The functional effect of curcumin, a free radical scavenger and an herbal medicine from Indian yellow curry spice, Curcuma longa, on protease-mediated retinal ganglion cell death was investigated. These results show, for the first time, that curcumin indeed prevents the protease-mediated death of RGCs, both in vitro and in vivo.
Abstract
Purpose.
Staurosporine (SS) causes retinal ganglion cell (RGC) death in vivo, but the underlying mechanisms have been unclear. Since previous studies on RGC-5 cells indicated that SS induces cell death by elevating proteases, this study was undertaken to investigate whether SS induces RGC loss by elevating proteases in the retina, and curcumin prevents SS-mediated death of RGCs.
Methods.
Transformed mouse retinal ganglion-like cells (RGC-5) were treated with 2.0 μM SS and various doses of curcumin. Two optimal doses of SS (12.5 and 100 nM) and curcumin (2.5 and 10 μM) were injected into the vitreous of C57BL/6 mice. Matrix metalloproteinase (MMP)-9, tissue plasminogen activator (tPA), and urokinase plasminogen activator (uPA) activities were assessed by zymography assays. Viability of RGC-5 cells was assessed by MTT assays. RGC and amacrine cell loss in vivo was assessed by immunostaining with Brn3a and ChAT antibodies, respectively. Frozen retinal cross sections were immunostained for nuclear factor-κB (NF-κB).
Results.
Staurosporine induced uPA and tPA levels in RGC-5 cells, and MMP-9, uPA, and tPA levels in the retinas and promoted the death of RGC-5 cells in vitro and RGCs and amacrine cells in vivo. In contrast, curcumin attenuated RGC and amacrine cell loss, despite elevated levels of proteases. An NF-κB inhibitory peptide reversed curcumin-mediated protective effect on RGC-5 cells, but did not inhibit protease levels. Curcumin did not inhibit protease levels in vivo, but attenuated RGC and amacrine cell loss by restoring NF-κB expression.
Conclusions.
The results show that curcumin attenuates RGC and amacrine cell death despite elevated levels of proteases and raises the possibility that it may be used as a plausible adjuvant therapeutic agent to prevent the loss of these cells in retinal degenerative conditions.
Irreversible loss of retinal ganglion cells (RGCs) is a major cause of blindness in glaucoma. Although the only proven risk factor to date is elevated intraocular pressure (IOP), the events that cause the damage to RGCs in glaucoma are unclear. Several hypotheses have been proposed and continue to be investigated to explain the mechanisms underlying RGC death in glaucoma. These include mechanical damage,1–3 insufficient retrograde transport of trophic factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF),4 insufficient vascular nutrition,5–7 glutamate neurotoxicity,8–10 nitric oxide synthesis,11,12 hypoxia-inducible factor,13 inflammatory cytokines,14,15 altered immunity,16 accumulation of advanced glycation products,17 endothelin-mediated ischemia,18,19 glial cell activation,12 β-amyloid neurotoxicity,20,21 and autonomous axonal self-destruction.22–24 Despite these various hypotheses, the mechanisms connecting IOP to RGC loss in POAG are still unclear.
Recent studies suggest that curcumin, a free radical scavenger and herbal medicine extracted from the Indian yellow curry spice, Curcuma longa, prevents the damage associated with several of the triggers mentioned above, including NMDA-induced excitotoxicity in the retina.25
By employing some of the animal models related to glaucoma, such as retinal ischemia26 and excitotoxicity,27 we found that elevated levels of the three proteases matrix metalloproteins (MMP)-9, tissue plasminogen activator (tPA), and urokinase plasminogen activator (uPA) promotes the death of RGCs. Furthermore, by employing undifferentiated and transformed retinal ganglion-like cells (RGC-5), and by treating them with the protein kinase inhibitor staurosporine (SS), we found that RGC-5 cells differentiated on treatment with SS and produced elevated levels of tPA and uPA and that elevated levels of tPA and uPA directly caused the death of the RGC-5 cells.28,29
Despite recent reports indicating that SS induces significant loss of RGCs in vivo,30–32 the downstream mechanisms causing RGC loss remain unclear. Therefore, in this study, we used SS to induce loss of RGCs in vivo and loss of RGC-5 cells in vitro, and investigated whether SS induces elevated levels of proteases in vivo, and whether curcumin attenuates SS-mediated loss of RGC-5 cells in vitro and RGCs in vivo.
Materials and Methods
Materials
The following materials were obtained for use in this study: the RGC-5 cell line from Neeraj Agarwal (University of North Texas Health Science Center, Fort Worth, TX), Dulbecco's modified Eagle's medium (DMEM), Dulbecco's phosphate-buffered saline (DPBS), and penicillin and streptomycin (Invitrogen, Carlsbad, CA); SS (Alexis Biochemicals; San Diego, CA); human glu-plasminogen and human fibrinogen (American Diagnostica; Stamford, CT); gelatin (Bio-Rad Laboratories, Hercules, CA); curcumin (extracted from Curcuma longa) and DMSO (Sigma-Aldrich, St. Louis, MO); cell-permeable NF-κB inhibitor peptide (SN50) and NF-κB inactive control peptide (SN50M) (EMD Chemicals, Inc.; Gibbstown, NJ); antibodies against Brn3a and NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA); antibodies against Thy-1 from BD PharMingen (San Jose, CA); antibodies against choline acetyltransferase (ChAT) (Novus Biologicals, Littleton, CO); secondary antibodies conjugated to AlexaFluor 568 and 488 (Invitrogen), and fetal bovine serum (Atlanta Biologicals, Norcross, GA).
Cell Culture
RGC-5 cells were cultured in DMEM containing glucose (1 g/L), fetal bovine serum (FBS; 10%), penicillin (100 U/mL), and streptomycin (100 μg/mL). This cell line has been reported to be originated from rat (Rattus norvegicus), but recent studies have suggested that this cell line seems to be originated from mouse (Mus musculus). Therefore, we have performed DNA sequence analysis on RGC-5 cells and found that this cell line indeed is of mouse origin (data not shown). Cells were cultured at 37°C in a humidified incubator with circulating 5% CO2. RGC-5 cells were cultured routinely in 100-mm tissue culture dishes. For the indicated experiments, RGC-5 cells were trypsinized, the cell count was determined by a cell counter (Beckman Coulter, Carlsbad, CA), and the cells were seeded at a density of 4 × 103 cells/mL in 96-well tissue culture plates (100 μL of cell suspension in each well). After overnight incubation, the medium was aspirated, and the cells were washed twice with DPBS to remove any remaining serum and incubated with SS (2 μM; dissolved in DMSO) in DMEM containing no FBS. Where indicated, the RGC-5 cells were also treated with the indicated concentrations of curcumin in DMEM containing no FBS.
Cell Morphology and Cell Viability
Morphology of the cells was assessed by using an inverted, phase-contrast, bright-field microscope (Nikon, Tokyo, Japan), and digitized images were obtained (Nikon). Cell viability was determined by incubating the cells with 1.2 mM MTT (3-[4,5-dimethylthiazol-2-yl]- 2,5-diphenyltetrazolium bromide) for 2 hours at 37°C. After incubation, the formazan product formed by the viable cells was dissolved in 0.01 M HCl, and the optical density was read at 570 nm by using an automated spectrophotometer.
