Optical coherence tomographic imaging of swollen optic discs caused by papilledema but not nonarteritic anterior ischemic optic neuropathy causes deformation of the RPE/Bruch's membrane borders of the neural canal. Elevated pressure in the subarachnoid space of the optic canal causes biomechanical alteration of load-bearing structures of the optic nerve head.
Abstract
Purpose.
To examine the biomechanical deformation of load bearing structures of the optic nerve head (ONH) resulting from raised intracranial pressure, using high definition optical coherence tomography (HD-OCT). The authors postulate that elevated intracranial pressure induces forces in the retrolaminar subarachnoid space that can deform ONH structures, particularly the peripapillary Bruch's membrane (BM) and RPE layers.
Methods.
The authors compared HD-OCT optic nerve and peripapillary retinal nerve fiber layer (RNFL) findings in eyes with papilledema caused by raised intracranial pressure to findings in eyes with optic disc swelling caused by optic neuritis and nonarteritic anterior ischemic optic neuropathy (NAION), conditions without intracranial hypertension. The authors measured average thickness of the RNFL and the angle of the RPE/BM at the temporal and nasal borders of the neural canal opening. The angle was measured as positive with inward (toward the vitreous) angulation and as negative with outward angulation.
Results.
Of 30 eyes with papilledema, 20 eyes (67%) had positive RPE/BM rim angles. One of eight optic neuritis (12%) eyes and 1 of 12 NAION (8%) eyes had positive angulation. In five eyes with papilledema, RNFL thickening increased, three of which developed positive RPE/BM angles. On follow-up, 22 papilledema eyes had a reduction of RNFL swelling, and 17 of these eyes had less positive RPE/BM angulation.
Conclusions.
In papilledema, the RPE/BM is commonly deflected inward, in contrast to eyes with NAION or optic neuritis. The RPE/BM angulation is presumed to be caused by elevated pressure in the subarachnoid space, does not correlate with the amount of RNFL swelling, and resolves as papilledema subsides.
Papilledema is caused by increased intracranial pressure. Histopathologic studies in animal models have shown that elevated pressure within the optic nerve sheath causes stasis of axoplasmic flow and decreased blood flow to axons in the neural canal.1–3 The ensuing optic disc swelling (papilledema) is a consequence of axonal distension of the prelaminar and peripapillary nerve fibers. Vascular congestion, leakage, and ischemia follow the acute axoplasmic flow stasis and are associated with interstitial edema. Although the proximate cause of early edema is stagnant axonal transport, there still remains some uncertainty about the relative effects of compression and ischemia.1–3
Burgoyne et al.4 and others5–11 have proposed a conceptual approach that analyzes the optic nerve head (ONH) as a biomechanical structure. They hypothesize that stress (force/cross-sectional area) and strain (local deformation) on the ONH may be key determinants of axonal, glial, and vascular dysfunction in the pathogenesis and progression of glaucoma. Although the forces acting on the nerve head in papilledema differ from those in glaucoma, the application of biomechanical principles may provide insights about the clinical effects of intracranial pressure on the ONH with respect to pathophysiology, etiology, treatment, and monitoring.
The connective tissues of the peripapillary sclera, the lamina cribosa, the sclera neural canal, vessels with adventitia, Bruch's membrane (BM), RPE, and the optic nerve sheath can be considered load-bearing structures of the ONH.11,12 Precisely how intracranial hypertension affects these structures is unknown; however, there is clinical and experimental evidence that biomechanical forces play an important role in the genesis of papilledema including posterior scleral flattening, distension of the nerve sheath,13,14 resolution of papilledema after optic nerve sheath fenestration,15–17 and the occurrence of choroidal and circumpapillary folds.18
Time domain optical coherence tomography (OCT) has been shown to be a clinically useful method of monitoring the severity of optic disc edema by quantifying the thickness of the peripapillary retinal nerve fiber layer (RNFL).19–21 Scott et al.20 has validated OCT methodology by correlating RNFL thickness with corresponding fundus photos using a modified Frisén grading scale. To our knowledge, high speed, high-resolution, spectral-domain OCT (HD-OCT) investigation of the effects of papilledema and intracranial hypertension on subsurface structures of the ONH and peripapillary region has not been reported.
The purpose of this study was to determine how increased intracranial pressure affects the peripapillary subsurface structures of the neural canal opening (NCO) of the ONH at the level of the RPE and basement membrane (RPE/BM) layer using HD-OCT.22 We hypothesize that alterations (both worsening and improvement) in the degree of RNFL thickening associated with intracranial hypertension correlates with the angulation of the RPE/BM at the NCO and that inward angulation of the RPE/BM borders (toward the vitreous) is specific to papilledema with intracranial hypertension but should be unaffected in patients with other forms of disc edema (e.g., optic neuritis and nonarteritic anterior ischemic optic neuropathy [NAION]), where intracranial pressure is normal.
