A population-based study of Latinos (LALES) evaluated the ability of various glaucoma screening tests to detect glaucoma in high-risk subgroups. When screening persons for glaucoma, the assessment of optic disc according to the cup-to-disc ratio was the optimal test.
Abstract
Purpose.
To evaluate the ability of various screening tests, both individually and in combination, to detect glaucoma in the general Latino population and high-risk subgroups.
Methods.
The Los Angeles Latino Eye Study is a population-based study of eye disease in Latinos 40 years of age and older. Participants (n = 6082) underwent Humphrey visual field testing (HVF), frequency doubling technology (FDT) perimetry, measurement of intraocular pressure (IOP) and central corneal thickness (CCT), and independent assessment of optic nerve vertical cup disc (C/D) ratio. Screening parameters were evaluated for three definitions of glaucoma based on optic disc, visual field, and a combination of both. Analyses were also conducted for high-risk subgroups (family history of glaucoma, diabetes mellitus, and age ≥65 years). Sensitivity, specificity, and receiver operating characteristic curves were calculated for those continuous parameters independently associated with glaucoma. Classification and regression tree (CART) analysis was used to develop a multivariate algorithm for glaucoma screening.
Results.
Preset cutoffs for screening parameters yielded a generally poor balance of sensitivity and specificity (sensitivity/specificity for IOP ≥21 mm Hg and C/D ≥0.8 was 0.24/0.97 and 0.60/0.98, respectively). Assessment of high-risk subgroups did not improve the sensitivity/specificity of individual screening parameters. A CART analysis using multiple screening parameters—C/D, HVF, and IOP—substantially improved the balance of sensitivity and specificity (sensitivity/specificity 0.92/0.92).
Conclusions.
No single screening parameter is useful for glaucoma screening. However, a combination of vertical C/D ratio, HVF, and IOP provides the best balance of sensitivity/specificity and is likely to provide the highest yield in glaucoma screening programs.
Open-angle glaucoma affects approximately 66.8 million people worldwide and is the second leading cause of blindness, affecting 6.7 million people.1 In the United States, it is the leading cause of blindness in African Americans.2 Its prevalence and severity exhibit large differences among various racial and ethnic groups. For example, the prevalence of glaucoma is evident at an earlier age, is four times more common and results in more visual loss in African Americans than in U.S. whites.3,4
Latinos are the largest minority group in the United States and also the fastest growing segment, representing 12.5% (35 million) of the population.5 By 2050, an estimated 25% of the population in the United States will be of Hispanic origin.5 Data from the Los Angeles Latino Eye Study (LALES) suggest that the prevalence of glaucoma in Latinos is similar to that in African Americans.6 In addition, these data indicate that 75% of the glaucoma in Latinos is undiagnosed, compared with an estimated 50% among whites.3 Consequently, there is a need to identify screening tests and parameters that will help develop cost-effective screening strategies in Latinos.
Because the disease is asymptomatic, except in its late stages, many screening programs have been used to try to diagnose the disease in patients at an early stage and thus prevent irreversible vision loss. However, the current standards of screening, including tonometry and visual field examination, have poor sensitivity and specificity.7,8 Even examination of the optic nerve has been less than effective when used as a screening tool, because of the need for trained observers. However, the subjective nature of the examination leading to poor interobserver agreement, even in experts,9 further limits its usefulness as a sole screening measure.
In this article, we evaluate the relative performance of various screening parameters to detect glaucoma, as defined by the three different diagnostic criteria (glaucomatous appearance of the optic nerve alone, glaucomatous visual field, and having both a glaucomatous optic nerve and visual field). Screening tests included (1) Humphrey visual field (HVF) reading by glaucoma experts; (2) HVF parameters such as mean deviation (MD), pattern standard deviation (PSD), and glaucoma hemifield test (GHT); (3) frequency doubling technology (FDT); (4) intraocular pressure (IOP); (5) central corneal thickness (CCT); and (6) optic nerve vertical cup to disc ratio (C/D). Screening tests were evaluated for all participants, as well as for high-risk subgroups based on older age, family history of glaucoma, and presence of diabetes mellitus. To our knowledge, this is the first population-based study in which preset cutoffs were evaluated (as would be used in a population screening program), better cutoffs were developed from the multivariate data analysis via classification and regression tree (CART) analysis, CCT and FDT were included and a high-risk subgroup was analyzed in screening for glaucoma. It is also the first glaucoma screening study in Latinos.
Methods
The study population consisted of participants from the Los Angeles Latino Eye Study, a population-based study of eye disease among Latinos aged 40 years and older living in and around the city of La Puente in Los Angeles County. This research received Institutional Review Board approval, and all procedures adhered to the principles outlined in the Declaration of Helsinki for research involving human subjects. The details of the complete LALES study design and methods are reported elsewhere.10 All eligible participants underwent a detailed, standardized eye examination, including visual acuity, IOP, CCT, HVF testing, simultaneous stereoscopic optic disc photography, OCT imaging, and FDT perimetry.
HVF Testing
Visual field testing was performed with standard achromatic perimetry, using the SITA standard 24-2 program on an automated perimeter (Humphrey Automated Field Analyzer II; Carl Zeiss Meditec, Dublin, CA). If the visual field results were unreliable or abnormal, a repeat SITA standard 24-2 or full-threshold test program was performed. The following criteria were used to determine whether a visual field required a repeat test: GHT borderline, outside normal limits, or generalized reduction in sensitivity or two or more adjacent points depressed to a probability level <5%. An unreliable test result was defined as either false positives or false negatives >33% or fixation losses >50%. A total of 5781 participants had HVF data on both eyes. Three glaucoma experts reviewed the visual fields while masked to other patient data and graded the readings as to the presence and congruence of defects and whether the defects were consistent with or characteristic of glaucoma.
FDT Perimetry
In the FDT (Carl Zeiss Meditec) testing, we used the screening C-20-1 stimulus pattern, which determines contrast sensitivity to detect the frequency-doubling stimulus at 17 locations within the central 20° of the visual field. The pattern consists of sixteen 10° squares (four in each quadrant) and a central 5° circular area. Results of FDT were classified as any defect or no defect.
