Treatment of posterior segment disease is becoming more dependent on the use of biologics. Targets include the macula and specific retinal layers. In this study, the authors compared pharmacokinetics of direct intravitreal injections with suprachoroidal injections.
Abstract
Purpose.
To compare the pharmacokinetics and tissue response between intravitreal and microcannulation injections into the suprachoroidal space using bevacizumab.
Methods.
Sixty-two pigs were studied. Either a pars plana intravitreal bevacizumab or a viscoelastic-enhanced microcannula suprachoroidal injection was performed with either 1.25 mg (group 1) or 3 mg (group 2). In group 1, six animals were euthanatized at 0.5, 7, 30, 60, 90, and 120 days after injection (n = 36). In group 2, six animals were euthanatized at 0.5, 7, 14, and 32 days (n = 24). Eyes were enucleated, dissected, and snap-frozen, or they were fixed for histology. Analysis of drug tissue levels was performed at two separate laboratories using masked specimens.
Results.
Both laboratories were confirmatory. Intravitreal bevacizumab pharmacokinetics demonstrated a gradual decline in tissue levels over 30 to 60 days in both groups 1 and 2. In addition, suprachoroidal bevacizumab tissue levels declined rapidly and were not measurable at or beyond 7 days. Vitreitis and granulomatous vasculitis were noted in 7 of 30 intravitreal injection eyes. Immunohistology suggested a distinctive drug distribution.
Conclusions.
Direct intravitreal injection of bevacizumab has a more sustained pharmacologic profile than does a similar dose delivered to the suprachoroidal space. Intravitreal injections distributed more to the inner retina, whereas suprachoroidal delivery occurred primarily at the choroid, retinal pigment epithelium, and photoreceptor outer segments. Sustained release formulation of larger biological molecules should be considered to optimize suprachoroidal delivery. Inflammation from injections is granulomatous, seen only with intravitreal injections, and may result from either an altered immune response or a dose-related effect.
Age-related macular degeneration (AMD) is a leading cause of legal blindness in persons over the age of 65 in the developed world.1 In 2004, primary treatment of exudative, or wet, AMD (eAMD) transitioned from laser-based therapies to pharmacotherapy.2–4 In June 2006, intravitreal ranibizumab rapidly become the standard of care for eAMD.3,4 The off-label use of bevacizumab, originally introduced systemically,5,6 has been widely adopted as an intravitreal injection to treat eAMD,7 largely because it costs less than ranibizumab.8
We began to explore alternative routes of drug delivery to the retina and specifically to the macular region by initially investigating transscleral drug delivery routes.9,10 The benefit of transscleral delivery is the added safety of avoiding an intraocular, invasive procedure. For small molecules, diffusion is rapid through the sclera.10 However, for larger biological agents, such as ranibizumab or bevacizumab, there are significant limitations to the transscleral route, and effective levels may be suboptimal. The retinal pigment epithelium-choroid may be a major barrier, especially to hydrophilic compounds and macromolecules.11 In 2002, Einmahl et al.12 first investigated the feasibility and tolerance of suprachoroidal injections in the rabbit model. They used poly-ortho ester as a sustained drug delivery system with a solid, olive-tipped cannula inserted into the suprachoroidal space. The authors demonstrated that material remained in the suprachoroidal space for 3 weeks; however, there were retinal pigment epithelial irregularities associated with the injections. In 2006, we described the use of a flexible fiberoptic microcannula that has the ability to access the suprachoroidal space, and we demonstrated the method to be safe in the pig model.13 This cannula has been used to access Schlemm's canal for circumferential viscodilation during canaloplasty surgery.14 In our studies accessing the suprachoroidal space, we examined 94 porcine eyes and demonstrated safety, sustained local delivery, and excellent pharmacokinetics of triamcinolone for at least 120 days with few complications.
Herein, we sought to determine the kinetics of bevacizumab injections into the suprachoroidal space using the flexible microcannula (iScience Interventional, Inc; Menlo Park, CA) method previously described.13 Clearly, intravitreal injections of both ranibizumab and bevacizumab are effective for the management of exudate AMD. The route of delivery depends on the target tissue and varies with the pharmacologic agent and disease state. Anatomically, there are several advantages to the suprachoroidal route. More specifically, diffusion into the choroid and through a damaged Bruch's membrane may offer more direct delivery to the disease-affected tissue than diffusion across the neurosensory retina. We examined the pharmacokinetics of bevacizumab, comparing intravitreal injections with suprachoroidal injections. We also demonstrated a significant difference in the immune response to these two routes of administration.
Methods
Two studies were undertaken sequentially. Data are presented separately, and there was no pooling of data. In the first study, analysis laboratories at the National Eye Institute were asked to determine tissue levels of bevacizumab in masked tissue samples. Next, we repeated the assays in a commercial laboratory using optimized tissue extraction techniques. Studies were conducted with approval of the institutional animal care and use committee (IACUC) at the University of Minnesota (protocol 0501A66950). Animals were treated in accordance with the guidelines outlined in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Animal Studies
During surgery, eyes were dilated with a combination of phenylephrine 2.5% and atropine 1%, monitored using a pulse-oximeter (TPR V3395; Surgivet, Inc., Waukesha, WI), supported with oxygen at 4 L/min with a nasal cannula, monitored with a rectal probe to maintain a core temperature of 37°C, and warmed with a thermal water blanket. After surgery, animals were treated with atropine 1%, an antibiotic-steroid ointment, and pain medication as needed.