Methods and Subjects
Methods
HD-OCT scans were performed using a Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA). Sharply focused, uniformly illuminated images obtained at baseline and, where possible, at subsequent visits, included optic disc cube 200 × 200 (×2) where the laser scanned a 6- × 6-mm area, capturing of a cube of data consisting of 200 A-scans from 200 linear B-scans of 2 mm per A-scan (40,000 A-scans) and average thickness for the entire circumference of the RNFL was calculated using software provided with the HD-OCT, and the HD 5 Line Raster scan, consisting of five speckle-reduced B-scans of 9 mm long, each consisting of 1024 A-scans (each with an axial depth of 2 mm and 1024 pixels per A-scan). Only scans centered on the optic disc with a signal strength of six or higher were analyzed.
Axial views were evaluated from the individual B-scan (horizontal image) through the center of each optic disc. The horizontal image was 9 mm long for the five-line raster scan and 6 mm long for the optic disc cube scan. The axial image was used to identify the temporal and nasal RPE/BM borders of the neural canal. The angle formed between a line drawn tangential to the curve of the unaltered RPE/BM in the peripapillary retina furthest from the ONH and the altered border adjacent to the NCO was measured on the nasal and temporal sides of the optic nerve (Fig. 1). We evaluated the direction of the RPE/BM angle measured with the image centered on the center of the optic disc and for all horizontal images above and below the midline. Because the angle direction was the same for all images at each NCO border in individual eyes, we analyzed on the image positioned in the middle of the optic disc. The angle was measured along the NCO relative to the RPE/BM, increasing further away from the NCO. We realize that a limitation of our study is that given our 9-mm image use, we did not always reach a nondeformed region of the RPE/BM, but the direction of the angle measured and the changes over time were consistent. The expected position of the RPE/BM was based on the fellow eye for nonpapilledema eyes and based on an interpolated line from the observed outer RPE/BM position in eyes with papilledema. We recognized a limitation in current clinically available methodology related to degradation of signal intensity in deeper layers of the nerve by optic disc edema. This can make evaluation of the architecture of the sclera directly underlying the most edematous portion of the nerve difficult.
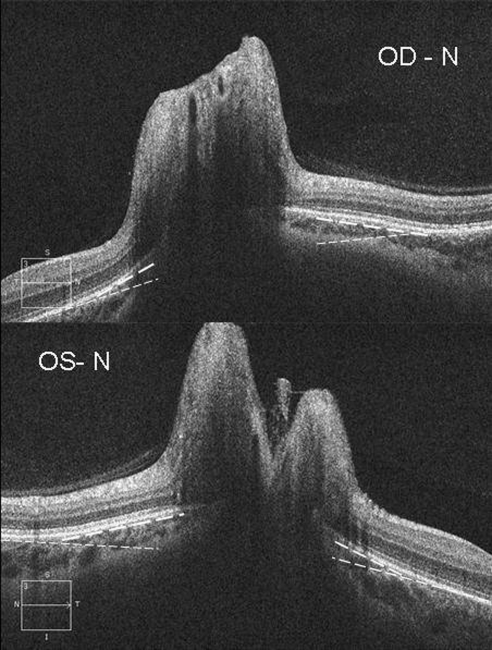
Figure 1.
The right (OD, top) and left (OS, bottom) eye from one patient with papilledema showing inward positive angulation (toward the vitreous) of the peripapillary RPE/BM layer relative to the more peripheral peripapillary regions of the retina (broken lines). Note the angle on the nasal side (N) of the neural canal is greater than on the temporal angle for each eye.