IOP, CCT, and C/D Ratio
The IOP was measured with Goldmann applanation tonometry by a trained ophthalmic technician with the participant under topical anesthesia with proparacaine 1%. The mean of three measurements rounded to the nearest 1 mm Hg was recorded as the Goldmann IOP. The CCT measurements were performed with an ultrasonic corneal pachymeter (DGH Ophthalmics, Inc., Exton, PA). After instillation of topical proparacaine 1%, the probe was placed on the center of the cornea, and the automatic readings were taken and displayed in micrometers of corneal thickness. An average of three consecutive readings rounded to the nearest micrometer was used for the final value. Horizontal and vertical C/D ratios were determined by a board-certified ophthalmologist, who examined all participants at a slit lamp biomicroscope with a 78-D lens. This assessment was independent of the optic nerve analysis performed by the two glaucoma specialists for the diagnosis of glaucoma.
Diagnosis of Open-Angle Glaucoma
A two-step process was used to diagnose open-angle glaucoma (OAG). First, the clinical history, including any history of treatment for glaucoma, family history of glaucoma, and treatment for other ocular diseases such as cataract, diabetic retinopathy, or age-related maculopathy, was determined. Also, a detailed clinical evaluation of visual acuity, gonioscopy, and IOP and an examination of the anterior and posterior segments of the eye were performed. Gonioscopy was performed by a four-mirror examination (Carl Zeiss Meditec) and all patients with angle closure (grading 1 or less on the Schaffer scale) were excluded from this analysis and were not considered part of the OAG group.
For the second step, two glaucoma specialists reviewed the data, as well as the optic disc photographs and visual fields, to determine a diagnosis of glaucoma. The specialists independently graded the optic disc and visual field for each eye, then arrived at a diagnosis of normal, suspected glaucoma, or glaucoma, based on standardized criteria.6 Optic disc photographs were used only for the glaucoma diagnosis; the vertical C/D ratio used as a screening parameter was measured by a different ophthalmologist during the clinical examination. The FDT data were not used in the diagnosis of glaucoma. The primary definition of OAG required an open angle, visual field, and optic disc damage characteristic or compatible with glaucoma. However, in cases in which glaucomatous optic neuropathy was identified in the absence of visual field abnormality, a diagnosis of glaucoma was made. If the two specialists agreed, the diagnosis was assigned to that specific eye. If the two disagreed, a third glaucoma specialist reviewed the data, and agreement between two of the three specialists was used to assign the diagnosis. Analysis of photographic vertical C/D ratios showed interobserver reliability of 0.89. The κ statistic for agreement between the first and second reviewer for HVF reading was 0.47.
For the purposes of this study, analysis was performed on the basis of three definitions of glaucoma. The first definition required an optic nerve and visual field characteristic of or compatible with OAG. The second required only glaucomatous optic nerve appearance. The third group was defined by the presence of typical and repeatable glaucomatous visual field loss with no other identifiable cause of the defect (such as retinal disease). In the latter two groups, a glaucomatous visual field or optic nerve, respectively, could also be present, but was not required.
High-Risk Groups
Three high-risk groups were defined for analysis: (1) individuals who were 65 years of age or older, (2) individuals with a family history of glaucoma, and (3) individuals with diabetes mellitus. We did not include risk factors that can be determined only by an eye examination, such as high IOP, large vertical C/D ratio, and thin CCT, because in a population glaucoma screening program, these cannot be identified before the examination.
Statistical Analysis
All analyses were conducted on a per-person basis on one eye of each participant. If only one eye had visual field testing, that eye was chosen. If both eyes completed full clinical testing and only one eye was judged to have OAG, then that eye was chosen. If both eyes were found to have OAG, then the eye with the worse MD was chosen. If OAG was not diagnosed in either eye, then one eye was randomly selected.
The following definitions were used as cutoff points for normal versus abnormal screening parameters. The HVF expert reading was classified as glaucomatous or nonglaucomatous by agreement of two of the three glaucoma expert evaluators on the basis of the HVF results only. The HVF parameters were dichotomized into the following categories: (1) false negatives (≥33% vs. <33%); (2) MD and PSD (<5% vs. ≥5%); and (3) GHT (outside normal limits versus within normal, borderline, or generalized reduction in sensitivity). As previously mentioned, FDT perimetry was dichotomized into any defect (mild, moderate, or severe damage) versus no defect. IOP was dichotomized as ≥21 mm Hg versus <21 mm Hg, CCT as ≤504 versus >504 μm, and vertical C/D ratio as <0.8 versus ≥0.8. These cutoffs were chosen based on historical data and generally accepted standards. The CCT value of 504 μm is 1 SD below the mean population CCT in the metaanalysis by Doughty and Zaman.11 The value for vertical C/D ratio was chosen as 2 SD from the mean. Sensitivity and specificity were calculated for each of these parameters for each of the three definitions of glaucoma.
Stepwise logistic regression analyses were performed to evaluate the independent association of each of the screening parameters in predicting the diagnosis of glaucoma using each of the three definitions of glaucoma. To evaluate the ability of the continuous variables that were significantly associated with glaucoma in the stepwise logistic regression models to correctly predict glaucoma or nonglaucoma diagnosis, receiver operating characteristic (ROC) curves were generated, and the areas under the curve (AUC) were calculated. Graphs of sensitivity and specificity according to possible cutoffs of IOP, CCT, and vertical C/D ratio were used to illustrate the trade-offs between sensitivity and specificity in the choice of cutoff. The point where the lines for sensitivity and specificity cross may or may not be the best cutoff. Analyses were conducted at the 0.05 significance level (SAS, ver. 9.2; SAS, Cary, NC).