Intravitreal Injections
All experiments on animals were performed under general anesthesia according to the protocols approved by Oakland University's Animal Care and Use Committee and according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Normal adult C57BL/6 mice (6–8 week-old; Charles River Breeding Laboratories, Wilmington, MA) were anesthetized by an intraperitoneal injection of 1.25% Avertin (2,2,2-tribromoethanol in tert-amyl alcohol; 17 μL/g body weight). After a drop of topical anesthetic agent (proparacaine) was instilled, SS (12.5 and 100 nM) or curcumin (2.5 and 100 μM) were injected into the vitreous humor with a syringe (NanoFil; World Precision Instruments, Sarasota, FL) equipped with a 36-gauge beveled needle. Unless otherwise indicated, intravitreal injections were performed in a final volume of 2 μL. For control experiments, the eyes (n = 6 retinas each; three independent experiments) were injected with 2 μL of DMSO alone, and in the treatment groups, the eyes (n = 6 retinas each; three independent experiments) were injected with SS (12.5 and 100 nM), with or without curcumin (2.5 and 10 μM) prepared in DMSO. At various time points after injection, the eyes were enucleated and processed for protein extraction or embedded in optimal temperature cutting (OCT) compound for preparation of retinal cross sections. In some experiments, whole retinas were dissected and processed for immunohistochemistry.
Extraction of Total Proteins from Retinas
At 48 and 72 hours after intravitreal injection, the animals were euthanatized with an overdose of CO2, and the eyes were enucleated. The enucleated eyes were cut in half at the equator, and the lenses were removed. The retinas were carefully peeled off with forceps and washed three times in PBS (pH 7.4) to remove any vitreous that may have adhered to the retina. Four retinas each were placed in tubes (Eppendorf, Fremont, CA) containing 40 μL extraction buffer (1% Nonidet-P40, 20 mM Tris-HCl, 150 mM NaCl, and 1 mM Na3VO4 [pH 7.4]), and the tissues were homogenized. Tissue homogenates were centrifuged at 10,000 rpm for 5 minutes at 4°C, and supernatants were collected. The protein concentration in the supernatants was determined by using a commercial assay kit (Bio-Rad Laboratories).
Zymography Assays
Proteolytic activity of MMP-9, tPA, and uPA was determined by substrate zymography according to methods described previously.28,33,34 Briefly, aliquots containing equal amounts of conditioned medium (20 μL) or retinal protein extracts (50 μg total protein) were mixed with 4× SDS gel-loading buffer and the samples were loaded (without reduction or heating) onto 10% SDS polyacrylamide gels containing gelatin (2.6 mg/mL) to detect MMP-9, and fibrinogen (5.5 mg/mL) and plasminogen (50 μg/mL) to detect uPA and tPA. After electrophoresis, the gels were washed three times with 2.5% Triton X-100 (15 minutes each time), placed in 0.1 M glycine buffer (pH 8.0 [for tPA and uPA]) or in 10 mM calcium chloride buffer (pH 7.4 [for MMP-9]), and incubated overnight at 37°C to allow proteolysis of the substrates in the gels. After overnight incubation, the gels were stained with 0.2% Coomassie brilliant blue R250 (4 minutes) and destained with a solution containing 50% methanol and 10% acetic acid in deionized water. Samples containing standard recombinant MMP-9, tPA, and uPA were electrophoresed for comparison (data not shown).
Immunohistochemistry
RGC-5 Cells.
RGC-5 cells were seeded at a density of 2 × 103 cells/mL in eight-well chamber slides (200 μL of cell suspension in each chamber). After overnight incubation, the medium was aspirated, the cells were washed twice with PBS to remove any remaining serum and incubated overnight with SS (2 μM; dissolved in DMSO) in DMEM containing no FBS. After overnight incubation, the cells were washed twice with PBS and fixed for 30 minutes with 4% paraformaldehyde. The cells were washed three times with PBS and permeabilized for 15 minutes with 0.2% Triton X-100. After three washes in PBS, the cells were incubated in blocking buffer for 1 hour at room temperature (5% BSA in PBS). After they were blocked, the cells were washed three times with PBS and incubated for 2 hours at room temperature with primary antibodies against Brn3a and Thy-1 (1:200 dilution in blocking buffer). They were washed three times with PBS and then incubated for 1 hour at room temperature with secondary antibodies conjugated to AlexaFluor 568 and finally were washed three times with PBS, mounted with aqueous mounting medium (Gel Mount; Sigma-Aldrich) and observed by microscope (Nikon) equipped with epifluorescence. Digitized images were obtained (SPOT digital camera; Diagnostic Instruments, Sterling, MI) and then processed and compiled (Photoshop, ver. 5.5 and 7.0; Adobe Systems Inc., San Jose, CA).
Radial Sections.
Eyes enucleated at 48 and 72 hours after SS or curcumin was injected were fixed in 4% paraformaldehyde for 1 hour at room temperature and embedded in OCT compound (Tissue-Tek; Sakura Finetek USA, Torrance, CA). Ten-micrometer-thick radial sections were prepared by using a cryostat and placed onto slides (Super-Frost Plus; Fisher Scientific, Pittsburgh, PA). The sections were then immunostained by using antibodies against NF-κB (1:100 dilution in PBS) and ChAT (1:100 dilution in PBS). The sections were washed three times with PBS and incubated with secondary antibodies conjugated to AlexaFluor 568 for 1 hour at room temperature, washed three times with PBS, and mounted with a coverslip. NF-κB-positive immunoreactivity was assessed by observing the sections with a microscope equipped and images obtained as described above. Images for ChAT-positive immunoreactivity were converted to gray scale and inverted. For quantitative analysis of ChAT-positive amacrine cells, the total number of cells was counted both in the GCL and the INL; no attempts were made to differentiate the location of these cells in the GCL and the INL during counting. The total number of ChAT-positive amacrine cells in the retinas, located approximately the same distance from the optic disc (7200 μm2, 40× magnification), was quantitated (Scion Image Analysis; Scion Corp., Frederick, MD). For quantitative analysis, ChAT-positive cells were counted in four to six microscope fields of identical size (n = 6 retinas, three independent experiments) located approximately at the same distance from the optic disc. Statistical significance was analyzed by using a nonparametric Newman-Keuls analogue procedure (GB-Stat Software; Dynamic Microsystems, Silver Spring, MD) and expressed as the mean ± SEM.
Flat-mounted Retinas
Eyes enucleated at 48 and 72 hours after injection of SS or curcumin were fixed in 4% paraformaldehyde for 30 minutes at room temperature. The corneas and lenses were removed and the remaining eye cups were incubated in 4% paraformaldehyde for another 30 minutes. Retinas were peeled off carefully and washed three times with PBS, and ganglion cells remaining in the retinas were identified according the methods described by Nadal-Nicolas et al.35 Briefly, whole retinas were permeabilized in 0.5% Triton X-100 (in PBS) for 15 minutes at room temperature. The retinas were washed three times with PBS and incubated overnight at 4°C in polyclonal antibodies against Brn3a (1:100 dilution in blocking buffer [2% bovine serum albumin, 2% Triton X-100, and PBS]). After overnight incubation, the retinas were washed three times with PBS and incubated for 2 hours at temperature with secondary antibodies conjugated to AlexaFluor 568 (1:200 dilution in blocking buffer). Subsequently, the retinas were washed three times with PBS and mounted on slides, vitreous side facing upward. Brn3a-positive RGCs in whole-mounted retinas were assessed by observing flat-mounted retinas under a microscope equipped with epifluorescence (Nikon), and digitized images were obtained (SPOT digital camera; Diagnostic Imaging) and then processed and compiled (Photoshop, ver. 5.5 and 7.0; Adobe Systems Inc.). The total number of Brn3a-positive cells in the retinas, located approximately the same distance from the optic disc (7200 μm2, 40× magnification) was quantitated (Scion Image Analysis; Scion Corp.). For quantitative analysis, the Brn3a-positive cells were counted in four to six microscope fields of identical size located at approximately the same distance from the optic disc. Statistical significance was analyzed by using a nonparametric Newman-Keuls analog procedure (GB-Stat Software; Dynamic Microsystems) and expressed as the mean ± SEM.