Injection Technique
Animals were preanesthetized using tiletamine/zolazepam (Telazol; Fort Dodge Animal Health, Fort Dodge, IA) at 4 mg/kg. An ear vein was used for intravenous access and propofol (Diprivan; AstraZeneca, Wilmington, DE) was titrated as a drip for sedation. Right eyes were prepared with topical anesthetic (proparacaine HCl) and povidine-iodine solution (5% Betadine).
Suprachoroidal Delivery.
An identical injection technique was used and has been previously described.13 After placement of a sterile drape and lid speculum, a conjunctival peritomy was made superiorly near 12 o'clock. A radial incision was made through the sclera. The cannula (PDS; iScience Interventional Inc.) was primed with bevacizumab (Genentech Inc., San Francisco, CA) concentrated in hyaluronic acid (Healon 10 mg/mL; Abbott Medical Optics, Abbott Park, IL) for a total delivery of either 1.25 mg (group 1) or 3 mg (group 2) of bevacizumab in 0.05-mL stock solution plus 0.02 mL hyaluronic acid for a total volume injection of 0.07 mL. The cannula was passed into the suprachoroidal space. A wide-angle surgical viewing system was used to visualize the fiberoptic tip of the cannula in the suprachoroidal space. Once the tip was in the region of the area centralis, the injection was delivered using a viscous injector system in a slow, deliberate manner. Time was allowed for the material to dissect into the tissue planes before the cannula was removed. The sclerotomy site was closed with a single 7–0 Vicryl suture, and the conjunctiva was closed with an interrupted 6–0 plain gut suture.
Intravitreal Injections.
After the lid speculum was inserted, a caliper was used to measure 2.5 mm posterior to the surgical limbus near 12 o'clock, and a 30-gauge needle on a tuberculin syringe was used to inject either 1.25 mg bevacizumab (group 1) or 3 mg (group 2) in a total volume of 0.05-mL stock solution. A cotton-tip applicator was rolled over the needle track as the needle was removed to minimize reflux and vitreous incarceration.
Pharmacokinetic Dissections
Animals were euthanatized using an overdose of pentobarbital sodium (100 mg/kg), and the eyes were enucleated for either pharmacokinetics or histopathologic evaluation on postoperative days 0.5, 7, 30, 90, and 120 (group 1) and 0.5, 7, 14, and 32 (group 2). Eyes were processed for histopathology by immersion in 10% formalin. Eyes for pharmacokinetics evaluation were immediately dissected after enucleation (time from enucleation to freezing was approximately 5 minutes). The anterior segment was removed with a sharp blade and scissors circumferentially at the pars plana. A vitreous cutting system was used to obtain a 1-mL anterior and a separate 1-mL posterior vitreous sample. The remaining vitreous was removed by using forceps to strip the vitreous base, en bloc. A 6-mm corneal trephine was used to punch the area centralis (macular equivalent) approximating, but not including, the optic nerve. Samples were then frozen in liquid nitrogen and stored at −80°C. Tissue layers were dissected and separated as follows: central neurosensory retina, central RPE-choroid, central sclera, anterior vitreous, posterior vitreous, remainder vitreous, peripheral retina, peripheral RPE-choroid, peripheral sclera, and anterior segment. Venous blood samples were obtained from animals in the pharmacokinetics group.
Histopathology
Globes were enucleated and sectioned in an anterior-posterior (AP) manner; the region of the posterior injection was the target of the AP section, whereas the anterior segment bisected the cornea (JDC, SP). DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) staining of the retinal and RPE cell nuclei was performed by using a dilution of the DAPI stock solution (Molecular Probes, Eugene OR) to 300 nM in phosphate-buffered saline (PBS). Approximately 300 μL diluted DAPI staining solution was added to the coverslip preparation, making certain that the cells were completely covered. The solution was allowed to incubate for 5 minutes. Slides were rinsed in PBS, excess buffer was drained, and a mounting medium with antifade reagent was added. Slides were viewed with a fluorescence microscope with appropriate filters. The secondary antibody used was fluorescence-conjugated donkey anti-human AlexaFluor incubated for 2 hours at room temperature in the dark.
Group 1
Six animals were enucleated at each interval; three were intravitreal and three were suprachoroidal specimens. At the National Institutes of Health laboratory, the pig retinal tissue was dissected into two separate fractions, the neural retina (retina) and RPE-choroid (RPE/Ch). Tissues were homogenized with tissue disrupter (Polytron; Brinkman Instruments, Inc., Westbury, NY) in 1-mL solution consisting of 1.0 M NaCl, 20 mM Tris-HCl (pH 7.4), 5 mM EDTA, and protease inhibitor cocktail (Halt; Pierce, Rockford, IL). After three sonications at 30 seconds each (power setting, 10) and centrifugation at 13,000g for 30 minutes at 4°C, the supernatant was analyzed for bevacizumab by ELISA. Protein concentrations were measured using a protein assay (Coomassie Brilliant Blue G-25-based; Bio-Rad, Hercules, CA). For ELISA, the samples were loaded into each well in the same amount of total protein (70 μg) and were adjusted to 50 μL by extraction solution. ELISA was performed in the usual manner. Amounts of bevacizumab in the samples were presented as micrograms of bevacizumab per milligrams of total protein.