CART analysis is a recursive method of predicting outcome based on several decision points (nodes) determined by predictor variables that form a tree, with the final nodes containing the outcome prediction.12 In these analyses, we used CART to predict glaucoma based on optic nerve and visual field. We made no distinction between the costs of a false-negative versus a false-positive classification. Potential predictors included all the screening parameters shown in Table 2, but allowed vertical C/D ratio, IOP, and CCT to take on any of their continuous values. A multivariate CART model included all the potential predictors, and completeness was determined by 10-fold cross validation with a maximum of 10 nodes and a minimum node size of 10 for further splitting. No pruning or growing of the tree was preformed. CART analyses were completed by using multivariate analysis (CART ver. 6.0; Salford Systems, San Diego, CA).
Table 2.
Frequency Distribution of Glaucoma Screening Test Parameters Stratified by Preselected Cutoff Values Based on Three Different Definitions of Glaucoma in Participants in the LALES
| Screening Parameters | Preselected Cutoffs | Basis of Glaucoma Diagnosis |
||
|---|---|---|---|---|
| Optic Nerve (n = 270) | Visual Field (n = 231) | Optic Nerve and Visual Field (n = 216) | ||
| HVF, expert reading | Glaucomatous | 174 | 185 | 173 |
| Nonglaucomatous | 96 | 46 | 43 | |
| HVF, false negatives | ≥33% | 11 | 11 | 11 |
| <33% | 257 | 219 | 204 | |
| HVF, MD | <5% | 211 | 205 | 190 |
| ≥5% | 58 | 26 | 26 | |
| HVF, PSD | ≥5% | 177 | 178 | 165 |
| <5% | 92 | 53 | 51 | |
| HVF, GHT | Outside normal limits | 204 | 208 | 195 |
| Other | 65 | 23 | 21 | |
| FDT perimetry | Any defect | 150 | 149 | 138 |
| No defect | 104 | 69 | 68 | |
| IOP | ≥21 mm Hg | 57 | 53 | 52 |
| <21 mm Hg | 212 | 176 | 163 | |
| CCT | ≤504 μm | 38 | 41 | 34 |
| >504 μm | 232 | 190 | 182 | |
| Vertical C/D ratio | ≥0.8 | 155 | 133 | 130 |
| <0.8 | 115 | 98 | 86 | |
Data are the number of subjects fitting each screening parameter.
Results
Of the 6357 participants in the LALES cohort, 215 were unable to travel to the clinic and did not undergo any clinical testing except for an in-home examination, and 60 were unable to complete visual field testing. A total of 6082 participants completed a full glaucoma evaluation consisting of HVF, IOP, CCT, and optic nerve evaluation, and FDT data were obtained on 5854 of the participants. There was no difference in rates of diabetes and family history of glaucoma between those with and without FDT data; however, 19.4% of those with FDT data were 65+ years of age, whereas 30.3% of those without FDT data were 65+ (P < 0.0001).
The demographic characteristics of those included versus excluded are as follows: 58% versus 62% were female (P = 0.18); 20% versus 29% were ≥65 years of age (P = 0.0003); 10% versus 17% had a self-reported history of untreated cataract (P = 0.001); and 3% for each group had a self-reported history of glaucoma (P = 0.99). Means and standard deviations for continuous screening parameters are shown in Table 1.
Table 1.
Values for Various Screening Parameters in Participants in the LALES Population
| Screening Parameters | Mean (SD) |
|---|---|
| HVF: MD, dB | −2.75 (5.03) |
| HVF: PSD | 2.73 (2.21) |
| IOP, mm Hg | 14.5 (3.2) |
| CCT, μm | 550 (35) |
| Vertical C/D ratio | 0.34 (.22) |
Of the 6082 participants, 286 (4.7%) were diagnosed by the glaucoma experts as having open-angle glaucoma; the remaining 5796 (95.3%) eyes were considered nonglaucomatous. The agreement between observers showed a correlation coefficient = 0.89 and a weighted κ = 0.64. Glaucoma based on optic nerve appearance alone (with or without a glaucomatous visual field) was seen in 270 individuals. Glaucoma based on visual field (with or without a glaucomatous optic nerve) was seen in 231 participants. According to the definition of glaucomatous optic nerve and visual field, 216 participants were identified. High-risk participants comprised 37% of the total population sample and 75% of the glaucoma cases based on glaucomatous optic nerve and visual field.
Table 2 displays the univariate association (frequency and prevalence rate) for each of the glaucoma screening parameters for each of the three definitions of glaucoma. Table 3 shows the sensitivity and specificity of each of the screening parameters for the three definitions of glaucoma. Preset cutoffs for screening parameters never resulted in both sensitivity and specificity exceeding 80%. Sensitivities and specificities were quite similar regardless of which definition of glaucoma was used, with the exception of the HVF parameters that showed lower sensitivity when glaucoma was determined based on the optic nerve alone.
Table 3.
Sensitivity and Specificity with 95% CI for Various Glaucoma Screening Test Parameters Based on Three Different Definitions of Glaucoma in the Participants in the LALES*
| Screening Test Parameters | Basis of Glaucoma Diagnosis |
||
|---|---|---|---|
| Optic Nerve (n = 270) Sensitivity (95% CI)/Specificity (95% CI) | Visual Field (n = 231) Sensitivity (95% CI)/Specificity (95% CI) | Optic Nerve and Visual Field (n = 216) Sensitivity (95% CI)/Specificity (95% CI) | |
| Vertical C/D ratio, ≥0.8 | 0.57 (0.52–0.63)/0.98 (0.979–0.986) | 0.58 (0.51–0.64)/0.98 (0.973–0.981) | 0.60 (0.54–0.67)/0.98 (0.975–0.982) |
| HVF GHT | 0.76 (0.71–0.81)/0.72 (0.71–0.73) | 0.90 (0.86–0.94)/0.72 (0.71–0.74) | 0.90 (0.86–0.94)/0.71 (0.71–0.72) |
| HVF MD, <5% | 0.78 (0.74–0.83)/0.65 (0.64–0.66) | 0.89 (0.85–0.93)/0.64 (0.63–0.65) | 0.88 (0.84–0.92)/0.64 (0.63–0.65) |
| HVF expert reading | 0.64 (0.59–0.70)/0.89 (0.88–0.90) | 0.80 (0.75–0.85)/0.89 (0.88–0.90) | 0.80 (0.75–0.85)/0.89 (0.89–0.90) |
| HVF PSD, ≥5% | 0.66 (0.60–0.71)/0.78 (0.77–0.79) | 0.77 (0.72–0.82)/0.78 (0.77–0.79) | .076 (0.71–0.82)/0.78 (0.77–0.79) |
| FDT perimetry | 0.59 (0.53–0.65)/0.79 (0.78–0.80) | 0.68 (0.62–0.75)/0.80 (0.79–0.81) | 0.67 (0.61–0.73)/0.79 (0.78–0.80) |
| IOP, ≥21 mm Hg | 0.21 (0.16–0.26)/0.97 (0.97–0.97) | 0.23 (0.18–0.29)/0.97 (0.97–0.98) | 0.24 (0.18–0.30)/0.97 (0.97–0.97) |
| CCT, ≤504 μm | 0.14 (0.10–0.18)/0.91 (0.90–0.92) | 0.18 (0.13–0.23)/0.91 (0.90–0.92) | 0.16 (0.11–0.21)/0.91 (0.90–0.92) |
| HVF false negatives, ≥33% | 0.04 (0.02–0.06)/0.98 (0.98–0.99) | 0.05 (0.02–0.08)/0.98 (0.98–0.99) | 0.05 (0.02–0.08)/0.98 (0.98–0.99) |
n = 6082.
