Pupil constriction to brief flashes can be used to assess the function of retinal ganglion cells in patients blinded from diseases of the receptors. This study establishes the bases of a clinical protocol for assessing the health of the rod, cone, and melanopsin (intrinsic ganglion cell pigment) contributions to the pupil response.
Abstract
Purpose.
To better understand the relative contributions of rod, cone, and melanopsin to the human pupillary light reflex (PLR) and to determine the optimal conditions for assessing the health of the rod, cone, and melanopsin pathways with a relatively brief clinical protocol.
Methods.
PLR was measured with an eye tracker, and stimuli were controlled with a Ganzfeld system. In experiment 1, 2.5 log cd/m2 red (640 ± 10 nm) and blue (467 ± 17 nm) stimuli of various durations were presented after dark adaptation. In experiments 2 and 3, 1-second red and blue stimuli were presented at different intensity levels in the dark (experiment 2) or on a 0.78 log cd/m2 blue background (experiment 3). Based on the results of experiments 1 to 3, a clinical protocol was designed and tested on healthy control subjects and patients with retinitis pigmentosa and Leber's congenital amaurosis.
Results.
The duration for producing the optimal melanopsin-driven sustained pupil response after termination of an intense blue stimulus was 1 second. PLR rod- and melanopsin-driven components are best studied with low- and high-intensity flashes, respectively, presented in the dark (experiment 2). A blue background suppressed rod and melanopsin responses, making it easy to assess the cone contribution with a red flash (experiment 3). With the clinical protocol, robust melanopsin responses could be seen in patients with few or no contributions from the rods and cones.
Conclusions.
It is possible to assess the rod, cone, and melanopsin contributions to the PLR with blue flashes at two or three intensity levels in the dark and one red flash on a blue background.
The pupillary light reflex (PLR) is a reflex that controls the constriction and dilation of the pupil in response to changes in light intensity. The PLR has been used in the clinic and in clinical research as an objective measure of retinal and optic nerve function. Most clinical studies have measured the latency and amplitude of pupil constriction to a brief, light stimulus.1–7 There is also a slow and sustained miosis after stimulus offset that was well known, although not necessarily well understood, at a neurophysiological level.8 The discovery of a class of retinal ganglion cells (RGCs) containing the photopigment melanopsin, which could be directly activated by light and which provide input to the pupillomotor center, provided a new understanding of the sustained PLR.9
The melanopsin-expressing intrinsic photosensitive RGCs (ipRGCs) show a delayed latency and a prolonged response that extend well after stimulus offset,10 similar to the sustained PLR. More recent studies, using pharmacologic blockades of the rod and cone input in primates11 and knockout mice,12,13 support the claim that the sustained PLR is mainly dependent on the ipRGCs.
The use of the PLR as assay of the ipRGC is of potential clinical value.5,14 First, a PLR technique to measure rod sensitivity to light has been developed as an objective measure of the effect of treatment in a clinical trial.15–17 Second, a protocol to evaluate the melanopsin contribution versus rod and cone contributions to the PLR may be of use in estimating the degree of damage to the RGCs versus to the retinal photoreceptors. Third, the presence of intact ipRGCs may help us to understand the currently unexplained phenomena (e.g., circadian rhythm) in some patients with little or no receptor function.18 Finally, considering the recent developments in retinal prosthesis (see, for example, Refs. 19–21), there is a need to know which patients with little or no receptor function have functioning RGCs and thus are viable candidates for recovery of vision.
Kardon et al.14,22 provided evidence that a clinical protocol could assess the contributions of the rods, cones, and melanopsin to the PLR in patients with outer retinal disease. Although an important proof of concept, their original design was suboptimal. First, they did not establish conclusively that they had isolated the rod, cone, and melanopsin contributions to the human PLR, though their results did show evidence for discriminating patients with photoreceptor disease from healthy subjects. Second, they used a staircase stimulus paradigm of increasing intensity (13-second duration stimulus) to facilitate patient comfort and to observe both transient and sustained stimulus-on pupil responses. However, the 13-second duration stimuli, even at the highest stimulus intensity of blue light (100 cd/m2), did not reveal obvious sustained PLR after light termination, which is the characteristic electrophysiological signature of a melanopsin-mediated contribution. Recently, Kankipati et al.23,24 showed that there is a significant decrease in melanopsin-driven PLR in patients with glaucoma, a disease known to decrease the number of RGCs.
The purpose here was to better understand the relative contributions of rod, cone, and melanopsin to the human PLRs and to determine the optimal conditions for assessing the health of the rod, cone, and melanopsin pathways with a relatively brief clinical protocol.
Methods
The study consisted of four experiments. Experiment 1 measured PLRs to red and blue stimuli of various durations to determine the optimal parameters, particularly for eliciting the melanopsin-mediated sustained pupil contraction after light termination. Experiment 2 measured the pupil response versus stimulus intensity (RvI) functions for red and blue stimuli in the dark. Experiment 3 measured similar RvI functions under light adaptation with a rod-suppressing blue background. Experiment 4 explored clinical protocols based on the results from experiments 1 to 3.