The relative RPE/BM angulation inward or toward the vitreous was measured as a positive angle and outward was measured as a negative angle. For each eye, the angulation was considered positive if there was 5° or greater inward angulation of unadjusted images (measured with Adobe Photoshop [Adobe, San Jose, CA]) of either the temporal or nasal border of the RPE/BM immediately around the ONH. The angle was considered negative if it was less than or equal to 5° and neutral if it fell between the two. The angle was relative and not absolute because the HD-OCT images used for the determinations are scaled differently for the vertical and horizontal dimensions. For a 9-mm HD 5 Line Raster scan, the horizontal dimension is decreased by a factor of three relative to the vertical dimension, because the aspect ratio presented is 2:3, while the true acquired aspect ratio is 2:9. Although this introduces some error—because the conversion for aspect ratio depends on the proportion of horizontal and vertical components—for angles 20° or smaller, the conversion is nearly linear, and the true angle is approximately one-third of the measured angle. For the cube scans, with a 6-mm horizontal extent, the converted angle will be roughly half of the relative angle. To show this mathematically the conversion is expressed as:
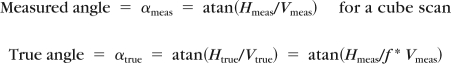 |
where Htrue is the true horizontal extent of the right triangle that defines the angle, Vtrue is the vertical extent, Hmeas is the horizontal extent of the right triangle that defines the measured angle, Vmeas is the vertical extent, and f is the ratio of the aspect ratios, equal to 3 for a 9-mm long B-scan and 2 for a 6-mm long B-scan. Solving for the relationship of the measured angle to the true angle gives:
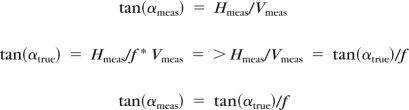 |
For small angles, this implies that the true angle is proportional to the measured angle, so the sign of the angle will be correct. Therefore, in no case should the direction of the angular deviation be affected by this aspect ratio conversion. Because we used unaltered exported images to derive the angle, we adjusted the angle sizes after measurements were obtained from the unaltered images accordingly and used the term relative angle with respect to the plane of the peripheral peripapillary retina away from the nerve head in reporting angle measurements for the study findings. We determined whether the angle was positive, negative, or at neutral (called zero).
A review of 246 subjects (491 eyes with images that could be evaluated) in the INN Neuro-Ophthalmology Cirrus database, who at the time of imaging had no clinically swollen optic nerves or chronic open angle glaucoma (mean age, 53 years [SD, 17]), revealed only nine eyes in five subjects who met our criteria for having positive RPE/BM angulation. Out of one image for each of 491 eyes, the median relative angle for the temporal and nasal borders was –1.0 (SD, 1.7) and –1.0 (SD, 1.6) degrees, respectively, and six eyes (1%) had an angle of 5°. In the 491 eyes and patients in this study, on the unadjusted images, the RPE/BM temporal and nasal angles on repeat scans at each visit were within 5°, and none were positive on one side and negative on the other, nor positive on one and negative on the repeat scan. In addition, we prospectively performed five-line HD scans horizontally through the middle of the optic disc in the right eye of 30 subjects without optic nerve disease. Without correction for the aspect ratio, for the temporal and nasal neural canal angles the mean was –7.2° (SD, 6.6; range, 0 to –22) and –3.8° (SD, 3.7; range, 0 to –15), respectively. No eyes had a positive angle or inward deflection. We used this information to require on unadjusted images at least 5° change for eyes with more than one examination visit over time to determine that the angle was altered. In addition, because the variability for at least two optic cube images per eye at the same examination was <10 μm (data not shown) and the reproducibility of OCT RNFL measurements (shown in many previous peer reviewed journals) is within 10 μm, we required the average RNFL to differ by >10 μm (increase for thickening and decrease for thinning) between visits to determine that the RNFL was changed.
Subjects
This study was conducted with New York Eye and Ear Infirmary Institutional Review Board approval. As part of an exploratory project to develop a protocol for an HD-OCT substudy as part of the National Eye Institute–supported “Idiopathic Intracranial Treatment Trial” conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium, we prospectively evaluated patients with active idiopathic intracranial hypertension ([IIH] modified Dandy criteria) with clinically apparent papilledema (some treated and some at baseline). All subjects were women with a mean age of 34 years.
Once we began to explore the data from the patients with IIH, we prospectively evaluated patients with acute (evaluated within 14 days of vision loss) NAION (using accepted diagnostic criteria) and clinically apparent optic disc swelling (Table 1). We also retrospectively evaluated patients with acute (evaluated within 14 days of vision loss) optic neuritis (meeting accepted clinical criteria, all with magnetic resonance imaging verification), and clinically apparent optic disc swelling. No patient in any group had an IOP >20 mm Hg, and none had signs of glaucoma in either eye.
Table 1.
Patients with Acute NAION and Clinically Apparent Optic Disc Swelling
| Papilledema | NAION | Optic Neuritis | |
|---|---|---|---|
| Age, y | 38 | 60 | 30 |
| Sex | 15 women | 8 women | 5 women |
| Mean RNFL thickness at presentation, μm | 221 (SD, 102) | 260 (SD, 82) | 176 (SD, 59) |
Results
We found a positive angulation of the RPE/BM borders in 20 of 30 eyes (67%) among 10 of the 15 patients with papilledema (Fig. 1). The mean inward RPE/BM angle was +1.5° (SD, 1.5°) temporally and +2.5° (SD, 1.8°) nasally. Among the remaining 10 eyes, the mean inward angle was –1.06° (SD, 2.6°) temporally and –0.92° (SD, 1.6°) nasally. For the 20 eyes with positive angles, the larger angle was nasal in nine eyes (Figs. 2A, 3) and was temporal in four eyes and equal in seven eyes. Temporal and nasal border angles were moderately correlated (r ≥ 0.63; P = 0.01) for each eye and between eyes in each papilledema subject. No patient had positive angulation in one eye and negative angulation in the other. Two patients had a positive angle in one eye and a zero angle in the contralateral eye, despite slightly more RNFL swelling in the zero angle eyes (average RNFL 350 μm and 242 μm vs. 401 μm and 263 μm, respectively).