Results
RGC-5 Cells Express Markers for Retinal Ganglion Cells
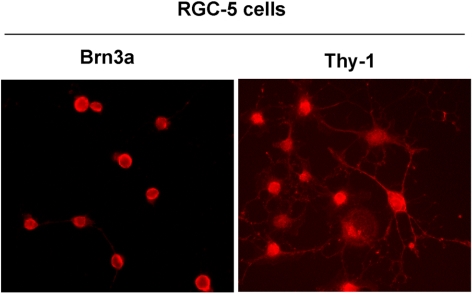
To determine whether RGC-5 cells express markers characteristic of retinal ganglion cells, RGC-5 cells were plated in eight-well chamber slides, treated overnight with 2.0 μM SS, and immunostained by using antibodies against Brn3a and Thy-1. Results presented in Figure 1 indicate that RGC-5 cells indeed expressed both markers characteristic of retinal ganglion cells.
Figure 1.
RGC-5 cells expressed RGC markers. RGC-5 cells were plated in eight-well chamber slides, treated overnight with 2.0 μM SS, and immunostained with antibodies against Brn3a and Thy-1. The results showed that the RGC-5 cells differentiated after SS treatment and expressed Brn3a and Thy-1, markers characteristic of RGCs. Magnification, ×40.
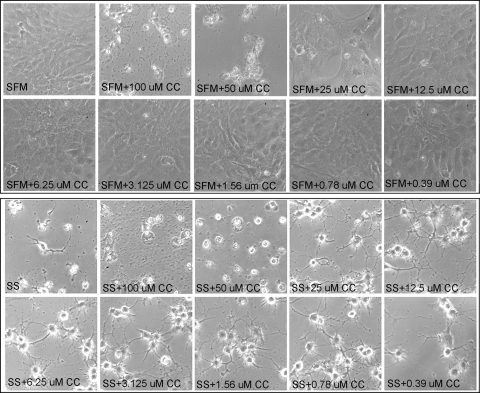
Effect of Curcumin and SS on Morphology of RGC-5 Cells
We have previously reported that 2.0 μM SS reduces the viability of transformed RGC-like cells (RGC-5) significantly within 48 hours.28 To investigate the effect of curcumin on SS-induced death of the RGC-5 cells, the cells were left untreated or treated with various doses of curcumin, SS (2.0 μM), or curcumin plus SS. At 48 hours after treatment, cell morphology was assessed by phase-contrast microscopy. The results presented in Figure 2 indicate that compared with untreated cells (undifferentiated), cells treated with higher doses of curcumin (50 and 100 μM) showed pyknotic morphology, whereas the cells treated with lower concentrations of curcumin (<50 μM) showed normal fibroblast morphology. When compared with untreated cells (undifferentiated), the cells treated with SS showed differentiation along with an extension of neuritis. Differentiated cells treated with higher doses of curcumin (50 and 100 μM) showed pyknotic morphology and lost their neurite extensions. When differentiated cells were treated with lower doses of curcumin (< 50 μM), the RGC-5 cells seemed healthy and showed elongated neurites.
Figure 2.
Curcumin improved the survival of SS-treated RGC-5 cells. RGC-5 cells were left untreated (serum-free medium; SFM) or treated with the indicated concentrations of curcumin in the absence (top) or presence (bottom) of SS. Forty-eight hours after treatment, the morphology of RGC-5 cells was observed under a phase-contrast microscope. Morphologic observations indicate that 0.78 to 25 μM of curcumin (CC) improved the survival of RGC-5 cells in the absence (top) or presence (bottom) of SS (SS). In addition, curcumin increased neurite outgrowth (bottom) suppressed by SS.
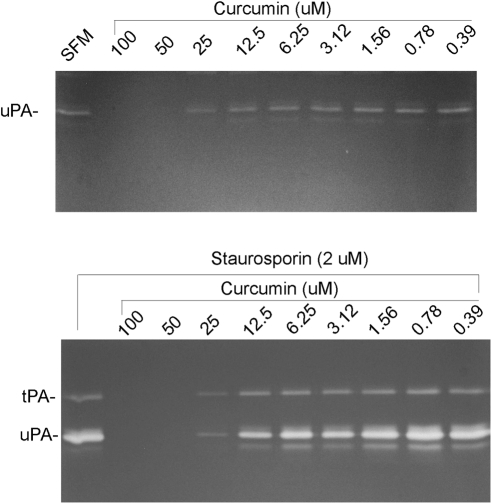
Effect of Curcumin and SS on Protease Levels Secreted by RGC-5 Cells
Our previous studies have shown that untreated cells secreted a low and constitutive level of uPA alone, whereas cells treated with 2 μM SS secreted elevated levels of uPA and tPA.28 We have also reported that constitutive levels of uPA did not affect cell survival, whereas elevated levels of uPA and tPA, acting in an autocrine fashion, promoted cell death. To investigate the effect of curcumin on uPA and tPA levels secreted by both undifferentiated and differentiated RGC-5 cells, the cells were treated with various doses of curcumin, SS, and curcumin plus SS. At 48 hours after treatment, conditioned medium was collected, and protease levels were assessed by fibrinogen/plasminogen zymography. The zymography results presented in Figure 3 indicate that untreated RGC-5 cells secreted low levels of uPA. When cells were treated with higher doses of curcumin (50 and 100 μM), tPA levels reduced to undetectable levels, whereas cells treated with lower doses of curcumin (<50 μM) and 2.0 μM SS secreted uPA similar to the levels observed in untreated cells. In contrast, cells treated with 2.0 μM SS alone secreted elevated levels of both uPA and tPA. uPA and tPA levels were decreased to undetectable levels in cells treated with higher doses of SS and curcumin (50 and 100 μM), but their levels were restored to normal levels with lower doses of curcumin (<25 μM). Note that low doses of curcumin did not affect protease levels, but the same doses were effective in attenuating cell death.
Figure 3.
Curcumin induces SS-mediated expression of uPA and tPA. RGC-5 cells were left untreated or treated with the indicated concentrations of curcumin in the absence (top) or presence (bottom) of SS. Forty-eight hours after the treatment, conditioned medium was collected and proteolytic activities of tPA and uPA were determined by zymography assays. Zymography assays indicate that untreated RGC-5 cells expressed low levels of uPA (but not tPA; top), whereas SS-treated cells expressed increased levels of both tPA and uPA (bottom). In addition, whereas higher concentrations of curcumin (>25 μM) decreased the expression of uPA and tPA, lower concentrations (<25 μM) increased their expression.