Sensitivity is the proportion of participants having glaucoma according to the glaucoma diagnostic criteria who test positive by the given screening parameter. Specificity is the proportion of participants not having glaucoma according to the glaucoma diagnostic criteria who test negative by the given screening parameter.
Analysis of the high-risk parameters showed a statistically significant association between diabetes mellitus, age, and family history glaucoma (data not shown). The best balance between sensitivity and specificity was for age greater than 65 years. However, separate analyses of the overall high-risk group (n M= 161) and each of the three high-risk subgroups (n = 65–126) did not show an improvement in the sensitivity and/or specificity performance of the glaucoma screening parameters (Table 4). For example, vertical C/D ratio showed sensitivity and specificity of 0.60 and 0.98, respectively, in the full population group, while sensitivity ranged from 0.58 to 0.63 and specificity from 0.96 to 0.97 in the high-risk subgroups (Table 3).
Table 4.
Sensitivity/Specificity with 95% CI for Various Glaucoma Screening Parameters in High-Risk Subgroups of the LALES*†
| Screening Parameters | History of Diabetes (n = 65/1040)‡ | Family History of Glaucoma (n = 26/488) | Age 65+ (n = 126/1204) | All High Risk (n = 161/2263) |
|---|---|---|---|---|
| Vertical C/D ratio, ≥0.8 | 0.63 (0.51–0.75)/0.97 (0.96–0.98) | 0.58 (0.39–0.77)/0.97 (0.96–0.99) | 0.58 (0.49–0.67)/0.96 (0.95–0.98) | 0.60 (0.53–0.68)/0.97 (0.97–0.98) |
| HVF, PSD, ≥5% | 0.74 (0.63–0.85)/0.69 (0.66–0.72) | 0.73 (0.56–0.90)/0.81 (0.77–0.84) | 0.81 (0.74–0.88)/0.64 (0.61–0.67) | 0.79 (0.73–0.85)/0.70 (0.68–0.72) |
| HVF, GHT | 0.86 (0.78–0.95)/0.61 (0.58–0.64) | 0.81 (0.66–0.96)/0.72 (0.68–0.76) | 0.96 (0.93–0.99)/0.56 (0.53–0.59) | 0.91 (0.87–0.96)/0.62 (0.60–0.64) |
| HVF, MD, <5% | 0.94 (0.88, 1.00)/0.50 (0.47–0.53) | 0.81 (0.66–0.96)/0.69 (0.65–0.74) | 0.90 (0.85–0.96)/0.45 (0.42–0.48) | 0.91 (0.87–0.96)/0.53 (0.51–0.55) |
| HVF expert reading | 0.75 (0.65–0.86)/0.84 (0.82–0.86) | 0.85 (0.71–0.98)/0.90 (0.88–0.93) | 0.83 (0.76–0.89)/0.80 (0.77–0.82) | 0.81 (0.75–0.87)/0.84 (0.82–0.85) |
| FDT perimetry | 0.73 (0.61–0.85)/0.66 (0.63–0.70) | 0.52 (0.32–0.72)/0.82 (0.78–0.85) | 0.70 (0.62–0.78)/0.61 (0.58–0.64) | 0.71 (0.64–0.78)/0.69 (0.67–0.71) |
| (IOP, ≥21 mm Hg) | 0.20 (0.10–0.30)/0.96 (0.95–0.97) | 0.23 (0.07–0.39)/0.95 (0.92–0.97) | 0.26 (0.19–0.34)/0.95 (0.94–0.97) | 0.27 (0.20–0.34)/0.96 (0.95–0.96) |
| (CCT, ≤504 μm) | 0.14 (0.05–0.22)/0.92 (0.90–0.94) | 0.19 (0.04–0.34)/0.91 (0.89–0.94) | 0.18 (0.12–0.25)/0.89 (0.87–0.91) | 0.17 (0.11–0.23)/0.91 (0.89–0.92) |
| HVF, false negatives (≥33%) | 0.06 (0–0.12)/0.97 (0.96–0.98) | 0.08 (0–0.19)/0.99 (0.98, 1.00) | 0.07 (0.03–0.12)/0.96 (0.95–0.97) | 0.06 (0.03–0.10)/0.97 (0.97–0.98) |
Sensitivity is the proportion of participants who had glaucoma according to the glaucoma diagnostic criteria and who tested positive by the given screening parameter. Specificity is the proportion of participants not having glaucoma according to the glaucoma diagnostic criteria, who tested negative for the given screening parameter.
The definition of glaucoma is based on glaucomatous optic nerve and visual field examinations.
The numerator is the number of glaucoma cases among those with the risk factor; the denominator is the total number with that risk factor.