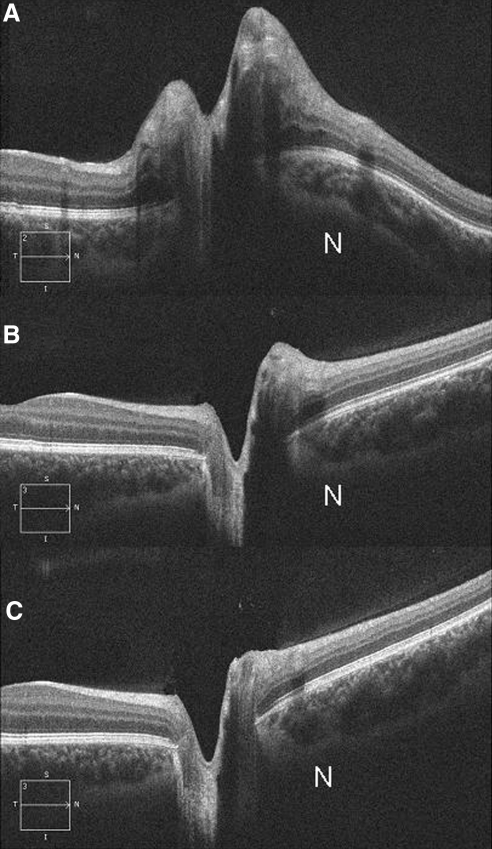
Figure 2.
Sequential HD-OCT of the right eye from a patient with papilledema followed for a period of 12 weeks. (A) Photograph obtained at baseline showing marked elevation of the optic disc, thickening of the RNFL, and inward angulation of the temporal RPE/BM at the margin and severe inward deformation and displacement of the nasal RPE/BM. Note that the sclera/choroid are also deflected inward (worse at nasal border [N]). (B) Photograph obtained 8 weeks after a 15-lb weight loss revealing a significant decrease in disc elevation, RNFL thickening, and straightening of the RPE/BM layer. (C) Photograph obtained at 12 weeks after a 24-lb weight loss reveals resolution of papilledema with a negative RPE/BM. At each time point, the left eye (not shown) had similar findings.
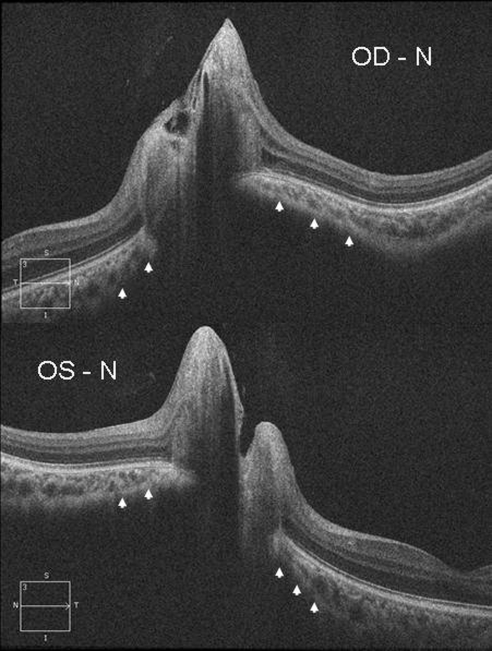
Figure 3.
For each eye (OD, top and OS, bottom), the RPE/BM angle is positive and the sclera is bowed inward (arrowheads). The RPE/BM angle and sclera have more inward angulation on the nasal side (N) of the optic canal of each eye.
There were two patterns of inward angulation. Twelve eyes had a positive RPE/BM angle with mild deformation of the RPE/BM and choroid (Fig. 1) confined to the NCO margin. The second pattern observed in six of the eyes showed a large inward bowing of the RPE/BM and choroid (Figs. 2A, 3) that extended well into the surrounding peripapillary region.
