS; Promega). It was found that the VLA-1 siRNA treatment led to a 30% reduction of cell proliferation compared with the control condition (Fig. 5), suggesting that LEC proliferation is also in part regulated by VLA-1.
Figure 5.
VLA-1 depletion inhibits lymphatic endothelial cell proliferation. Summarized data showing significant inhibition of LEC proliferation after transfection with VLA-1 siRNA, as revealed by MTS proliferation assay. *P < 0.05.
Effect of VLA-1 Depletion on LEC Tube Formation
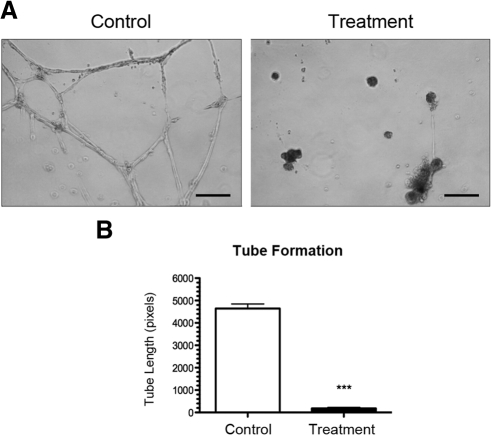
We also investigated the effect of VLA-1 siRNA treatment on the ability of LECs to form capillary tubes in vitro using the three-dimensional culture system. Forty-eight hours after transfection with either VLA-1 or scrambled siRNA, LECs were seeded on basement membrane matrix (Matrigel; BD Biosciences) to allow for tube formation. Surprisingly, VLA-1 depletion showed a dramatic 95% reduction in total tubule length (Figs. 6A, 6B). These results indicate a critical role of this factor in LEC capillary tube formation function.
Figure 6.
VLA-1 depletion inhibits lymphatic endothelial cell tube formation. (A) Representative micrographs showing dramatic inhibition of LEC capillary tube formation in basement membrane matrix after transfection with VLA-1 siRNA. Scale bars, 200 μm. (B) Summarized data on total tubule length measurement. ***P < 0.0001.
Discussion
With this study, we provide the first evidence that VLA-1 directly mediates LG in vivo and in vitro. We also identified several important LEC functions that are mediated by the VLA-1 pathway. At least two important conclusions can be drawn from our data: VLA-1 is critically involved in corneal LG during inflammatory response, and anti–VLA-1 strategies are effective in suppressing LG processes both in vivo and in vitro.
Our data demonstrating that VLA-1 mediates corneal LG is new but consistent with previous reports on another integrin family member, integrin alpha 5, which is involved in corneal inflammatory LG and transplant rejection.16,34 These data together indicate that in addition to the VEGF family, which has been studied extensively, future studies on integrin family members may shed some new light on our understanding and management of corneal LG. Moreover, the results of this study align well with our previous finding that transplantation-associated LG is suppressed in VLA-1 knockout mice.22 However, to date, there have been no studies to reveal the underlying mechanisms of this phenomenon. In the present study, using both in vivo and in vitro models, we have shown, for the first time, VLA-1 mediates LG through interference with the processes of macrophage infiltration and LEC adhesion, proliferation, and tube formation.
Studies on the mechanisms of corneal LG are important because LG accompanies many corneal diseases after inflammatory, chemical, infectious, or traumatic damage.3,35,36 LG also bears direct implication in transplant rejection, especially in “high-risk” conditions in which grafts are placed in inflamed and lymphatic-rich corneas and the rejection rate can be as high as 90%, irrespective of the treatment delivered.3,14,15 Unfortunately, many patients who are blind from corneal diseases fall into this category. To date, there is little effective treatment for these diseases, so it is a field with urgent demand for new therapeutic strategies. Our finding that inflammatory LG in the adult cornea can be inhibited by interfering with the VLA-1 pathway indicates VLA-1 as a promising target for the development of novel therapeutic protocols for patients at high risk for transplant rejection, which warrants further investigation.