A stepwise logistic regression analysis was performed to identify independent predictors of glaucoma diagnosis (data not shown). The best predictor for each category of glaucoma diagnosis was the vertical C/D ratio. The rank order varied depending on the glaucoma category, but all models included vertical C/D ratio, HVF visual field result, IOP, FDT, and GHT. CCT was a significant predictor only in the model based on a definition of glaucoma by visual field alone.
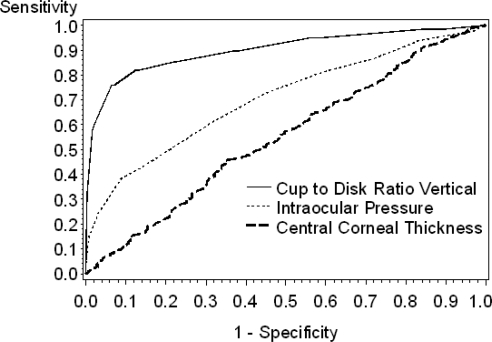
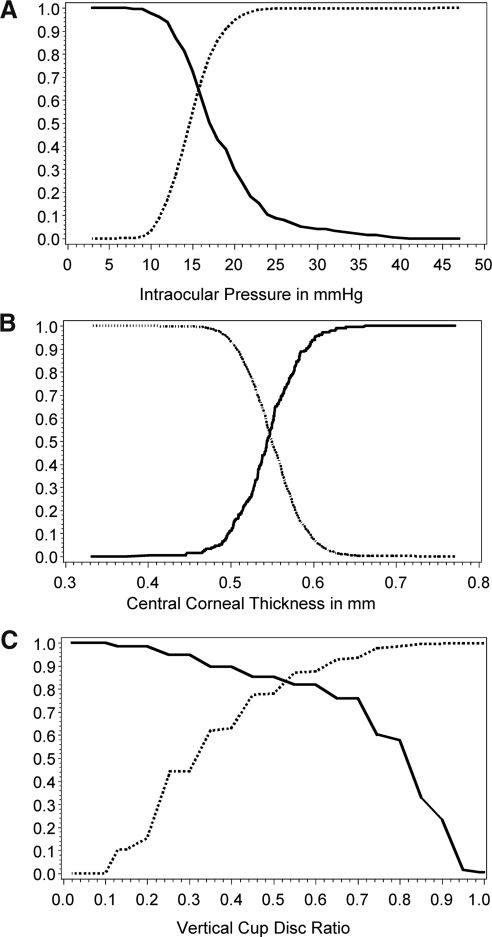
The AUC for the ROC for continuous screening parameters are summarized in Table 5 for the study cohort and the high-risk subgroups. The AUCs for the three independent predictors were vertical C/D ratio = 0.900, IOP = 0.705, and CCT = 0.549 (Fig. 1). Pair-wise comparisons revealed significant differences in the AUCs (P < 0.0001). A more in-depth analysis of sensitivity and specificity for continuous variables is presented in Figure 2. These represent calculations of continuously varying sensitivity and specificity at different cutoff points in the vertical C/D ratio, IOP, and CCT. Of note, the best balance achieved by IOP reading (Fig. 2A) was between 15 and 16 mm Hg, suggesting the need for vigilance for glaucoma presenting with IOP in the mid teens. The best balance for CCT (Fig. 2B) was at 559 μm; but as with IOP, this still resulted in poor sensitivity and specificity. The best balance for vertical C/D ratio as a single parameter (Fig. 2C) was achieved between 0.5 and 0.6.
Table 5.
AUC for Glaucoma Screening Parameters in High-Risk Subgroups of the LALES
| Screening Parameter | History of Diabetes (n = 1040) | Family History of Glaucoma (n = 488) | Age >65 y (n = 1204) | Any High Risk Group (n = 2263) | Total Population (N = 6082) |
|---|---|---|---|---|---|
| Vertical C/D ratio | .918 | .900 | .885 | .895 | .900 |
| MD | .826 | .833 | .799 | .835 | .861 |
| IOP | .612 | .624 | .668 | .668 | .705 |
| CCT | .586 | .553 | .572 | .578 | .549 |
| FN | .674 | .666 | .588 | .612 | .646 |
| PSD | .809 | .860 | .806 | .835 | .868 |
n, total number of participants with the risk factor. The ROC for each screening parameter plots sensitivity on the y-axis and 1 - specificity on the x-axis. The sensitivity and specificity of each potential cutoff for the screening parameter is plotted to give a curve extending from the lower left corner to the upper right corner of the graph. A perfectly diagonal line would indicate that any gain in sensitivity is exactly offset by a decrease in specificity. A better performing test would rise rapidly so that the curve approaches the upper left corner of the figure. In the case of a diagonal line, the AUC would be 0.5; the maximum AUC would be 1.0. There were modest differences in the AUCs for each of the risk groups, but regardless of risk group, vertical C/D ratio is consistently the best performing parameter. MD and PSD show relatively good AUCs, but were not independent predictors in our logistic regression model, whereas IOP and CCT show rather weak AUCs, but were independent predictors in the logistic regression model, indicating that MD and PSD, but not IOP and CCT, are closely associated with other independent predictors in the model.
Figure 1.
ROC curves for continuous parameters identified as independently associated with a diagnosis of glaucoma based on characteristic glaucomatous optic nerve and visual field changes.
Figure 2.
Sensitivity and specificity for continuous parameters identified as independently associated with a diagnosis of glaucoma based on both a characteristic glaucomatous optic nerve and visual field: (A) IOP, (B) CCT, (C) vertical C/D ratio. Solid line: sensitivity; dotted line: specificity.
The results of the CART analysis are shown in Figure 3. The CART model revealed that the best initial screening variable was vertical C/D ratio, using the CART-based cutoff of 0.7. If an individual has a vertical C/D ratio <0.7, the second screening variable was expert assessment of the visual field. If the HVF was normal, then the individual was judged not to have glaucoma; if the HVF was abnormal, then the third screening variable was IOP, using the CART-based cutoff of 14 mm Hg. If an individual had IOP ≥14 mm Hg, then the individual was judged to have glaucoma; otherwise the individual was glaucoma-free.