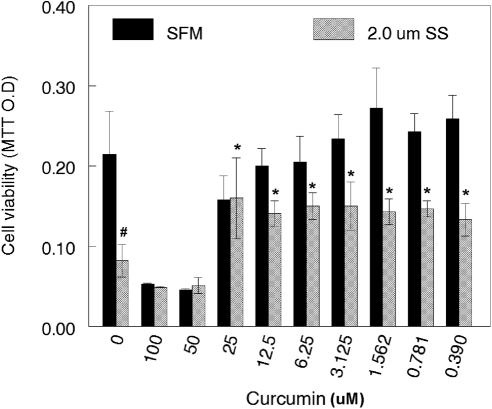
Effect of Curcumin and SS on RGC-5 Cell Death
To determine the effect of curcumin on SS-induced cell death, we left cells untreated or treated them with various doses of curcumin. In addition, cells were treated with 2.0 μM SS along with various doses of curcumin. At the end of 48 hours, cell viability was assessed by MTT assays. Compared with untreated RGC-5 cells (Fig. 4), the viability of cells treated with higher doses of curcumin (50 and 100 μM) alone decreased by ∼63% and 75%, respectively (P < 0.05; untreated versus curcumin treated). However, when the cells were treated with <25 μM curcumin, their viability increased from ∼25% to 30% (P < 0.05; untreated versus curcumin treated).
Figure 4.
Curcumin attenuated SS-induced death of RGC-5 cells. RGC-5 cells were left untreated or treated with the indicated concentrations of curcumin in the absence or presence of SS for 48 hours. At 48 hours after treatment, cell viability was determined by MTT assay. Results indicate that SS reduced the survival of RGC-5 cells significantly (#P < 0.05), when compared with untreated cells. In addition, whereas lower concentrations of curcumin (<25 μM) improved the survival of RGC-5 cells significantly (*P < 0.05), higher concentrations (>25 μM) decreased their survival further.
Compared with the untreated cells, the viability of the cells treated with 2.0 μM SS alone decreased by ∼77% (P < 0.05). The viability of RGC-5 cells treated with 2.0 SS and higher doses of curcumin (50 and 100 μM) decreased further (∼38% and ∼40%, respectively; P < 0.05). In contrast, when the cells were treated with <25 μM curcumin and SS (2 μM), their viability increased from 60% to 95% (SS treated versus SS and curcumin treated; P < 0.001). Although cell viability was increased in 2.0 SS-treated and <25 curcumin-treated cells, the trend plateaued with subsequent concentrations of curcumin.
Effect of NF-κB Inhibitors on RGC-5 Cell Death
Curcumin has been shown to either activate or inactivate the NF-κB pathway based on the tissue studied. Since the above results suggest that curcumin attenuates the protease-mediated (SS-induced) death of RGC-5 cells, additional experiments were performed to determine whether inhibition of NF-κB activation would reverse the curcumin-mediated protective effect. Before proceeding to the next experiments, we tested various doses of curcumin and NF-κB inhibitors on RGC-5 cells, to determine the optimal concentrations. Our results suggested that 6.25 μM curcumin and 75 μg/mL NF-κB inhibitors were optimal for having any significant effect on RGC-5 cells (data not shown). Therefore, in the remaining experiments, we used 6.25 μM curcumin and 75 μg/mL NF-κB inhibitors for the treatments.
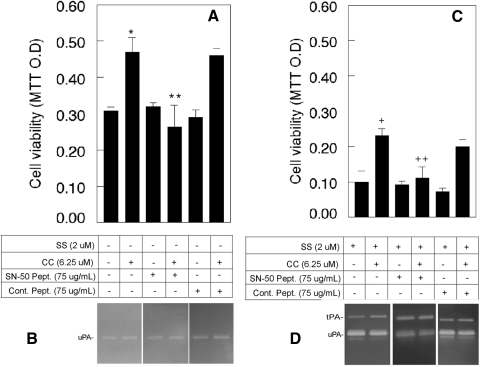
RGC-5 cells were left untreated or treated with curcumin, with or without NF-κB inhibitory peptide (SN-50) or inactive control peptide. The results presented in Figure 5A show that, compared with untreated cells, the viability of cells treated with curcumin increased by ∼50% (P < 0.05). When cell viability was compared among cells treated with curcumin, curcumin plus NF-κB inhibitory peptide, or curcumin plus NF-κB inactive peptide, the cell viability decreased by ∼68% (P < 0.05) in curcumin plus inhibitory peptide–treated cells, but not in curcumin plus inactive peptide–treated cells. Cell viability was unaffected when compared among cells untreated or treated with or without NF-κB active peptide or inactive peptide.
Figure 5.
NF-κB inhibitor reversed the curcumin-mediated protective effect on RGC-5 cells. RGC-5 cells were left untreated or were treated with the indicated concentrations of curcumin (CC), cell-permeable NF-κB-inhibitory peptide (SN-50), or cell-permeable NF-κB inactive peptide (Cont. Pept.) in the absence or presence of SS. Forty-eight hours after treatment, conditioned medium was collected, and the proteolytic activity of uPA and tPA was measured by zymography assays. Cell viability was determined by MTT assay. Zymography results indicate that regardless of the addition of NF-κB active or inactive peptides, RGC-5 cells expressed low levels of uPA (B), whereas SS-treated cells expressed increased levels of both uPA and tPA (D). MTT assays (A) indicated that the addition of curcumin alone increased cell survival (*P < 0.05). This protective effect was reversed by NF-κB-inhibitory peptide (**P < 0.05), whereas NF-κB inactive peptide had no effect (C). In addition, curcumin-mediated protective effect (+P < 0.05) was also decreased by NF-κB-inhibitory peptide (++P < 0.05), but not by NF-κB inactive peptide (C).
To determine whether NF-κB peptides affect uPA levels, conditioned medium was collected from untreated or cells treated with curcumin, active peptide, curcumin plus active peptide, control peptide, and curcumin plus control peptide, and zymography assays were performed. Results presented in Figure 5B indicate that uPA levels were unaltered with any of the treatments. To determine the effect of NF-κB inhibitors on SS and curcumin-treated cells, RGC-5 cells were treated with SS (2 μM) or SS plus curcumin (6.25 μM), with or without NF-κB inhibitory peptide or inactive control peptide. At the end of 48 hours, cell viability was assessed by MTT assays. Results presented in Figure 5C indicate that the viability of cells treated with SS alone decreased by 68% when compared with untreated cells. When cell viability was compared between the SS-treated and SS plus curcumin–treated cells, viability increased by ∼130% (SS vs. SS and curcumin treated; P < 0.05). In contrast, when cell viability was compared among the SS plus curcumin, SS alone, curcumin alone, and inhibitory peptide–treated cells, viability decreased by ∼92% (SS plus curcumin treated and SS, curcumin, and inhibitory peptide treated; P < 0.05). Cell viability was unaffected when the cells were treated with inhibitory peptide alone or with SS, curcumin, and inactive peptide.
To determine whether NF-κB peptides affects uPA and tPA levels (increased by SS), zymography assays were performed on conditioned medium collected from SS, SS plus curcumin, SS plus inhibitory peptide, SS plus curcumin plus inhibitory peptide, SS plus control peptide, or SS, curcumin, and control peptide-treated cells. Zymography results (Fig. 5D) indicate that none of these treatments had any effect on elevated levels of uPA and tPA observed in SS-treated cells.
Effect of SS and Curcumin on RGCs In Vivo
Since the results on RGC-5 cells indicate that curcumin attenuates SS (and protease)-mediated cell death, in vivo experiments were performed on C57BL/6 mice to investigate whether SS promotes RGC loss, SS induces the expression of proteases, and curcumin attenuates the SS-induced loss of RGCs.