Group 2
Eyes of six animals were enucleated at each interval; three were intravitreal and three were suprachoroidal delivery. An objective of this study was to quantify bevacizumab, a humanized monoclonal antibody, in plasma and in eye tissues of pigs. Bevacizumab recognizes all isoforms of VEGF-A. This humanized antibody contains >95% human immunoglobulin G (IgG) sequences, including the Fc portion. With these intrinsic properties, bevacizumab can bind its antigen VEGF, and it interacts with any antibody that recognizes the Fc portion of the human IgG molecule. As such, VEGF and an anti-human Fc IgG antibody can be used to capture bevacizumab and quantify it in ELISA. When VEGF is used in the assay, only the free fraction of bevacizumab can be measured, whereas the anti-hIgG (Fc) antibody can capture both the free bevacizumab and the bound bevacizumab-VEGF complex, enabling the total amount of bevacizumab to be measured. Two direct ELISAs were developed to measure bevacizumab in pig eye tissues and plasma. In the assay to measure total bevacizumab, the tetramethylbenzidine (TMB) assay, a goat polyclonal antibody that recognizes the Fc portion of human IgG, was used to capture bevacizumab. This assay uses the colorimetric substrate TMB for quantification. In the ELISA to measure free bevacizumab, the chemiluminescence assay, recombinant hVEGF-165, was used to capture bevacizumab, and the chemiluminescence substrate was used for quantification. The sensitivity of the assay was defined as the lowest level of bevacizumab that gives at least twice the absorbance or chemiluminescence value as background; that is, the absorbance (or luminescence) at the lowest bevacizumab level with background subtracted is the same as the absorbance (or luminescence) of the background. The assays were validated by measuring the stability of bevacizumab in plasma or eye tissue homogenates on storage at 4°C and were subjected to cycles of freeze-thaws.
Tissue Homogenates
Retina, choroid, sclera, and vitreous were prepared from enucleated pig eyes and stored at −80°C. For these tissue homogenates, several samples were prepared simultaneously by using a bead mill (Precellys 24; Bertin Technologies, Tarnos, France). To each preloaded Omni tube containing 2.8 mm steel beads, ≤0.4 g choroid, and 1 mL lysis buffer (+PI) were added. To each preloaded Omni tube containing 1.4 mm zirconium silicate beads, ≤0.7 g retina, and 0.7 mL lysis buffer (+PI) was added. Tubes (up to 12) were milled at 6,500 rpm for 2 minutes (i.e., two cycles of 60 seconds with a 40-second pause between cycles, stored on ice for 5 minutes, and centrifuged at top speed [14,500 rpm] in the microfuge at 4°C for 10 minutes). For the sclera homogenate, 12 small sclera pieces weighing 80 to 113 mg were prepared. To each reinforced Omni 2-mL tube, three steel beads of 3.2-mm diameter, sclera, and 1.4 mL lysis buffer (+PI) were added. Tubes containing sclera mixture were milled at 6,500 rpm for 6 minutes (i.e., six cycles of 60 seconds with 40-second pauses between two consecutive cycles, stored on ice for 5 minutes, and centrifuged at top speed in the microfuge at 4°C for 5 minutes). For the vitreous stored in 15-mL conical tubes, protease inhibitor cocktail was added to yield a final concentration of 1×, mixed thoroughly by several inversions, dispensed in 12 aliquots measuring 1 mL each (in 1.5-mL Eppendorf tubes), and centrifuged at top speed in the microfuge at 4°C for 10 minutes to remove particulates. In a separate experiment to compare the efficiency of mixing the protease inhibitors manually by inversion to mechanically mixing by milling with zirconium beads for 1 minute at 6,500 rpm, it was found that the protein concentrations remained the same in both samples. For all homogenates, clarified supernatants were pooled and assayed for protein concentrations, using the BCA assay and BSA (in 5%–10% lysis buffer) as standard, as suggested by the manufacturer. Typical yields for these tissue homogenates were 40 to 50 mg/g retina, 20 to 60 mg/g choroid, 25 to 60 mg/g sclera, and 2 to 5 mg/g vitreous.
ELISA
Typical steps of direct ELISA are as follows: coating the plate with the capture molecule, blocking nonspecific binding, binding the bevacizumab analyte, detecting the captured analyte with detection antibody conjugated with an enzyme (horseradish peroxidase [HRP]), and developing signals proportional the amount of enzyme bound to the analyte. Between the sequential steps, wells (usually the entire plate of 96 wells) were washed thoroughly to remove reactants of each step before the addition of reactants of the next step. For these assays, HRP-conjugated antibody was used as the detection antibody. The antibody pair was based on the human IgG detection kit of Bethyl Laboratories (Montgomery, TX). Different conditions of the assay, such as concentrations of the antibody pair, blocking buffers, incubation time, incubation temperature, and wash buffers, were examined to yield the highest signal-to-noise ratio. The final protocol for each assay is described.