Figure 3.
CART algorithm for screening for glaucoma. The vertical C/D ratio with a cutoff of 0.7 was identified as the best initial parameter for classifying individuals as likely to have glaucoma or nonglaucoma. Those with vertical C/D ratio <0.7 are then further classified according to whether they had a normal versus abnormal visual field. Those with normal visual field are classified as not having glaucoma. Those with an abnormal visual field are further separated into those with IOP <14 mm Hg (classified as not having glaucoma) or those with IOP ≥14 mm Hg (classified as having glaucoma). Participants with vertical C/D ratio ≥0.7 are classified according to HVF analyzer GHT results. Those with abnormal GHT were classified as having glaucoma, whereas those with normal GHT are further classified by expert visual field assessment as normal (not having glaucoma) or abnormal (having glaucoma). The algorithm illustrated here has both a sensitivity and specificity of 0.92. Fifty-two participants are not included in this analysis due to missing data on one or more of the parameters.
If an individual had a vertical C/D ratio ≥0.7, the second screening variable was based on the GHT. If this was abnormal, then the individual was given a diagnosis of glaucoma. Otherwise, an HVF test was needed to make the decision. As before, if the HVF results were normal, then the individual did not have glaucoma; but if the HVF was abnormal, the individual was judged to have glaucoma, and IOP was not needed. Sensitivity, specificity, and AUC for the CART model were excellent (0.92, 0.92, and 0.96, respectively). These values were almost identical for the training and the validation subgroups (0.89, 0.92, and 0.95, respectively).
CART results for the high-risk subgroup were similar, with the initial branch determined at the same vertical C/D ratio cutoff of 0.7. Although the full tree results were simpler for the high-risk subgroup than for the total population, the model fit remained high (sensitivity = 0.98, specificity = 0.80, and AUC = 0.93).
Discussion
We analyzed the ability of various screening tests (visual field testing, vertical C/D ratio, FDT, IOP, and CCT) to diagnose glaucoma in a large population-based study of Latinos (LALES) and in three subgroups of high-risk subjects: those with a positive family history of glaucoma, history of diabetes, or age ≥65 years. This is the first population-based glaucoma screening to include such a wide variety of screening parameters (including FDT and CCT) and to separately analyze groups of high-risk individuals to determine whether the performance of screening tests can be improved if applied to these subgroups. We used the CART analysis to find the ideal cutoffs for each of the continuous parameters. The appeal of CART is that it enables consideration of several potential predictor variables to arrive at a final prediction of outcome through the use of a decision tree which is easy to understand and implement. Furthermore, CART can fully account for complex interactions of the variables. The final classification by CART has a clearly defined sensitivity and specificity, and thus, comparisons with other predictive models can be readily made.
The diagnosis of glaucoma requires expert evaluation, which cannot be replicated in a setting appropriate for large-scale population screening. Potential screening parameters would be factors that the ophthalmologist would consider when making a diagnosis. No matter how objectively or independently these screening parameters are measured, there will necessarily be bias toward any parameter that the ophthalmologist considers to be highly important in making the diagnosis.
This study included a large proportion of the eligible population of LALES (95.7%) and is representative of the study population.6,10 To reduce bias toward any one screening parameter, we created three separate definitions of glaucoma from the original glaucoma cohort for analysis. As expected, we found that vertical C/D ratio performed equally well as a screening parameter, regardless of the method used to define glaucoma, but that the visual field parameters had decreased sensitivity when the definition of glaucoma was based on the appearance of the optic nerve alone.
We used the third group (in which glaucoma was defined by the requirement of both a glaucomatous optic nerve and a glaucomatous visual field) to assess the performance of each of the screening parameters in high-risk groups, defined by an age of 65 years or older, history of diabetes mellitus, and family history of glaucoma. The screening parameters performed similarly in each of the high-risk groups and in the full study population. A total of 37% of study participants and 75% of glaucoma cases (identified with glaucomatous optic nerve and glaucomatous visual field) fell into one or more of the high-risk groups.
Vertical C/D Ratio
When we used a predetermined cutoff of ≥0.8 for vertical C/D ratio, there was excellent specificity (0.98) but moderate sensitivity (0.57–0.60). Using the cutoff point of 0.8 revealed that vertical C/D ratio showed the best positive likelihood ratio of 28.24 (the probability of testing positive for glaucoma when glaucoma is present divided by the probability of testing positive for the disease when glaucoma is not present) compared with CCT at 1.73 and IOP at 8.13. In general, a more stringent screening cutoff would result in higher positive likelihood ratios. Likelihood ratios can be calculated directly from sensitivities and specificities and, unlike false-positive and -negative rates, have the advantage of not being dependent on the proportion of ocular disease in the population. They also can be used with pretest probabilities of disease to calculate posttest probabilities of disease. However, when we used a lower cutoff of ≥0.7 (as suggested by the CART analysis) as a single screening measure, the sensitivity, while significantly improved (from 0.60 to 0.76), was still not optimal, and the specificity was reduced (from 0.98 to 0.94). Thus, whereas using a vertical C/D ratio cutoff of 0.7 allowed identification of glaucoma in more participants, with only a small increase in the rate of false positives, there would continue to be a significant number of individuals with glaucoma who would remain undetected if the vertical C/D ratio were used as a single screening measure.
Central Corneal Thickness
Because the Ocular Hypertensive Treatment Study reported that CCT is a significant independent risk factor in the development of glaucoma13,14 and CCT has been identified as a risk factor for advanced glaucomatous damage,15 we included CCT in our analysis. The high specificity indicates that low CCT is a significant risk factor for glaucoma; but the low sensitivity suggests that a large number of persons with glaucoma are likely to be undetected if this is used as a screening measure.
HVF Perimetry
We found HVF SITA standard testing to be associated with the diagnosis of glaucoma but with a relatively low sensitivity and good specificity. Based on the logistic regression analysis and CART results, we concluded that visual field expert reading and GHT were useful tools in glaucoma diagnosis but were inadequate as single screening parameters.