We explored the relationship between changes in the average RNFL thickness and RPE/BM angulation over multiple visits in 13 patients with papilledema. In five eyes, the average RFNL thickness increased (Table 2) and at least one of the RPE/BM angles increased in the inward direction for three of these eyes (Fig. 4). The average RNFL decreased or was less thick (including two eyes that had previously had increasing thickness) in 22 eyes (Table 2) and the RPE/BM angle of at least one border was less positive or became negative in 17 of these eyes (Figs. 2 and 4). For all 30 papilledema eyes, the amount of change in the nasal RPE/BM angle (Spearman r = 0.63; P = 0.01) but not the temporal angle (r = 0.13; P = 0.51) correlated with the change in average RNFL (examples in Figs. 2 and 4). Our prototypical case in Figure 4 shows how changes in optic disc swelling or elevation and RPE/BM angle changes correlated, worsening as medical treatment failed. The optic disc swelling resolved after ventricle shunt (performed for worsening visual fields) placement to reduce the intracranial pressure. The average RNFL and RPE/BM angles also changed in parallel as seen in Figure 4 for our prototypical case. Another example of normalization of optic disc swelling and RPE/BM angle normalization is seen in Figures 2B, 2C after dramatic weight reduction.
Table 2.
Relationship between Changes in the Average RNFL Thickness and RPE/BM Angulation over Multiple Visits in 13 Patients with Papilledema
| No. | No. with More Positive RPE/BM Angle | No. with Less Positive or More Negative RPE/BM Angle | Mean of Average RNFL at Follow-Up | Change in Average RNFL at Follow-Up | |
|---|---|---|---|---|---|
| Eyes where RNFL thickness increased | 5 (3 patients) | 3 | 0 | 252 μm (SD, 80) | 142 μm (SD, 86) |
| Eyes where RNFL thickness decreased | 22 (12 patients) | 0 | 17 | 122 μm (SD, 86) | −110 μm (SD, 73) |
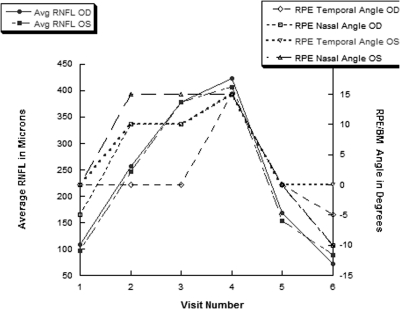
Figure 4.
For our prototypical case, the average RNFL and RPE/BM angles at neural canal borders changed in parallel over six visits. The RPE/BM angles became positive as the RNFL swelling increased. Time point five is 1 week after a ventricular shunt was placed to normalize the intracranial hypertension and reveals normalization of the RPE/BM angles in both eyes.
For papilledema patients, there was no correlation of having positive (inward) with the lumbar puncture opening cerebrospinal fluid (CSF) pressure (mean, 35 cm H20 [SD, 8]) but the CSF pressure determination was frequently remote in time from the first HD-OCT measurements.
One eye out of eight (12%) with optic neuritis had positive angulation (5° for both temporal and nasal borders) and this optic nerve had magnetic resonance imaging evidence of retrobulbar inflammation up to and including the sclera and an average RNLF thickness of 294 μm. The opposite eye did not have a positive angle. Within 2 weeks of the first examination, the average RNFL was 138 μm and both angles were zero.
One eye of 12 (8%) with acute NAION had positive angulation (5° temporal border only) and the unaffected fellow eye also had positive angulation (5° temporal border only). The affected eye had an average RNFL of 275 μm at presentation and 133 μm at 1 month. The angulation did not change in either eye at 1 month.
Among all papilledema, optic neuritis, and NAION eyes, there was no correlation of the amount or direction of RPE/BM angulation with the amount of total RNFL thickening for all etiologies.
Discussion
This study confirms that in addition to revealing peripapillary RNFL thickening associated with papilledema,19–21 HD-OCT is able to detect changes in the subsurface architecture of the ONH and peripapillary region23 (Williams GSN, et al. IOVS 2009;50:ARVO E-Abstract 2253). Papilledema with intracranial hypertension results in an inward bowing of the RPE/BM layer at the NCO not typically seen in normal eyes or other forms of disc edema. Although the sclera is not imaged clearly or completely by HD-OCT, the angulation and displacement of the RPE/BM presumably reflects deformation of the underlying peripapillary sclera and lamina cribosa in response to an elevated pressure gradient between the retrolaminar subarachnoid perioptic nerve sheath compartment and the globe. This deformation is dynamic and in the opposite direction in glaucoma. In experimental acute IOP elevation, outward bowing of the RPE/BM occurs but the amount of displacement is small.24 In chronic glaucoma and chronic experimentally induced elevated IOP, the IOP gradient exceeds the perioptic subarachnoid pressure more than under normal conditions. This may induce changes in load-bearing connective tissues that results in laminar thinning, peripapillary scleral bowing posteriorly, and expansion of the scleral canal.4,25–30
Our findings in humans are consistent with experimental studies in dogs. Using confocal scanning laser tomography, Morgan31,32 has shown that intracranial hypertension displaces the optic disc surface and lamina cribosa anteriorly (toward the vitreous), whereas ocular hypertension displaces the disc surface posteriorly. An increase in CSF pressure resulted in greater anterior displacement of the disc surface than the posterior displacement induced by a corresponding increase in IOP. The normal lamina cribrosa is already slightly bowed backwards (as is the RPE/BM in our controls). This makes it difficult to show an additional increase in the bowing backward until the pressure gradient increases significantly and damages support structures of the ONH, as in untreated glaucoma. However, in the case of raised intracranial pressure, a reversal in the direction of this bowing is seen and may be easier to detect. In addition, the retrolaminar tissue pressure was shown to be determined by the CSF pressure and the translaminar pressure gradient was related to the difference between IOP and CSF pressure across a wide range of both IOP and CSF pressure.