TMB ELISA.
The TMB assay uses an antibody that recognizes the Fc portion of human IgG to capture bevacizumab and an HRP-conjugated antibody that recognizes another site of the Fc portion of human IgG to detect and quantitate bevacizumab. The colorimetric substrate was TMB. Reactions were developed for 30 minutes at room temperature, gently rotated, and quenched with acidic solution to enhance the absorbance of the reactions.
TMB Assay for Pig Plasma.
Capture antibody (A80-104; 0.5 μg/mL in coat buffer; Bethyl Laboratories) was added to all wells of microtiter plates (MaxiSorp; Thermo Scientific, Pittsburgh, PA), and plates were incubated overnight at 4°C. Plates were washed three times with wash buffer 2, then blocked with block buffer (2.5% casein in wash buffer 2) for 30 minutes at room temperature and washed three times again. Bevacizumab standards, prepared in block buffer and in a twofold serial dilution range (0.5–32 ng/mL), were added to designated wells. Bevacizumab, in the same concentration range as standards, was prepared in different concentrations of pig plasma (diluted with block buffer) and was added to designated wells. Plates were incubated at 37°C, gently rotating for 2 hours. Samples were removed, and plates were washed five times with wash buffer 2. The detection antibody, HRP-conjugated goat anti-hIgG (Fc, A80-104P, 20 ng/mL in block buffer; Bethyl Laboratories), was added to each well, and plates were incubated at 37°C for 1 hour. Samples were removed, and plates were washed five times with wash buffer 2. TMB was added to each well, and plates were rotated gently at room temperature for 30 minutes. Stop solution was added to each well. Absorbance at 450 nm was determined for each well with a spectrophotometer (M5; Molecular Devices, Eugene, OR).
TMB Assay for Pig Eye Tissue.
Capture antibody (A80-104, 0.5 μg/mL in coat buffer; Bethyl Laboratories) was added to all wells of microtiter plates (MaxiSorp; Thermo Scientific), and plates were incubated at 37°C for 1 hour. Plates were washed three times with wash buffer 2, then blocked with block buffer (2.5% casein in wash buffer 2) for 30 minutes at room temperature, and washed three times again. Bevacizumab standards, prepared in block buffer, in a twofold serial dilution range of 0.16 to 10 ng/mL, were added to designated wells for standards. Bevacizumab, in the same concentration range as standard, was prepared in different concentrations of pig tissue homogenates (diluted with lysis buffer +PI) were added to designated wells. Plates were incubated overnight at 4°C. Samples were removed, and plates were washed five times with wash buffer 2. The detection antibody, preadsorbed HRP-conjugated goat anti-hIgG (A80-219P, 100 ng/mL in block buffer; Bethyl Laboratories) antibody, was added to each well, and plates were incubated at 37°C for 1 hour. Samples were removed and plates were washed 5 times with wash buffer 2. TMB was added to each well, and plates were rotated gently at room temperature for 30 minutes. Solution (STOP Solution; Cell Signaling, Danvers, MA) was added to each well. Absorbance at 450 nm was determined for each well with a spectrophotometer (M5; Molecular Devices).
Chemiluminescence ELISA.
The Chemi assay uses recombinant hVEGF-165 to capture bevacizumab and an HRP-conjugated antibody that recognizes the heavy and light chains of human IgG to detect and quantitate bevacizumab. This detection antibody was preadsorbed with pig IgG to prevent its nonspecific binding in the pig eye tissue homogenates. Light-emitting substrate, a luminol and peroxide mixture (SuperSignal; Pierce), was the chemiluminescence substrate. Immediately after substrate was added to all wells of the assay plate, the plate was placed in the plate reader (M5; Molecular Devices) and agitated for 1 minute, and light emission from each well was collected for 1 minute.
Chemiluminescence Assay for Pig Plasma.
Recombinant hVEGF-165 (0.1 μg/mL in coat buffer; HumanZyme, Chicago, IL) was added to all wells of microtiter plates (Microlite2+; Fisher Scientific, Pittsburgh, PA) and incubated for 1 hour at 37°C. Plates were washed three times with wash buffer 2, then blocked with block buffer (2.5% casein in wash buffer 2) for 30 minutes at room temperature, and were washed three times again. Bevacizumab standards, prepared in block buffer and in a twofold serial dilution range of 0.016 to 1 ng/mL, were added to designated wells for standards. Bevacizumab, in the same concentration range of standards, was prepared in different concentrations of pig plasma (diluted with block buffer) and added to designated wells. Plates were incubated at 37°C, gently rotating for 2 hours. Samples were removed and plates were washed five times with wash buffer 2. The detection antibody, HRP-conjugated goat anti-hIgG (Fc, A80-104P, 20 ng/mL in block buffer; Bethyl Laboratories), was added to each well, and plates were incubated at 37°C for 1 hour. Samples were removed, and plates were washed five times with wash buffer 2. Chemiluminescent substrate was added to all wells. The plate was placed in the spectrophotometer (M5; Molecular Devices) and agitated for 1 minute, and the luminescence of each well was collected for 1 minute.