We performed several preliminary experiments to determine the optimal concentrations of SS and curcumin and the optimal time periods for observing loss of RGCs and found that more than 100 nM SS caused severe damage to the entire eye, while less than 12.5 nM caused very little damage to the retina. We found that more than 10 μM curcumin also caused severe damage to the eye, while less than 2.5 μM curcumin had little effect. In addition, we observed that RGC loss did not occur before 48 hours. Therefore, for the in vivo experiments, we chose 12.5 and 100 nM SS, 2.5 and 10 μM curcumin, and 48 and 72 hour time points for further experiments.
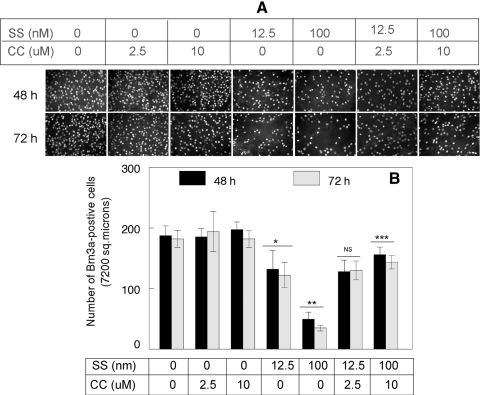
To determine whether SS causes RGC loss in vivo, C57BL/6 mice were treated with intravitreal injections of DMSO (1–2 μL; solvent used to dissolve SS and curcumin), curcumin (2.5 and 10 μM/1 μL), SS (12.5 and 100 nM/1 μL), or both SS and curcumin. At 48 and 72 hours after injection, the eyes were enucleated and fixed in 4% paraformaldehyde, and the whole retinas were immunostained with antibodies against Brn3a to detect the remaining RGCs. Results presented in Figure 6A indicate that at 48 and 72 hours after injection, the density of the Brn3-positive RGCs was reduced significantly in the retinas of SS or SS plus a lower dose of curcumin (2.5 μM)–treated animals, when compared that in placebo-treated or curcumin-treated animals. Furthermore, when the remaining Brn3a-positive RGCs in the retinas were compared between 100 nM SS– and a high-dose curcumin (10 μM) treated animals, an increased number of Brn3a-positive RGCS were observed in the SS- and 10 μM curcumin–treated, but not in the 12.5 nM SS– and 2.5 μM curcumin–treated animals.
Figure 6.
Curcumin attenuated SS-induced loss of retinal ganglion cells. Indicated concentrations of SS, curcumin (CC), or vehicle (DMSO) were injected into the vitreous humor of C57BL/6 mice, and loss of retinal ganglion cells in flat-mounted retinas was determined by immunofluorescence staining with antibodies against Brn3a (A). Immunofluorescence staining and quantification of cells indicate that, whereas Brn3a-positive RGCs remained similar in vehicle or curcumin-treated animals (A, B), Brn3a-positive RGCs were reduced significantly in animals treated with SS alone (*,**P < 0.05). In contrast, SS-induced cell loss was reversed (***P < 0.05) when the animals were treated with SS (100 nM) plus curcumin (10 μM), but not with SS (100 nM) or curcumin (2.5 μM) alone (A, B). NS, not significant (12.5 nM vs. 12.5 nM SS plus 2.5 curcumin).
Quantitative analysis of RGC loss (Fig. 6B) indicated that when compared with DMSO-treated animals (at 48 hours), the number of Brn3a-positive RGCs was reduced by ∼30% and 73% (P < 0.05) in 12.5 and 100 nM SS–treated animals, respectively; at 72 hours, the number of Brn3a-positive cells decreased further by ∼32% and 80% (P < 0.05). When compared between 100 nM SS-treated and 100 nM SS plus 10 μM curcumin–treated animals, the number of Brn3a-positive RGCs was increased further at both at 48 and 72 hours. There was no change in the number of Brn3a-positive cells when compared between 12.5 nM SS and 12.5 nM SS plus 2.5 μM curcumin-treated animals.
The Effect of SS and Curcumin on Amacrine Cells In Vivo
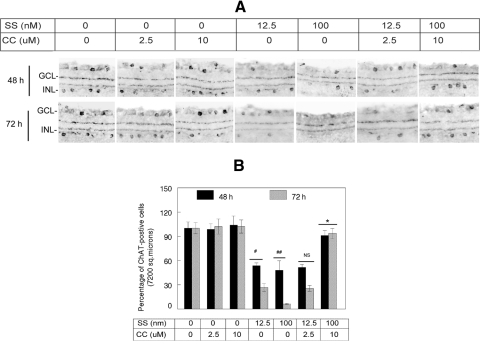
Our previous studies have indicated that once RGC death is initiated in the retina, the death of other cells, in particular amacrine cells, also takes place due to secondary damage. To determine whether SS promotes loss of amacrine cells, we treated C57BL/6 mice with intravitreal injections of DMSO (1–2 μL; solvent used to dissolve SS and curcumin), curcumin (2.5 and 10 μM/1 μL), SS (12.5 and 100 nM/1 μL), or both SS and curcumin. At 48 and 72 hours after injection, the eyes were enucleated and fixed in 4% paraformaldehyde, and retinal cross sections were prepared. Retinal cross sections were then immunostained with antibodies against ChAT, a marker for amacrine cells. For quantitative analysis of ChAT-positive amacrine cells, the total number of cells was counted both in the GCL and the INL; during counting, no attempt was made to differentiate the location of these cells in the GCL and the INL. The results presented in Figure 7 indicate that at 48 and 72 hours after injection, the density of ChAT-positive amacrine cells was reduced significantly in the retinas of SS or SS plus low-dose curcumin (2.5 μM)–treated animals, when compared with placebo- or curcumin-treated animals. Furthermore, when remaining ChAT-positive amacrine cells in the retinas were compared between 100 nM SS and the high-dose curcumin (10 μM)–treated animals, an increased number of ChAT-positive amacrine cells were observed in SS and 10 μM curcumin–treated, but not in 12.5 nM SS and 2.5 μM curcumin–treated animals.
Figure 7.
Curcumin attenuated SS-induced loss of amacrine cells both in the GCL and INL. After injecting indicated concentrations of SS, curcumin (CC), or vehicle (DMSO) into the vitreous humor of C57BL/6 mice, loss of amacrine cells in radial sections was determined by immunofluorescence staining using antibodies against ChAT (A). Immunofluorescence staining and quantification of cells indicate that, whereas ChAT-positive amacrine cells remained similar in vehicle- or curcumin-treated animals (A, B), ChAT-positive amacrine cells were reduced significantly in animals treated with SS alone (#, ##P < 0.05). In contrast, SS-induced cell loss was reversed (*P < 0.05) when animals were treated with both SS (100 nM) and curcumin (10 μM), but not with SS (12.5 nM) and curcumin (2.5 μM). NS, not significant.
Effect of Curcumin and SS on Protease Expression in the Retina
Although a few studies have reported that SS causes the death of RGCs in vivo, the mechanisms underlying SS-induced cell death was unclear. To determine whether SS increases protease levels similar to that observed in SS-treated RGC-5 cells (Fig. 5), retinal proteins were extracted from animals treated with DMSO, curcumin (2.5 and 10 μM), SS (12.5 and 100 nM), and curcumin (12.5 and 10 μM). At the end of 48 and 72 hours after treatment, retinal proteins were extracted and zymography assays were performed. Our previous studies indicated that RGC-5 cells treated with SS secrete elevated levels of uPA and tPA, but not MMP-9, whereas damaged retinas (induced by excitotoxicity) synthesize elevated levels uPA, tPA, and MMP-9.33 Therefore, gelatin zymography assays were performed to detect MMP-9, and fibrinogen–plasminogen zymography assays were performed to detect both uPA and tPA.