The inward angulation of the peripapillary RPE/BM cannot be explained solely by the degree of disc edema or RNFL thickening. With two exceptions (one with optic neuritis and one with NAION), the inward angulation of the peripapillary RPE/BM layer was confined to those patients with papilledema and absent among those with other forms of disc edema in the patients sampled in this study. The average RNFL thickness did not correlate with a positive RPE/BM angle or the degree of positive angulation or inward bowing of the RPE/BM across all patients with papilledema, optic neuritis, and NAION. However, for individual patients with papilledema, angulation changed with alterations in the RNFL thickness. Seventy-five percent of eyes with papilledema that had a measurable decrease in RNFL thickness became more negative or less positive. In a few cases with increased thickening of the RNFL, there was an associated increase in positive angulation. It is likely that the single case of ischemic optic neuropathy with inward angulation of the RPE/BM border was pre-existent because the unaffected eye had a similar inward angulation and the affected eye remained unchanged after the disc edema resolved. In contrast, in the patient with optic neuritis who had a positive RPE/BM angle (in the affected eye only), the angle became 0° as the RNFL thickening resolved. We have no definitive explanation, but the finding suggests that the inflammation extended into the NCO to alter the inward pressure gradient.
Our findings also suggest that there may be regional differences in the deformation of the peripapillary RPE/BM in patients with papilledema. The mean angle was greater nasally than temporally and the change in the nasal RPE/BM angle but not the temporal angle correlated with the increase in average RNFL. The difference may relate to regional variations in the compliance or elasticity of the lamina cribosa and peripapillary sclera. Downs et al.33,34 has shown that the nasal peripapillary sclera in monkeys is thinner and possibly more compliant than the temporal sclera to extraocular pressure forces. We considered whether the degree of myopia might be another factor to influence the compliance of the RPE/BM border but found no correlation with the presence or degree of positive displacement and refractive error (data not shown).
We cannot explain why 33% of the eyes with papilledema did not exhibit positive angulation or displacement of the RPE/BM. The HD-OCT and lumbar puncture in many of our patients were performed at different times and, in some cases, the HD-OCT was obtained after treatment was initiated. Presently, there are no data correlating the lumbar intracranial pressure with the retrolaminar pressure or the degree of RFNL thickening or the pattern of swelling of the optic disc. Eyes without positive RPE/BM angulation did not have less RNFL swelling. The degree of structural stiffness is a structural property that can vary among eyes and patients that can influence the deformation of the optic nerve. This is determined by a complex interaction of numerous factors, including size, shape of the scleral canal, scleral thickness, orientation and density of the lamina cribosa, scleral insertion of the optic nerve sheath, and material properties of the lamina and peripapillary sclera.4,7,9,11,35 The trabecular architecture of the distal optic nerve may asymmetrically affect translaminar pressure gradients or structural stiffness.36 Therefore, two patients or each eye in the same patient may respond quite differently to the same perioptic nerve sheath pressure. Moreover, the absence of a measurable deformation by HD-OCT does not necessarily indicate an absence of stress and strain on the load bearing structures of the optic nerve.11 Lastly, without knowing the premorbid position of the RPE/BM, we cannot rule out that small incremental inward deformations of the RPE/BM might have occurred.
It has been suggested that that the stability of the RPE/BM position at the neural canal may serve as reference plane from which other structural parameters may be derived.22,37–41 While this may be correct in a slowly progressive condition like glaucoma, our findings in papilledema shows substantial deformations of the peripapillary RPE/BM, in some cases over short time intervals, relative to other surface and subsurface regions of the optic disc. There may be limits to the use of the RPE/BM as a reference plane in patients with intracranial hypertension. The inward angulation of the RPE/BM must be considered when measuring other structural parameters of the optic disc in papilledema.