FDT Perimetry
FDT perimetry, based on the premise that glaucomatous patients may be less able to detect the frequency-doubling phenomenon in the early stages of the disease,16,17 can identify defects in patients that are not apparent with standard automated perimetry.18–23 In our study, FDT perimetry showed fair sensitivity and specificity, and an FDT defect was identified as the fourth or fifth significant predictor of glaucoma on stepwise logistic regression analysis. Overall, our experience with the FDT as a screening device did not replicate the promising results noted in other studies.20–23
Intraocular Pressure
As expected, IOP was correlated highly with glaucoma and maintained a high ranking in stepwise logistic regression analysis; but IOP was not a good screening parameter on its own. IOP showed excellent specificity but very poor sensitivity, indicating that if an IOP cutoff of 21 mm Hg is used as a screening measure, a large number of individuals with glaucoma will not be detected. The multivariate CART found a cutoff of 14 mm Hg in those with an abnormal visual field to be the best indicator of glaucoma. This finding underscores the need for screening in persons with IOP in the mid teens. Similarly, a significant number of false positives based on IOP reflect the presence of persons with ocular hypertension without glaucoma. These findings regarding the poor performance of IOP as a screening parameter for glaucoma in a population-based study are consistent with those of the Baltimore Eye Survey.24
Screening High-Risk Populations
The glaucoma screening parameters were also evaluated on subgroups of the population meeting criteria for a high-risk population based on age, family history of glaucoma, and history of diabetes. The analysis of these subgroups showed a very similar result to the general population and did not improve the performance of the screening parameters. Although the high-risk subgroups, being of smaller size than the full study cohort, are subject to less certainty in statistical estimates, these results suggest that screening of high-risk groups based on these criteria may not improve over screening of the general population over age 40. This is not to suggest that patients in these risk groups should not be evaluated, but that a general screening may not be as effective as individual examinations by a trained ophthalmologist. That sensitivities and specificities did not vary by high-risk subgroup suggests that any possible bias due to the excluded subjects (who, although small in number, tended to be older than the included population) would be minor.
Positive likelihood ratios for the high-risk subgroups based on history of diabetes, family history of glaucoma, age 65+, and any high-risk classification were 21.21, 20.50, 16.44, and 21.83 for vertical C/D ratio; 5.11, 4.24, 5.68, and 6.05 for IOP; and 1.71, 2.17, 1.68, and 1.81 for CCT, respectively.
Comparison with Other Studies
Glaucoma screening has historically been challenging because of a poor balance of sensitivity and specificity of screening procedures. IOP, automated visual field testing, frequency-doubling perimetry, optic disc evaluation, and nerve fiber analysis by photographs and automated methods have all been evaluated as potential screening devices, with variable results.
The meta-analysis performed by Mowatt et al.25 reviewed 40 studies of nine screening tests for the detection of glaucoma. In judging the performance of these tests by diagnostic odds ratios, these researchers found that FDT, oculokinetic perimetry, and HRT II (Retinal Tomograph II; Heidelberg Engineering, Heidelberg, Germany) were promising tests, whereas ophthalmoscopy, standard automated perimetry, retinal photography, and IOP performed relatively poorly as single tests. They concluded that no test or group of tests was clearly superior for glaucoma screening.
The Baltimore Eye Survey was a population-based study that evaluated IOP, vertical C/D ratio, and narrowest neuroretinal rim width as screening parameters for glaucoma.24 The investigators found no cutoff points that gave a good balance between sensitivity and specificity for any parameter or combination thereof—this despite the use of expert evaluation of stereoscopic disc photography as one of the screening parameters.
A study in the United Kingdom found that by adding visual field results, optic disc evaluation, and IOP, a sensitivity and specificity of >0.9 were obtained.26 Tests of visual function such as visual acuity, contrast sensitivity, and color discrimination did not improve the model. However, this was not a population-based glaucoma screening study, since the participants included a small group of persons without glaucoma and about half that number with suspected glaucoma. Thus, the results cannot be directly applied to population screening for glaucoma, because most persons do not have glaucoma.
In our study, while the vertical C/D ratio has the best balance of specificity and sensitivity, it does not achieve levels that would make it a useful screening test for population-based glaucoma screening in the general population or in high-risk subgroups. Nevertheless, we have shown that a combination of screening tests can be used in a simple decision algorithm to screen and identify persons with glaucoma, with high sensitivity and specificity. While our results do not suggest that glaucoma screening in Latino populations is materially different from screening in other populations, that possibility should be considered when extrapolating our results to other population groups. We calculated likelihood ratios which have the advantage of consistency across different levels of disease prevalence in the population being tested. However, there are also known differences in the levels of these screening parameters seen in various populations. For example, even within the Latino community, Latinos in Arizona (Proyecto VER) had higher levels of IOP among participants with OAG than were found in Latinos in Los Angeles among participants with OAG. It is not clear whether this difference is due to different study methods in measurement or determining glaucoma or to population differences within the Latino community (e.g., percentage with Native American ancestry, which was substantially different in the two studies). Given these limitations, an appropriate interpretation of these results is that our study is exploratory and provides a different methodological approach to screening for glaucoma. The use of a multiparameter algorithm such as we have developed herein may eliminate some of the ambiguity found with single-parameter evaluations; however, further studies are necessary to validate these findings. Indeed, a generalized algorithm may include race/ethnicity as a parameter subject to the CART decision process. A remaining limitation of this algorithm, however, is that from a practical standpoint, not all the required parameters may be readily available. In a future analysis, we propose an objective measure of optic disc parameters with automated testing to determine whether there is good a balance between sensitivity and specificity. The strategy of screening a high-risk population neither improved nor detracted from the sensitivity and specificity of the various glaucoma screening parameters, suggesting that a screening program would be equally effective in both settings.
In summary, we have validated the findings of previous studies highlighting that no single parameter is highly sensitive and specific for population-based screening of glaucoma. However, a combination of IOP, HVF, and optic disc assessments used in the algorithmic manner described herein yields the best approach to screening for glaucoma. Other population-based studies should develop similar algorithmic approaches to diagnosis based on parameters that are specific and applicable to their populations.