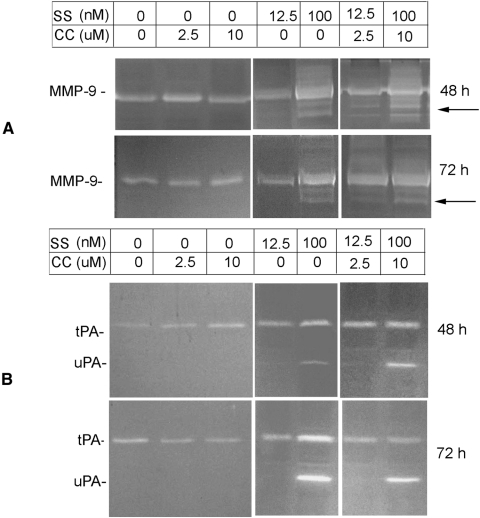
Gelatin zymography assays (Fig. 8A) indicated that a low level of MMP-9 was expressed constitutively in retinal proteins extracted from animals treated with DMSO, curcumin (2.5 and 10 μM), or a low dose of SS (12. 5 nM) both at 48 and 72 hours. In contrast, MMP-9 levels were elevated in retinal proteins extracted from animals treated with a higher dose of SS (100 nM) or SS (100 nM) and curcumin (10 μM). Note that, regardless of the dose, curcumin had no effect on elevated levels of MMP-9. Fibrinogen/plasminogen zymography assays (Fig. 8B) indicate that a low level of tPA (not uPA) was expressed constitutively in retinal proteins extracted from animals treated with DMSO, curcumin (2.5 and 10 μM), or a low dose of SS (12.5 nM) both at 48 and 72 hours. In contrast, uPA and tPA levels were increased in retinal proteins extracted from animals treated with a higher dose of SS (100 nM) or SS (100 nM) and curcumin (10 μM). Interestingly, curcumin, regardless of the dose, had no effect on increased levels of uPA and tPA.
Figure 8.
SS induced the expression of MMP-9, uPA, and tPA in the retina. The indicated concentrations of SS or curcumin (CC) were injected into the vitreous humor of C57BL/6 mice, and retinal proteins were extracted at 48 and 72 hours after injection. By using an equal amount of protein (50 μg), the proteolytic activity of MMP-9 (A) and uPA and tPA (B) was determined by zymography. Zymography indicated that while animals treated with vehicle (DMSO) or with CC (2.5 and 10 μM) expressed a low level of MMP-9 and tPA (but not uPA), animals treated with 100 nM SS expressed elevated levels of MMP-9, tPA, and uPA. Curcumin did not inhibit elevated levels of MMP-9, uPA, and tPA.
Effect of SS on NF-κB Expression in the Retina
Although numerous studies, mostly on tumor cells, showed that curcumin inhibits the activation of NF-κB, a recent study suggests that curcumin exerts biphasic effect on NF-κB activation. In addition, SS has been shown to inhibit the activation of NF-κB. Since the results indicated that a significant number of RGCs in C57BL/6 mice survived despite elevated levels of MMP-9, tPA, and uPA, additional experiments were performed to investigate the effect of SS and curcumin on NF-κB expression in the retina.
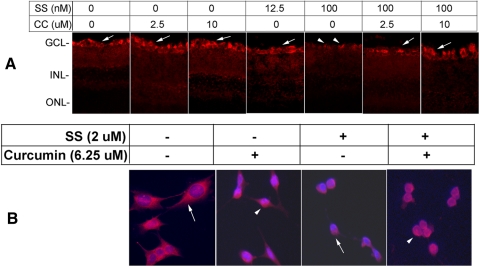
C57BL/6 mice were treated with intravitreal injections of DMSO (1–2 μL), curcumin (2.5 and 10 μM/1 μL), SS (12.5 and 100 nM/1 μL), or both SS (12. 5 and 100 nM) and curcumin (2.5 and 10 μM). At 48 hours after injection, the eyes were enucleated, fixed in 4% paraformaldehyde, processed for frozen retinal cross sections, and immunostained with antibodies against NF-κB. The results presented in Figure 9A indicate that NF-κB was expressed constitutively in the GCL of the control retinal sections. The level of NF-κB immunostaining remained similar to that of the controls in curcumin (2.5 and 10 μM) and 12.5 nM SS–treated animals. In contrast, the NF-κB immunostaining level decreased considerably in the GCL of the retinal cross sections prepared from 100 nM SS–treated animals. Interestingly, when animals were treated with a higher dose of curcumin (10 μM), but not with a lower dose (2.5 μM) the level of NF-κB was to the level observed in control retinal cross sections.
Figure 9.
Curcumin attenuated SS-induced downregulation of NF-κB. Indicated concentrations of SS, curcumin (CC), or vehicle (DMSO) were injected into the vitreous humor of C57BL/6 mice, and NF-κB expression was determined (at 72 hours after injection) by immunofluorescence staining of retinal cross sections with antibodies against NF-κB (A). Immunofluorescence staining indicates that cells in the GCL expressed NF-κB constitutively (arrows). The results also indicate that higher concentrations (100 nM) of SS reduced the expression of NF-κB (arrowheads), whereas lower concentrations (12.5 nM) had little effect. Furthermore, immunofluorescence analysis indicated that, whereas animals injected with higher concentrations of SS expressed very low levels of NF-κB, animals injected with higher concentrations of CC (10 μM) expressed NF-κB similar to that observed in untreated animals or animals treated with CC alone. To determine whether curcumin activates NF-κB in vitro, RGC-5 cells were left untreated or were treated with SS (2 μM) or SS and curcumin (6.25 μM) for 48 hours. At 48 hours after treatment, NF-κB expression was assessed by immunostaining. Results presented in (B) indicate a low level of NF-κB immunoreactivity was present constitutively in the cytoplasm of the RGC-5 cells that were left untreated (arrow); NF-κB expression was reduced further in cells treated with SS (arrow). In contrast when cells were treated with curcumin alone or SS plus CC, NF-κB expression was observed in the nucleus of RGC-5 cells (arrowhead).
To determine whether curcumin also activates NF-κB in RGC-5 cells, the cells were left untreated or treated with curcumin alone (6.25 μM) for 24 hours (Fig. 9B). In addition, cells were treated with SS or SS plus curcumin. Results presented in Figure 9B indicate that a low level of NF-κB immunoreactivity was observed in the cytoplasm of untreated cells (arrow) or cells treated with SS (double arrow). When cells were treated with curcumin alone or SS and curcumin, NF-κB-immunoreactivity was observed in the nucleus, suggesting that curcumin activates NF-κB.
Discussion
Previous studies have indicated that SS causes the death of RGCs in vivo,30–32 but the mechanisms underlying SS-mediated retinal damage were unclear. However, in an in vitro system, we previously showed that SS induces protease levels in transformed RGC-like cells (RGC-5) and that those elevated levels of proteases, in turn, promote the death of RGC-5 cells.28 Therefore, in this study, we investigated whether SS causes RGC death in vivo through elevated levels of proteases, and whether curcumin, a potent free radical scavenger, prevents SS-mediated death of RGC-5 cells in vitro and RGCs in vivo.