Chemiluminescence Assay for Pig Eye Tissue.
Recombinant hVEGF-165 (0.1 μg/mL in coat buffer; HumanZyme) was added to all wells of microtiter plates (Microlite2+; Fisher Scientific) and incubated at 37°C for 1 hour. Plates were washed three times with wash buffer 2, blocked with block buffer (2.5% casein in wash buffer 2) for 30 minutes at room temperature, and washed three times again. Bevacizumab standards, prepared in block buffer in a threefold serial dilution range (0.011-9 ng/mL), were added to designated wells for standards. Bevacizumab in different concentrations of pig tissue homogenates (diluted with lysis buffer +PI) were added to designated wells. Plates were incubated overnight at 4°C. Samples were removed, and plates were washed five times with wash buffer 2. The detection antibody, preadsorbed HRP-conjugated goat anti-hIgG (A80-219P, 100 ng/mL in block buffer; Bethyl Laboratories), was added to each well, and plates were incubated at 37°C for 1 hour. Samples were removed, and plates were washed five times with wash buffer 2. Chemiluminescent substrate was added to all wells. The plate was placed in the spectrophotometer (M5; Molecular Devices) and agitated for 1 minute, and the luminescence of each well was collected for 1 minute.
Results
Group 1
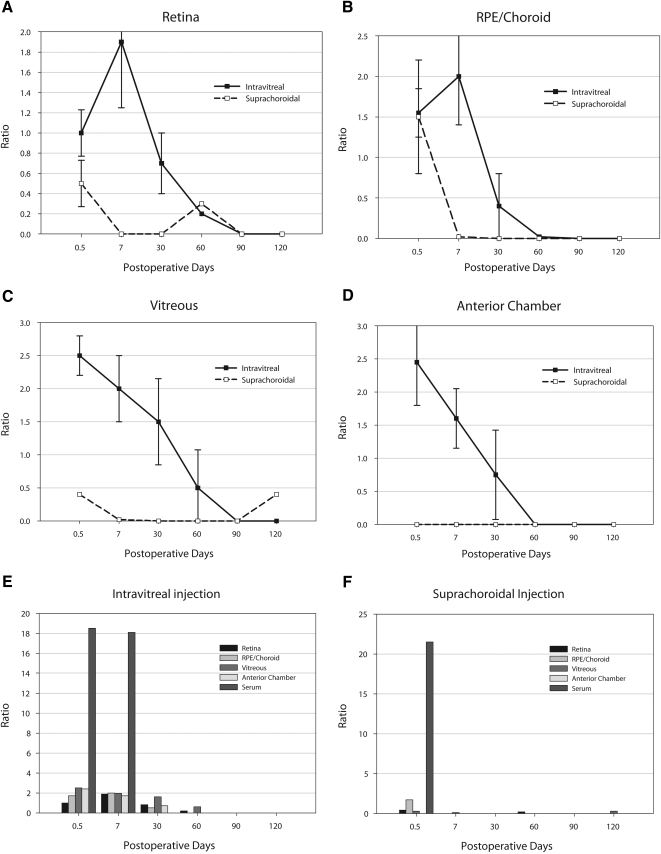
The pharmacokinetics of sustained delivery from the intravitreal injections are demonstrated (Figs. 1A–F). The 0.5-day or 12-hour samples between the two injection techniques were similar in the posterior RPE/choroid and in the posterior retina. Vitreous and anterior chamber measurements were dramatically lower in the suprachoroidal group than the intravitreal injection group. Systemic plasma levels were slightly higher (not statistically significant; P > 0.05) in the suprachoroidal group at 12 hours but undetectable thereafter. The intravitreal injection group had systemic levels that were measurable at day 7 but not thereafter. In fact, no significant levels of drug were detected from the suprachoroidal delivery route at any time point after 0.5 days in the bevacizumab group.
Figure 1.
Pharmacokinetic graphs from study 1 (1.25 mg bevacizumab). All y-axis measurements are presented as a ratio comparing units of bevacizumab measured (micrograms)/total protein (milligrams) with error bars as ±SD. The x-axis measurements are in days after the initial injection. The 0.5-day time point was 12 hours after the initial injection. (A) Comparison of the intravitreal route (solid line) with the suprachoroidal route (dashed line) in all extracted retinal tissue. (B) Similar comparison from the entire RPE-choroidal tissue specimen. (C) Comparison from the vitreous, clearly indicating higher levels from a direct intravitreal injection. (D) Comparison of aqueous fluid. Note that there was no measurable bevacizumab level at any time point in the aqueous from the suprachoroidal injection. (E) Bar graph of the various tissues with serum levels from an intravitreal injection. (F) Similar bar graph of the various tissues showing serum levels after suprachoroidal injection. Average SD (as a percentage of the mean) was approximately 34%.