Acknowledgments
The authors thank the LALES External Advisory Committee (members shown in the Appendix) for their advice and contributions.
Appendix
The Los Angeles Latino Eye Study Group
University of Southern California: Rohit Varma (Principal Investigator), Sylvia H. Paz (Project Director), LaVina Abbott, Stanley P. Azen, (Co-principal Investigator), Lupe Cisneros, Carolina Cuestas, Elizabeth Corona, Denise R. Globe, Sora Hahn, Mei Lai, George Martinez, Susan Preston-Martin, Ronald E. Smith, Mina Torres, Natalia Uribe, Joanne Wu, and Myrna Zuniga
Battelle Survey Research Center, St. Louis, MO: Sonia Chico, Lisa John, Michael Preciado, and Karen Tucker
University of Wisconsin: Ronald Klein, S. Tiffany Jan, Stacy M. Meuer, Scot E. Moss, Michael W. Neider, and Sandra C. Tomany
External Advisory Committee: Roy Beck (Chairman), Natalie Kurinij, Leon Ellwein, Helen Hazuda, Eve Higginbotham, Lee Jampol, M. Cristina Leske, Donald Patrick, and James M. Tielsch
Footnotes
Supported by Grants U10 EY-11753 and EY-03040 from the National Eye Institute, Bethesda, Maryland, and by an unrestricted grant from Research to Prevent Blindness, Inc, New York, New York.
Disclosure: B.A. Francis, None; R. Varma, None; C. Vigen, None; M.-Y. Lai, None; J. Winarko, None; B. Nguyen, None; S. Azen, None
References
- 1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–1417 [DOI] [PubMed] [Google Scholar]
- 3. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374 [PubMed] [Google Scholar]
- 4. Leske MC. The epidemiology of open-angle glaucoma: a review. Am J Epidemiol. 1983;118:166–191 [DOI] [PubMed] [Google Scholar]
- 5. U.S. Census Bureau: Census 2000: U.S. demographic profile and population center. Projections of the total resident population by 5-year age groups, race and Hispanic origin with special age categories. Washington, DC: U.S. Census Bureau; 2003. (NP-T4-F). Available at www.census.gov/population/pop-profile/2000/chap02.pdf [Google Scholar]
- 6. Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448 [DOI] [PubMed] [Google Scholar]
- 7. Eddy DM, Sanders LE, Eddy JF. The value of screening for glaucoma with tonometry. Surv Ophthalmol. 1983;28:194–205 [DOI] [PubMed] [Google Scholar]
- 8. Power EJ, Wagner JL, Duffy DM. Screening for open-angle glaucoma in the elderly. Washington, DC: Office of Technology Assessment, Congress of the United States; 1988 [Google Scholar]
- 9. Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992;99:215–221 [DOI] [PubMed] [Google Scholar]
- 10. Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–1131 [DOI] [PubMed] [Google Scholar]
- 11. Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408 [DOI] [PubMed] [Google Scholar]
- 12. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–181 [DOI] [PubMed] [Google Scholar]
- 13. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713 [DOI] [PubMed] [Google Scholar]
- 14. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720 [DOI] [PubMed] [Google Scholar]
- 15. Herndon LW, Weizer JS, Stinnet SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004;122:17–21 [DOI] [PubMed] [Google Scholar]
- 16. Quigley HA. Identification of glaucoma-related visual field abnormality with the screening protocol of frequency doubling perimetry. Am J Ophthalmol. 1998;125:819–829 [DOI] [PubMed] [Google Scholar]
- 17. Yamada N, Chen PP, Mills RP, et al. Screening for glaucoma with frequency-doubling technology and Damato campimetry. Arch Ophthalmol. 1999;117:1479–1484 [DOI] [PubMed] [Google Scholar]
- 18. Burnstein Y, Ellish NJ, Magbalon M, Higginbotham EJ. Comparison of frequency doubling perimetry with Humphrey visual field analysis in a glaucoma practice. Am J Ophthalmol. 2000;129:328–333 [DOI] [PubMed] [Google Scholar]
- 19. Tatemichi M, Nakano T, Tanaka K, et al. Performance of glaucoma mass screening with only a visual field test using frequency-doubling technology perimetry. Am J Ophthalmol. 2002;134:529–537 [DOI] [PubMed] [Google Scholar]
- 20. Wadood AC, Azuara-Blanco A, Aspinall P, Taguri A, King AJW. Sensitivity and specificity of frequency-doubling technology, tendency-oriented perimetry, and Humphrey Swedish interactive threshold algorithm-fast perimetry in a glaucoma practice. Am J Ophthalmol. 2002;133:327–332 [DOI] [PubMed] [Google Scholar]
- 21. Paczka JA, Friedman DS, Quigley HA, Barron Y, Vitale S. Diagnostic capabilities of frequency-doubling technology, scanning laser perimetry, and nerve fiber layer photographs to distinguish glaucomatous damage. Am J Ophthalmol. 2001;131:188–197 [DOI] [PubMed] [Google Scholar]
- 22. Wang YX, Xu L, Zhang RX, Jonas JB. Frequency-doubling threshold perimetry in predicting glaucoma in a population-based study: the Beijing Eye Study. Arch Ophthalmol. 2007;125:1402–1406 [DOI] [PubMed] [Google Scholar]
- 23. Iwase A, Tomidokoro A, Araie M, et al. Performance of frequency-doubling technology perimetry in a population-based survey of glaucoma: the Tajimi Study. Ophthalmology. 2007;114:27–32 [DOI] [PubMed] [Google Scholar]
- 24. Tielsch JM, Katz J, Singh K, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102–1110 [DOI] [PubMed] [Google Scholar]
- 25. Mowatt G, Burr JM, Cook JA, et al. Screening tests for detecting open-angle glaucoma: systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2008;49:5373–5385 [DOI] [PubMed] [Google Scholar]
- 26. Harper RA, Reeves BC. Glaucoma screening: the importance of combining test data. Optom Vis Sci. 1999;76:537–543 [DOI] [PubMed] [Google Scholar]