Furthermore, this study bears broader implications beyond the treatment of ocular diseases. As mentioned earlier, numerous diseases are associated with lymphatic dysfunction, which can be disfiguring, disabling, and even life threatening. During the past few years, LG has emerged as a focus of research to reduce cancer metastasis and to promote major organ (such as kidney and heart) transplant survival.1,37,38 It is hopeful that this study, combining both animal and human cell culture work, will also shed some light on the development of new VLA-1–based therapies for lymphatic disorders in general.
Supplementary Material
Acknowledgments
The authors thank Jeffrey LeDue (University of California at Berkeley) for technical assistance with the immunofluorescent microscopic studies and Covella Pharmaceuticals, Inc., and Biogen Idec, Inc., for providing the VLA-1 blocking antibody.
Footnotes
Supported in part by research grants from the National Institutes of Health, Department of Defense, and the University of California at Berkeley (LC).
Disclosure: S. Grimaldo, None; D. Yuen, None; T. Ecoiffier, None; L. Chen, None
References
- 1. Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476 [DOI] [PubMed] [Google Scholar]
- 2. Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225–234 [DOI] [PubMed] [Google Scholar]
- 3. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009;42:66–76 [PMC free article] [PubMed] [Google Scholar]
- 4. Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458 [DOI] [PubMed] [Google Scholar]
- 5. Kerjaschki D. Lymphatic neoangiogenesis in renal transplants: a driving force of chronic rejection? J Nephrol. 2006;19:403–406 [PubMed] [Google Scholar]
- 6. Folkman J, Kaipainen A. Genes tell lymphatics to sprout or not. Nat Immunol. 2004;5:11–12 [DOI] [PubMed] [Google Scholar]
- 7. Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783 [DOI] [PubMed] [Google Scholar]
- 8. Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Radhakrishnan K, Rockson SG. The clinical spectrum of lymphatic disease. Ann N Y Acad Sci. 2008;1131:155–184 [DOI] [PubMed] [Google Scholar]
- 10. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953 [DOI] [PubMed] [Google Scholar]
- 12. Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145 [DOI] [PubMed] [Google Scholar]
- 13. Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2009;207:101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281 [DOI] [PubMed] [Google Scholar]
- 15. Chong EM, Dana MR. Graft failure, IV: immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–222 [DOI] [PubMed] [Google Scholar]
- 16. Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berman AE, Kozlova NI, Morozevich GE. Integrins: structure and signaling. Biochemistry (Mosc). 2003;68:1284–1299 [DOI] [PubMed] [Google Scholar]
- 18. Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15 [DOI] [PubMed] [Google Scholar]
- 19. Ben-Horin S, Bank I. The role of very late antigen-1 in immune-mediated inflammation. Clin Immunol. 2004;113:119–129 [DOI] [PubMed] [Google Scholar]
- 20. Andreasen SO, Thomsen AR, Koteliansky VE, et al. Expression and functional importance of collagen-binding integrins, alpha 1 beta 1 and alpha 2 beta 1, on virus- activated T cells. J Immunol. 2003;171:2804–2811 [DOI] [PubMed] [Google Scholar]
- 21. Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400 [DOI] [PubMed] [Google Scholar]
- 22. Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007;125:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005;46:4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815 [DOI] [PubMed] [Google Scholar]
- 25. Zhang H, Hu X, Tse J, Tilahun F, Qiu M, Chen L. Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol Vis Sci. 2010;52:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuen D, Pytowski B, Chen L. Combined blockade of VEGFR-2 and VEGFR-3 inhibits inflammatory lymphangiogenesis in early and middle stages. Invest Ophthalmol Vis Sci. 2011;52:2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung ES, Chauhan SK, Jin Y, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225 [DOI] [PubMed] [Google Scholar]
- 31. Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–589 [DOI] [PubMed] [Google Scholar]
- 32. Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol. 2006;4:83–100 [DOI] [PubMed] [Google Scholar]
- 33. Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dietrich T, Onderka J, Bock F, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2009;207:101–115, S101–S102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ling S, Lin H, Liang L, et al. Development of new lymphatic vessels in alkali-burned corneas. Acta Ophthalmol. 2009;87:315–322 [DOI] [PubMed] [Google Scholar]
- 37. Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234 [DOI] [PubMed] [Google Scholar]
- 38. Geissler HJ, Dashkevich A, Fischer UM, et al. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg. 2006;29:767–771 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.