Group 2
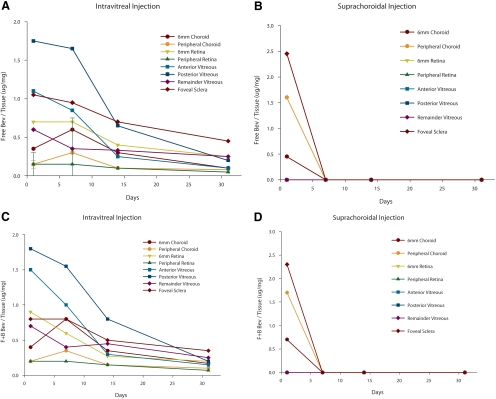
The pharmacokinetics are demonstrated (Figs. 2A–D). Suprachoroidal injections were not measurable at any point after the initial 12-hour analysis. Specifically, by day 7 and all points thereafter, no measurable bevacizumab was detectable. At the 12-hour data point, the levels of bevacizumab in the choroid that corresponded to the macular region or area centralis in the pig, were nearly equivalent using either injection technique.
Figure 2.
Pharmacokinetic graphs from study 2 (3 mg bevacizumab). (A, B) The y-axes represented the ratio of free bevacizumab (micrograms)/total protein (milligrams). Each data point represents three specimens (SD not included because of the number of lines present). (C, D) The y-axes represent the sum of free and bound bevacizumab (micrograms)/total protein (milligrams). (A) Pharmacokinetic data of the free intravitreal injections in various tissues from 0.5 to 32 days after injection. Note that there is a predictable, gradual decline in tissue levels over time. (B) Similar profile of free bevacizumab injected by the suprachoroidal route. Note that after the 12-hour measurement, there are no tissue levels of drug by day 7 or thereafter, suggesting rapid drug disappearance. (C) Similar profile of free plus bound bevacizumab after intravitreal injection. Pharmacokinetics is similar to that in Figure 2A. (D) Similar profile is seen when free plus bound bevacizumab is measured after the suprachoroidal method of injection. Pharmacokinetics is similar to that in Figure 2B. Average SD (as a percentage of the mean) was approximately 29%.
Immunohistology
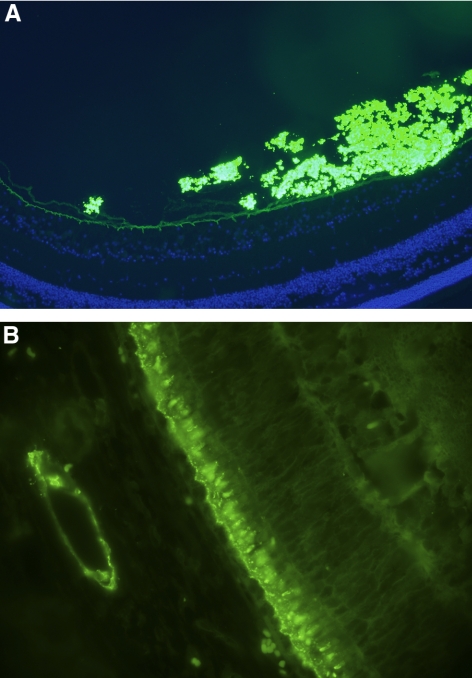
We performed immune staining using anti-human Fc antibodies with fluorescent labels and demonstrated that intravitreal bevacizumab was concentrated in the vitreous and layered along the posterior vitreous, near the internal limiting membrane of the retina (Fig. 3A). Anteriorly, the drug seemed to be limited by the ciliary epithelium. On the other hand, suprachoroidal delivery appeared to be localized at the endothelial aspects of the larger choroidal vessels, the inner choroid, the RPE, and the outer segments of the photoreceptors (Fig. 3B).
Figure 3.
Immunofluorescent bevacizumab staining (t = 12 hours). (A, green) Staining at 200× represents immunofluorescent antibodies to bevacizumab after intravitreal injection. (blue) DAPI staining of cell nuclei of the neurosensory retina. Note that the internal limiting membrane seems to limit the diffusion of bevacizumab. (B, green) Staining at 200× with immunofluorescent antibodies to bevacizumab after suprachoroidal injection. Note the staining pattern at the level of the RPE-photoreceptor complex and at the vascular endothelium of the larger choroidal vessels. Control tissue (not shown) had dim staining, similar to the inner retinal tissues.
Inflammation
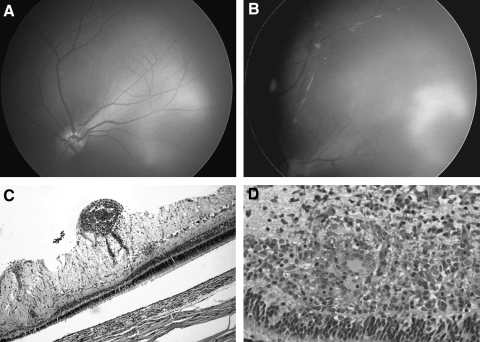
In 4 of the 18 intravitreal injections in study 1 and in 3 of the 12 intravitreal injections in study 2 (total, 7 of 30 total injections), we observed intraocular inflammation that appeared as vitreitis and peri-vasculitis (Fig. 4).
Figure 4.
Inflammation seen only with intravitreal injections. (A) Retcam fundus image of an eye after suprachoroidal injection. There is no sign of inflammation. (B) Retcam image approximately 30 days after intravitreal injection. Vitreitis and perivasculitis are indicated by a fluffy infiltrate near the vessels. (C) Photomicrograph (100×) of the perivascular inflammation near the inner retina. (D) Higher-power view (200×) of an adjacent area demonstrates granulomatous inflammation and the formation of giant cells along with anatomic disruption of the outer retinal structures.