This preliminary study was intended to determine whether HD-OCT was capable of analyzing additional structural parameters beyond RNFL thickness in patients with papilledema. By its very nature, there are a number of limitations. First, the sample was small and heterogeneous. Some of the patients underwent their HD-OCT assessment after treatment had been instituted, the follow-up was short, and treatment modalities varied. The depth penetration of commercially available HD-OCT is limited and, in the presence of severe disc edema, may be subject to artifacts that shadow subsurface structures. The output display image used to ascertain the angulation from the axial images from the HD-OCT on the Cirrus has an aspect ratio that differs for horizontal and vertical dimensions from the acquisition image. Although the reported angle sizes are calculated and relative, in no case was the direction of the angular deviation altered from the true direction.
This study provides HD-OCT evidence that intracranial hypertension alters the biomechanical environment of the peripapillary ONH at the level of the RPE/BM. The inward angulation of the NCO and the nasal peripapillary displacement of the RPE/BM presumably reflects pressure-induced stress and strain on the surrounding choroid, sclera, and lamina cribosa. Because papilledema resolves with treatment, we have shown an improvement of—and, in some cases, complete reversal of—this deformation that is not explained by the presence of disc edema or thickening of the RNFL alone. Previous studies have shown that intracranial hypertension impedes axonal transport, compromises blood flow, damages axons, and stimulates glial proliferation in the ONH. The extent to which these biomechanical changes in the ONH directly or indirectly affect or modulate cellular function and blood flow has yet to be established. It is also unknown if and how these structural deformations correlate with or predict a decline in vision. Nonetheless, HD-OCT may prove to be a clinically useful adjunct to the study and management of patients with papilledema that warrants further study. Additional studies with greater numbers of subjects will determine whether RPE/BM angulation will be a clinically useful finding to differentiate etiologies of ONH swelling with and without elevated intracranial pressure.
Footnotes
Supported by National Institutes of Health Grants U10 EY017281-01A1 and U10 EY017281-01A1S1 and the Veterans Affairs Rehabilitation Research and Development Division.
Disclosure: M.J. Kupersmith, None; P. Sibony, None; G. Mandel, None; M. Durbin, Zeiss-Meditec, Inc (E); R.H. Kardon, None
References
- 1. Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol. 1977;95:1553–1565 [DOI] [PubMed] [Google Scholar]
- 2. Watson PG, Hayreh SS, Awdry PN. Episcleritis and scleritis. II. Br J Ophthalmol. 1968;52:348–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977;95:1458–1462 [DOI] [PubMed] [Google Scholar]
- 4. Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73 [DOI] [PubMed] [Google Scholar]
- 5. Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci. 2000;41:2991–3000 [PubMed] [Google Scholar]
- 6. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Finite element modeling of optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2004;45:4378–4387 [DOI] [PubMed] [Google Scholar]
- 7. Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46:4189–4199 [DOI] [PubMed] [Google Scholar]
- 8. Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88:799–807 [DOI] [PubMed] [Google Scholar]
- 9. Sigal IA. Interactions between geometry and mechanical properties on the optic nerve head. Invest Ophthalmol Vis Sci. 2009;50:2785–2795 [DOI] [PubMed] [Google Scholar]
- 10. Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85:425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Downs JC, Roberts MD, Burgoyne CF. Mechanical strain and restructuring of the optic nerve head. In: Shaaraway TM, Sherwood MB, Hitchings RA, Crowston JG. eds. Glaucoma: Medical Diagnosis and Therapy. Philadelphia: Saunders Elsevier; 2009:67–90 [Google Scholar]
- 12. Zeimer R. Biomechanical properties of the optic nerve head. In: Drance SM, Anderson DR. eds. Optic Nerve in Glaucoma. Amsterdam: Kugler Publications; 1995:107–121 [Google Scholar]
- 13. Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–1693 [DOI] [PubMed] [Google Scholar]
- 14. Gass A, Barker GJ, Riordan-Eva P, et al. MRI of the optic nerve in benign intracranial hypertension. Neuroradiology. 1996;38:769–773 [DOI] [PubMed] [Google Scholar]
- 15. Knight RS, Fielder AR, Firth JL. Benign intracranial hypertension: visual loss and optic nerve sheath fenestration. J Neurol Neurosurg Psychiatry. 1986;49:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbett JJ, Nerad JA, Tse DT, Anderson RL. Results of optic nerve sheath fenestration for pseudotumor cerebri. The lateral orbitotomy approach. Arch Ophthalmol. 1988;106:1391–1397 [DOI] [PubMed] [Google Scholar]
- 17. Seiff SR, Shah L. A model for the mechanism of optic nerve sheath fenestration. Arch Ophthalmol. 1990;108:1326–1329 [DOI] [PubMed] [Google Scholar]