We have found that low levels of MMP-9 and tPA proteolytic activities were present constitutively in the retinas of animals treated with DMSO (an agent used to reconstitute SS). When C57BL/6 mice were treated with curcumin alone, MMP-9 and tPA levels were unaltered. In contrast, MMP-9 and tPA levels were increased in the retinas of C57BL/6 mice when treated with SS in a time and dose-dependent fashion. Of interest, uPA, absent in the control retinas was also increased in animals treated with SS. In addition, when animals were treated with both SS and curcumin, elevated levels of MMP-9, tPA, and uPA (induced by SS) were unchanged. Interestingly, despite elevated levels of three proteases, a higher dose of curcumin (10 μM) attenuated the protease-mediated death of RGCs and amacrine cells significantly. Furthermore, when animals were treated with curcumin and SS, NF-κB expression, downregulated in the GCL of SS-treated animals, was restored to the normal levels observed in DMSO-treated animals. Consistent with our previous findings, immunohistochemical analysis indicated that astrocytes expressed MMP-9 and uPA, whereas RGCs expressed tPA (data not shown). These results, for the first time, indicate that SS induces elevated levels of MMP-9, tPA, and uPA, and these proteases, in turn, promote the death of RGCs in C57BL/6 mice.
In vitro results indicate that undifferentiated RGC-5 cells express low levels of uPA constitutively. When cells were treated with SS, RGC-5 cells differentiated and expressed increased levels of both uPA and tPA, consistent with our previous findings.28 Of note, when cells were treated with both SS and various doses of curcumin, lower concentrations of curcumin did not reduce proteolytic activities of uPA and tPA, whereas higher concentrations of curcumin were found to be toxic. Cell viability assays indicated that despite elevated levels of tPA and uPA, RGC-5 cells treated with curcumin survived better and had extensive neurite outgrowth.
To date, there are numerous conflicting reports in the literature questioning the characteristics of the RGC-5 cells line and the methods used to differentiate them; some of those reports confirmed that these cells express markers characteristic of RGCs and others did not. For example, studies from Levin's laboratory (Frasetto et al.36) acknowledged that the RGC-5 cell line expresses neuronal markers characteristic of RGCs including Thy-1, Brn3, neuritin, synaptophysin, NMDA-R1, and GABA receptors.36 Although there is no doubt that RGC-5 cells can be differentiated into neuron-like cells after treating them with SS, a recent study by Wood et al.,37 indicated that RGC-5 cells do not express many (or any) markers characteristic of RGCs unlike the study by Van Bergen et al.,38 who have reported that RGC-5 cells express α- and β-tubulin and Tau, markers for RGCs. A recent report by Fragoso et al.39 clearly showed that RGC-5 cells express many RGC markers, including Brn3a, Thy-1, γ-synuclein, and neurofilament medium polypeptide (NFM). Although expression of Brn3a or Thy-1 can vary depending on the injury, these antibodies have been used by many investigators to identify RGCs in normal and degenerating retinas. Consistent with these findings, the results presented in this study indicate that RGC-5 expressed Brn3 a and Thy-1, markers characteristic of RGCs. These studies show that the issue with RGC-5 cells is not whether they express markers characteristic of RGCs. The expression of markers seems to depend on the culture conditions, method of differentiation, and antibodies used to determine the expression of RGC markers. Yet, we believe that the RGC-5 cell line is a plausible cell line for investigating certain signaling mechanisms involved in the death of RGCs.
NF-κB can be activated by two different pathways: classic and alternative.40 In a classic pathway, NF-κB (consisting of p50 [c-Rel] and p65 [RelA] dimers) is retained in the cytoplasm by binding to inhibitory proteins called IκBs. In response to various stimuli, IκB kinase (IKK) phosphorylates IκBs and targets them to proteasome-mediated degradation, whereas free NF-κB dimers translocate to the nucleus, regulate transcription of target genes, and induce apoptosis of cells through caspase activation. In an alternative pathway, IKK1 phosphorylates p100 protein and produces p52, which then dimerizes with RelB to activate NF-κB. A large body of literature on the central nervous system indicates that NF-κB acts as a regulator of growth, differentiation, neurogenesis,41–43 and growth of neuronal processes in maturing neurons.44–46 On the other hand, NF-κB has also been reported to be associated with neurodegeneration. This intriguing and bifunctional (neuroprotective and neurodegenerative) role of NF-κB has been widely studied in recent years. For example, it has been shown that tumor necrosis factor–mediated NF-κB activation protects hippocampal neurons from oxidative stress,47,48 while inhibition of NF-κB activation potentiates β-amyloid-induced apoptosis in neuronal cells.49 Based on further studies it has been proposed that contrasting effects of NF-κB depend on the type of stimuli and the target tissue. In support of this notion, previous studies have suggested that NF-κB can be neuroprotective when activated in neuronal cells and can be neurodegenerative when expressed in glial cells.50 Consistent with this hypothesis, the results presented in this study indicate that NF-κB is expressed in RGCs and not in astrocytes.
With regard to curcumin, some studies have suggested that curcumin exerts its effect primarily by downregulating NF-κB expression in tumor cells. A recent study, however, suggests that curcumin can either inhibit or activate NF-κB and can be protective or destructive, depending on the intracellular pathways that activate NF-κB.51 Consistent with this hypothesis, results presented in this study suggest that curcumin activates NF-κB in RGCs based on three key findings in this study: (1) curcumin prevented SS-mediated death of transformed RGC-like cells in vitro and RGCs in vivo; (2) curcumin activated NF-κB in the GCL; and (3) curcumin-mediated protective effect on RGC-5 cells was reversed by an NF-κB inhibitor.
How SS causes the death of RGCs and amacrine cells in vivo is unclear. One mechanism by which SS can inhibit NF-κB activation is by reducing IκB levels and by inhibiting Ikk-mediated phosphorylation of IκB.51 Consistent with these reports, results presented in this study indicate that SS downregulated NF-κB expression in both RGC-5 cells and the RGCs of C57BL/6 mice, while curcumin attenuated protease-mediated death of RGCs by restoring NF-κB. Furthermore, we have reported that activation of NF-κB prevents apoptotic death of human trabecular meshwork cells in response to oxidative tress.52 Studies in primates32 and rats30 have shown that SS causes the death of RGCs 2 hours after intravitreal injection. In this study, SS did not cause significant loss of RGCs before 48 hours. The discrepancy of these results is due to the assays used to determine the death of RGCs. Previous studies have used annexin V-labeling to determine the initiation of apoptotic cell death in the retina. In this study, we used antibodies against Brn3a to determine the loss of RGCs. Furthermore, while annexin V-labeling can be observed very early in degenerating retinas, loss of an entire cell body, detected by antibody immunostaining, takes a longer time.
There is one caveat regarding our study. We used an immunofluorescence method to detect NF-κB in the retinas, rather than the classic gel-shift method. It has to be noted that the gel-shift method does not indicate which cells in the retina express NF-κB. Since our focus is to investigate NF-κB activation in the GCL, we believe immunostaining is an appropriate method for this study. In addition, our in vitro and in vivo results showed that SS downregulated NF-κB, whereas curcumin restored its expression to normal levels.