Histopathology demonstrated a granulomatous reaction with giant cell formation along with nonspecific inflammatory cells at 30 days (Figs. 4C, 4D).
Discussion
Suprachoroidal drug delivery offers several interesting advantages over other delivery routes. We previously demonstrated sustained delivery of triamcinolone using this route with excellent tissue levels in the macula and low systemic levels for >120 days in the pig model.13 The suprachoroidal route avoids direct entry into the eye and decreases the risks for endophthalmitis, retinal detachment, and potentially cataract formation. It also provides access to the outer segment macular tissue with lower anterior segment tissue levels. Large biological agents, such as bevacizumab, are commonly injected using the intravitreal route. There is limited diffusion of large macromolecules when using the transscleral route of delivery, presumably because of the collagen matrix of the scleral tissue.10 Theoretically, when injected directly into the suprachoroidal space, these molecules could remain in the suprachoroidal space, providing sustained delivery to the macula, similar to the response we achieved using triamcinolone.
However, the kinetics of these large biological molecules in the suprachoroidal space are very different from those of small molecules suspended in a slow-release formulation. In fact, we found that suprachoroidal bevacizumab is rapidly cleared from this space, has a peak initial serum plasma level that is consistent with rapid systemic clearance, and has very low sustained tissue levels. These data are consistent with those of reports using computational models.15 Given that bevacizumab in hyaluronic acid is cleared rapidly from this space, it is not likely that this route, at the formulation presented in this series, would optimize drug delivery pharmacokinetics. The profile of intravitreal injections is more sustained and consistent with our current clinical treatment intervals, for both bevacizumab and presumably other large proteins. By examining the fluorescence-tagged antibodies, it appears that the drug rapidly diffuses away from the suprachoroidal space, directly into the choroid and RPE complex, and is swept out of the globe by the choroidal vasculature. Alternatively, the proteins may exit this space through more traditional uveoscleral outflow channels.16,17 The peak in serum levels early in the kinetics likely reflects a higher ratio of serum bevacizumab to total protein compared with tissue measurements. This may reflect either lower total protein in the serum, thus giving a higher ratio, or there may be some early binding of the antibody that leads to a transient retention of the antibody in the serum. The three peaks (Figs. 1E, 1F) were consistent and corresponded to what one would expect on a relative comparison; however, the absolute ratios seemed high compared with local tissue levels. We feel that these numbers are accurate given the consistency and the comparison with prepared serum standards.
Does the suprachoroidal route warrant further investigation for larger biological agents? Clearly, this study suggests that the answer is yes. The suprachoroidal route may still represent a more direct alternative route of delivery to these critical tissues. Histologic images using fluorescence-labeled antibodies to bevacizumab indicate higher initial levels of the agent in the RPE and photoreceptor regions than were seen with intravitreal injections. Gross kinetic data clearly show rapid loss of a depot effect when delivered to the suprachoroidal space. Lower anterior segment drug levels, avoidance of invasion of the optic axis, and more targeted delivery are all advantageous.
The histology also suggests that the vascular channels represent a primary exit route of the biological agent, as noted by the pronounced staining of the large choroidal vessel endothelium. Choroidal vessels lack the blood-retinal barrier tight junctions and certainly may be responsible for rapid removal of the proteins from the suprachoroidal space and the simultaneously high serum levels in the circulation. One could speculate that a sustained delivery formulation of the biological agent would optimize the pharmacokinetics so that it would mirror that of triamcinolone. Suprachoroidal delivery appears to directly target the choroid, RPE, and outer segments of the retina. Theoretically, a lower drug dose may be required when these tissues are the target of the intended therapy, especially in the setting of a sustained-release formulation. Future studies would require optimization of drug formulation and drug stabilization. Perhaps, by suspending the biological agent in nanoparticles or another sustained-release vehicle, a constant state of delivery would be advantageous in the suprachoroidal space, much like that seen using triamcinolone.13 In addition, the expansile nature of the suprachoroidal space, as evidenced by the size of choroidal detachments seen clinically, thereby offers a large potential depot for a sustained drug delivery site.
The strength of using several ELISAs in two separate studies is to verify and confirm our results. The second analysis (group 2) helped to quantify bevacizumab in pig plasma and specific ocular tissues, namely the retina, choroid, vitreous, and sclera. All data points are important to verify the findings from the initial study (group 1). The colorimetric (TMB) assay uses a polyclonal antibody that recognizes the Fc portion of human immunoglobulin G, and thus can detect all bevacizumab molecules, both free and VEGF-bound forms. This analysis diminished the risk for undetected bound forms of bevacizumab, leading to falsely low assay from tissue samples. The chemiluminescence assay uses VEGF-165 to capture bevacizumab and thus can only detect the free bevacizumab molecules.