- 18. Gittinger JW, Jr, Asdourian GK. Macular abnormalities in papilledema from pseudotumor cerebri. Ophthalmology. 1989;96:192–194 [DOI] [PubMed] [Google Scholar]
- 19. Rebolleda G, Munoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:5197–5200 [DOI] [PubMed] [Google Scholar]
- 20. Scott CJ, Kardon RH, Lee AG, Frisen L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128:705–711 [DOI] [PubMed] [Google Scholar]
- 21. Menke MN, Feke GT, Trempe CL. OCT measurements in patients with optic disc edema. Invest Ophthalmol Vis Sci. 2005;46:3807–3811 [DOI] [PubMed] [Google Scholar]
- 22. Strouthidis NG, Yang H, Fortune B, Downs JC, Burgoyne CF. Detection of optic nerve head neural canal opening within histomorphometric and spectral domain optical coherence tomography data sets. Invest Ophthalmol Vis Sci. 2009;50:214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strouthidis NG, Grimm J, Williams GA, Cull GA, Wilson DJ, Burgoyne CF. A comparison of optic nerve head morphology viewed by spectral domain optical coherence tomography and by serial histology. Invest Ophthalmol Vis Sci. 2010;51:1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fortune B, Yang H, Strouthidis NG, et al. The effect of acute intraocular pressure elevation on peripapillary retinal thickness, retinal nerve fiber layer thickness, and retardance. Invest Ophthalmol Vis Sci. 2009;50:4719–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF. Deformation of the normal monkey optic nerve head connective tissue after acute IOP elevation within 3-D histomorphometric reconstructions. Invest Ophthalmol Vis Sci. 2009;50:5785–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan DB, Coloma FM, Metheetrairut A, Trope GE, Heathcote JG, Ethier CR. Deformation of the lamina cribrosa by elevated intraocular pressure. Br J Ophthalmol. 1994;78:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan DB, Flanagan JG, Farra T, Trope GE, Ethier CR. Study of regional deformation of the optic nerve head using scanning laser tomography. Curr Eye Res. 1998;17:903–916 [DOI] [PubMed] [Google Scholar]
- 28. Levy NS, Crapps EE. Displacement of optic nerve head in response to short-term intraocular pressure elevation in human eyes. Arch Ophthalmol. 1984;102:782–786 [DOI] [PubMed] [Google Scholar]
- 29. Levy NS, Crapps EE, Bonney RC. Displacement of the optic nerve head. Response to acute intraocular pressure elevation in primate eyes. Arch Ophthalmol. 1981;99:2166–2174 [DOI] [PubMed] [Google Scholar]
- 30. Bellezza AJ, Rintalan CJ, Thompson HW, Downs JC, Hart RT, Burgoyne CF. Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2003;44:623–637 [DOI] [PubMed] [Google Scholar]
- 31. Morgan WH, Chauhan BC, Yu DY, Cringle SJ, Alder VA, House PH. Optic disc movement with variations in intraocular and cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2002;43:3236–3242 [PubMed] [Google Scholar]
- 32. Morgan WH, Yu DY, Alder VA, et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci. 1998;39:1419–1428 [PubMed] [Google Scholar]
- 33. Downs JC, Blidner RA, Bellezza AJ, Thompson HW, Hart RT, Burgoyne CF. Peripapillary scleral thickness in perfusion-fixed normal monkey eyes. Invest Ophthalmol Vis Sci. 2002;43:2229–2235 [PMC free article] [PubMed] [Google Scholar]
- 34. Downs JC, Ensor ME, Bellezza AJ, Thompson HW, Hart RT, Burgoyne CF. Posterior scleral thickness in perfusion-fixed normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2001;42:3202–3208 [PubMed] [Google Scholar]
- 35. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. 3D morphometry of the human optic nerve head. Exp Eye Res. 2010;90:70–80 [DOI] [PubMed] [Google Scholar]
- 36. Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87:777–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Downs JC, Yang H, Girkin C, et al. Three-dimensional histomorphometry of the normal and early glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. Invest Ophthalmol Vis Sci. 2007;48:3195–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strouthidis NG, White ET, Owen VM, Ho TA, Hammond CJ, Garway-Heath DF. Factors affecting the test-retest variability of Heidelberg retina tomograph and Heidelberg retina tomograph II measurements. Br J Ophthalmol. 2005;89:1427–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strouthidis NG, White ET, Owen VM, Ho TA, Garway-Heath DF. Improving the repeatability of Heidelberg retina tomograph and Heidelberg retina tomograph II rim area measurements. Br J Ophthalmol. 2005;89:1433–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strouthidis NG, Yang H, Reynaud JF, et al. Comparison of clinical and spectral domain optical coherence tomography optic disc margin anatomy. Invest Ophthalmol Vis Sci. 2009;50:4709–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strouthidis NG, Yang H, Downs JC, Burgoyne CF. Comparison of clinical and three-dimensional histomorphometric optic disc margin anatomy. Invest Ophthalmol Vis Sci. 2009;50:2165–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]