In summary the results presented in this study clearly indicate that activation of NF-κB attenuates SS (and protease)-mediated death of RGCs and amacrine cells and suggest the possibility of using curcumin as a neuroprotective agent for retinal degenerative conditions.
Acknowledgments
The authors thank Xiao Zhang, Mei Cheng, and Omar Yaldo for their technical assistance.
Footnotes
Supported, in part, by National Eye Institutes Project Grant EY017853-01A2 (SKC) and Vision Research Infrastructure Development Grant EY014803.
Disclosure: B. Burugula, None; B.S. Ganesh, None; S.K. Chintala, None
References
- 1. Morrison JC, Dorman-Pease ME, Dunkelberger GR, Quigley HA. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990;108:1020–1024 [DOI] [PubMed] [Google Scholar]
- 2. Johnson EC, Morrison JC, Farrell S, Deppmeier L, Moore CG, McGinty MR. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp Eye Res. 1996;62:663–674 [DOI] [PubMed] [Google Scholar]
- 3. Pena JD, Agapova O, Gabelt BT, et al. Increased elastin expression in astrocytes of the lamina cribrosa in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2001;42:2303–2314 [PubMed] [Google Scholar]
- 4. Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:764–774 [PubMed] [Google Scholar]
- 5. Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783 [PubMed] [Google Scholar]
- 6. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393 [DOI] [PubMed] [Google Scholar]
- 7. Schwartz B. Circulatory defects of the optic disk and retina in ocular hypertension and high pressure open-angle glaucoma. Surv Ophthalmol. 1994;38(suppl):S23–S34 [DOI] [PubMed] [Google Scholar]
- 8. Carter-Dawson L, Crawford ML, Harwerth RS, et al. Vitreal glutamate concentration in monkeys with experimental glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2633–2637 [PubMed] [Google Scholar]
- 9. Wamsley S, Gabelt BT, Dahl DB, et al. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch Ophthalmol. 2005;123:64–70 [DOI] [PubMed] [Google Scholar]
- 10. Siliprandi R, Canella R, Carmignoto G, et al. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8:567–573 [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Neufeld AH. Nitric oxide synthase-2 in human optic nerve head astrocytes induced by elevated pressure in vitro. Arch Ophthalmol. 2001;119:240–245 [PubMed] [Google Scholar]
- 12. Neufeld AH, Liu B. Glaucomatous optic neuropathy: when glia misbehave. Neuroscientist. 2003;9:485–495 [DOI] [PubMed] [Google Scholar]
- 13. Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122:1348–1356 [DOI] [PubMed] [Google Scholar]
- 14. Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000;118:666–673 [DOI] [PubMed] [Google Scholar]
- 15. Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794 [PubMed] [Google Scholar]
- 16. Schwartz M. Vaccination for glaucoma: dream or reality? Brain Res Bull. 2004;62:481–484 [DOI] [PubMed] [Google Scholar]
- 17. Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007;48:1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prasanna G, Hulet C, Desai D, et al. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51:41–50 [DOI] [PubMed] [Google Scholar]
- 19. Cioffi GA. Ischemic model of optic nerve injury. Trans Am Ophthalmol Soc. 2005;103:592–613 [PMC free article] [PubMed] [Google Scholar]
- 20. McKinnon SJ. Glaucoma: ocular Alzheimer's disease? Front Biosci. 2003;8:s1140–s1156 [DOI] [PubMed] [Google Scholar]
- 21. Normando EM, Coxon KM, Guo L, Cordeiro MF. Focus on: amyloid beta. Exp Eye Res. 2009;89:446–447 [DOI] [PubMed] [Google Scholar]
- 22. Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways: a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662 [DOI] [PubMed] [Google Scholar]
- 23. Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321 [DOI] [PubMed] [Google Scholar]
- 24. Hernandez MR, Miao H, Lukas T. Astrocytes in glaucomatous optic neuropathy. Prog Brain Res. 2008;173:353–373 [DOI] [PubMed] [Google Scholar]
- 25. Matteucci A, Cammarota R, Paradisi S, et al. Curcumin protects against NMDA-induced toxicity: a possible role for NR2A subunit. Invest Ophthalmol Vis Sci. 2011;52:1070–1077 [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Cheng M, Chintala SK. Optic nerve ligation leads to astrocyte-associated matrix metalloproteinase-9 induction in the mouse retina. Neurosci Lett. 2004;356:140–144 [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2374–2383 [DOI] [PubMed] [Google Scholar]
- 28. Harvey R, Chintala SK. Inhibition of plasminogen activators attenuates the death of differentiated retinal ganglion cells and stabilizes their neurite network in vitro. Invest Ophthalmol Vis Sci. 2007;48:1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rock N, Chintala SK. Mechanisms regulating plasminogen activators in transformed retinal ganglion cells. Exp Eye Res. 2008;86:492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004;101:13352–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maass A, von Leithner PL, Luong V, et al. Assessment of rat and mouse RGC apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr Eye Res. 2007;32:851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo L, Salt TE, Maass A, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mali RS, Cheng M, Chintala SK. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB J. 2005;19:1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chintala SK, Zhang X, Austin JS, Fini ME. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem. 2002;277:47461–47468 [DOI] [PubMed] [Google Scholar]
- 35. Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, et al. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868 [DOI] [PubMed] [Google Scholar]
- 36. Frassetto LJ, Schlieve CR, Lieven CJ, et al. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438 [DOI] [PubMed] [Google Scholar]
- 37. Wood JP, Chidlow G, Tran T, Crowston JG, Casson RJ. A comparison of differentiation protocols for RGC-5 cells. Invest Ophthalmol Vis Sci. 2010;51:3774–3783 [DOI] [PubMed] [Google Scholar]
- 38. Van Bergen NJ, Wood JP, Chidlow G, et al. Recharacterization of the RGC-5 retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2009;50:4267–4272 [DOI] [PubMed] [Google Scholar]
- 39. Fragoso MA, Yi H, Nakamura RE, Hackam AS. The Wnt signaling pathway protects retinal ganglion cell 5 (RGC-5) cells from elevated pressure. Cell Mol Neurobiol. 2011;31:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436 [DOI] [PubMed] [Google Scholar]
- 41. Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech Dev. 1993;43:135–147 [DOI] [PubMed] [Google Scholar]
- 42. O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258 [DOI] [PubMed] [Google Scholar]
- 43. West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931 [DOI] [PubMed] [Google Scholar]
- 44. Lezoualc'h F, Sagara Y, Holsboer F, Behl C. High constitutive NF-kappaB activity mediates resistance to oxidative stress in neuronal cells. J Neurosci. 1998;18:3224–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Middleton G, Hamanoue M, Enokido Y, et al. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J Cell Biol. 2000;148:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutierrez H, Hale VA, Dolcet X, Davies A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132:1713–1726 [DOI] [PubMed] [Google Scholar]
- 47. Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697 [DOI] [PubMed] [Google Scholar]
- 48. Tamatani M, Che YH, Matsuzaki H, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538 [DOI] [PubMed] [Google Scholar]
- 49. Kaltschmidt B, Uherek M, Wellmann H, Volk B, Kaltschmidt C. Inhibition of NF-kappaB potentiates amyloid beta-mediated neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:9409–9414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860 [DOI] [PubMed] [Google Scholar]
- 51. Grotterod I, Maelandsmo GM, Boye K. Signal transduction mechanisms involved in S100A4-induced activation of the transcription factor NF-kappaB. BMC Cancer. 2010;10:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]