An interesting, yet unexpected, finding in this study was the inflammatory reaction that was seen in approximately one-third of the cases injected using intravitreal administration. Because this is a human antibody injected into a porcine eye, one may well expect a xenograft immune rejection response to the foreign antibody and antigen. Why does this inflammation only occur in the intravitreal-injected eyes and not in the suprachoroid-injected eyes? Several explanations are possible. First, perhaps the rapid clearance of the suprachoroid drug minimized the time required for a delayed-type or cell-mediated immune reaction to occur. It is also possible that intravitreal injections have an altered immune response compared with injections either in the suprachoroidal space or even elsewhere in the body. The anterior chamber immune deviation could conceivably be induced by a suprachoroidal injection of antigen. Because this reaction occurred in both studies, was separated in time, and occurred using different batch numbers of bevacizumab, it is not likely that it represents a contaminant from the specific container used for aliquots of the intravitreal cases only. The reaction is granulomatous and may offer insight into the rare cases of intraocular inflammation reported in human eyes treated with bevacizumab.18–20
In summary, we demonstrate the pharmacokinetics and immunohistology of bevacizumab injected into the suprachoroidal space using controlled assays from two laboratories. We contrast the pharmacokinetics of a large biological agent that is suspended in hyaluronic acid with our previous work on a small molecule (triamcinolone) in a slow-release matrix and demonstrate distinctively different pharmacokinetic clearance profiles. The sustained-release small molecules may represent the ideal candidate drugs for suprachoroidal delivery. However, large biological agents have a more rapid clearance from the suprachoroidal space than comparable intravitreal injections of the same agent. The high-flow blood channels of the choroid and choriocapillaris are likely responsible for the systemic clearance, but we cannot rule out alternative pathways, such as uveoscleral outflow. Interestingly, the bevacizumab injected into the suprachoroidal space seems to diffuse more directly to the RPE and outer photoreceptors. In the future, we suggest that studies should examine a sustained-release formulation of bevacizumab or other biological agents. Such a study would have to be combined with maintained biological activity; as such molecules may be subject to degradation. The goal of formulation for the suprachoroidal space would be to develop sustained delivery plus sustained activity of large biological agents. Such measures may prove to support this unique potential space as an effective route for drug delivery to the macular and posterior segment tissues.
Acknowledgments
The authors thank Mike Nash, Stan Conston, Ron Yamamoto, and Tien Nguyen at iScience Interventional Inc. for their support of this study and for agreeing to adhere to institutional conflict of interest policies, and Tien Nguyen for the exceptional work on the technical aspects with the commercial laboratory.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, April 2010.
Supported in part by an industry-sponsored grant from iScience Interventional Inc. (approved through the Sponsored Projects Administration at the University of Minnesota) and an unrestricted grant from Research to Prevent Blindness to the Departments of Ophthalmology at Emory University and at the University of Minnesota.
Disclosure: T.W. Olsen, None; X. Feng, None; K. Wabner, None; K. Csaky, None; S. Pambuccian, None; J.D. Camero, None
References
- 1. Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 2. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816 [DOI] [PubMed] [Google Scholar]
- 3. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444 [DOI] [PubMed] [Google Scholar]
- 4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431 [DOI] [PubMed] [Google Scholar]
- 5. Moshfeghi AA, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002, e2001–2012 [DOI] [PubMed] [Google Scholar]
- 6. Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–1047 [DOI] [PubMed] [Google Scholar]
- 7. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenfeld PJ. Intravitreal Avastin: the low cost alternative to lucentis? Am J Ophthalmol. 2006;142:141–143 [DOI] [PubMed] [Google Scholar]
- 9. Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 1998;125:237–241 [DOI] [PubMed] [Google Scholar]
- 10. Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability: effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest Ophthalmol Vis Sci. 1995;36:1893–1903 [PubMed] [Google Scholar]
- 11. Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46:641–646 [DOI] [PubMed] [Google Scholar]
- 12. Einmahl S, Savoldelli M, D'Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002;43:1533–1539 [PubMed] [Google Scholar]
- 13. Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142:777–787 [DOI] [PubMed] [Google Scholar]
- 14. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open- angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009;35:814–824 [DOI] [PubMed] [Google Scholar]
- 15. Ranta VP, Mannermaa E, Lummepuro K, et al. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release. 2010;148:42–48 [DOI] [PubMed] [Google Scholar]
- 16. Bill A. The drainage of albumin from the uvea. Exp Eye Res. 1964;3:179–187 [DOI] [PubMed] [Google Scholar]
- 17. Bill A. Movement of albumin and dextran through the sclera. Arch Ophthalmol. 1965;74:248–252 [DOI] [PubMed] [Google Scholar]
- 18. Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246:779–781 [DOI] [PubMed] [Google Scholar]
- 19. Johnson D, Hollands H, Hollands S, Sharma S. Incidence and characteristics of acute intraocular inflammation after intravitreal injection of bevacizumab: a retrospective cohort study. Can J Ophthalmol. 2010;45:239–242 [DOI] [PubMed] [Google Scholar]
- 20. Georgopoulos M, Polak K, Prager F, Prunte C, Schmidt-Erfurth U. Characteristics of severe intraocular inflammation following intravitreal injection of bevacizumab (Avastin). Br J Ophthalmol. 2009;93:457–462 [DOI] [PubMed] [Google Scholar]