Light Stimuli
Light stimulation in all experiments was controlled by an LED-driven Ganzfeld system (Espion V5 system with the ColorDome LED full-field stimulator; Diagnosys LLC, Lowell, MA). This system generated a wide range of stimuli from −4 to 2.6 log cd/m2 for blue (467 ± 17 nm) lights and from −4 to >2.6 log cd/m2 for red (640 ± 10 nm) lights. Previous studies showed that melanopsin responses were observed in a range between ∼11 to >14 log quanta/cm2/s for a 470-nm light.10,11,25 After converting from quanta/s/deg2 to photopic trolands,26 the 11 and 14 log quanta/cm2/s of 470-nm light are equivalent to approximately −0.7 and 2.3 log cd/m2, respectively, assuming an average pupil diameter of 7 mm and a stimulus duration of 1 second. Our more intense lights are similar to those previously used to drive sustained pupil responses associated with melanopsin activity from human subjects.11
Because the stimuli were generated by LED lights, they were not strictly monochromatic. To check the conversion from photopic to scotopic cd/m2 calculated by the Ganzfeld software system, we used a full-field psychophysical test27 to obtain dark-adapted thresholds from 10 subjects with normal vision (7 males, 3 females; age, 28.4 ± 14.8 years). When equated for photopic cd/m2, the blue stimulus was 2.26 log units more effective than the red stimulus. This was essentially the same as the value of 2.3-log units calculated by the Ganzfeld system.
Pupil Recording
A binocular eye-tracking camera system with infrared illuminating diodes (Arrington Research, Scottsdale, AZ) was used for real-time pupil recording with a sample rate of 60 Hz. The miniature video camera system is attached to a plastic eye frame so that there is no physical contact between the camera and the eye and so that a wide visual field view is afforded. This system is the same as that described in Kardon et al.14,22
Data Analysis
The data were analyzed offline by customized scripts programmed in technical computing software (MATLAB; MathWorks Inc., Natick, MA), which allowed for semiautomatic analysis. First, a median filter with a 1-second (experiment 1) or a 500-ms (experiments 2, 3, and 4) time window was applied to remove the eye blinks. The filtered data were manually inspected to ensure that the filter had not distorted the waveform and to correct the machine-selected peak amplitude and latency where necessary. Responses irreparably contaminated by eye blinks or slow drifts in pupil size were rejected. Second, the filtered PLRs were normalized by the median pupil size during the 1 second before each stimulus onset (baseline pupil size): normalized pupil size = absolute pupil size/baseline pupil size. Peak normalized pupil size was defined as the minimum normalized pupil size after stimulus onset.
Subjects
Fifteen subjects with 20/20 corrected vision and no known visual abnormalities participated in one or more experiments (experiments 1–4). The number of subjects in each experiment will be described later. Persons with extreme eye blinking, which makes data analysis impossible, were not included. The dominant eye was determined by a simple finger position test and was used for the recording (monocular recording). The other eye was covered with a patch. In addition, five patients with retinitis pigmentosa (RP) and three with Leber's congenital amaurosis (LCA) were tested as part of experiment 4. All diagnoses were based on clinical examination and electrophysiological tests. No genetic tests were performed. The labels LCA1, LCA2, etc., or RP1, RP2, etc., refer to the first, second, etc., patient with LCA or RP. All patients had at least light perception, although the full-field ERG was nonrecordable in all cases. Patients details are summarized in Table 1.
Table 1.
Summary of Patient Characteristics
| Patient | Sex | Age (y) | Age at Diagnosis (y) | Diagnosis | Eye | Visual Acuity |
|---|---|---|---|---|---|---|
| RP1 | M | 39 | 21 | RP | OD | HM at 10 cm |
| RP2 | F | 54 | 16 | RP | OS | LP, cataract |
| RP3 | M | 46 | 27 | RP | OS | HM at 10 cm |
| RP4 | M | 34 | 18 | RP | OD | HM at 15 cm |
| RP5 | F | 40 | 25 | RP | OD | 20/800 |
| LCA1 | F | 41 | Birth | LCA | OD | LP |
| LCA2 | M | 19 | Birth | LCA | OD | LP |
| LCA3 | F | 22 | Birth | LCA | OD | LP |
RP, retinitis pigmentosa; LCA, Leber's congenital amaurosis; LP, light perception; HM, hand movement.
Informed consent was obtained from all subjects before their participation. Procedures adhered to the tenets of the Declaration of Helsinki, and the protocol was approved by the committee of the Institutional Board of Research Associates of Columbia University.
Experiment 1 Methods: Stimulus Duration Comparison
After 10-minutes of dark adaptation, the red and blue stimuli were presented in separate runs. Each run consisted of 2.5-log cd/m2 flashes of 8 durations (4 ms, 10 ms, 32 ms, 100 ms, 316 ms, 1 second, 3.16 seconds, and 10 seconds). In the first run (red condition), there was a 30-second recovery interval between stimuli (ISI), whereas in the second run (blue condition), the ISI increased from 30 seconds to 120 seconds as the stimulus durations increased. Pilot work indicated that the PLRs to blue flashes required longer recovery times to return to baseline. There was a 5-minute dark adaptation between two runs.
Two men and two women were run twice on two separate days. The mean age of subjects was 29 years (range, 19–43 years).
Experiment 2 Methods: Pupil Response versus Stimulus Intensity Function in the Dark
In this experiment, the dark-adapted RvI functions for the red and blue stimuli were measured over a wide range of intensities from −4 to 2.6 log cd/m2 in 0.5-log steps. Based on the results from experiment 1, a stimulus duration of 1 second was used. To avoid subject fatigue, the experiment was split into 2 runs. The intensities of the first run ranged from −4 to 2.6 log cd/m2 in 1-log steps. The intensities of the second run ranged from −3.5 to 2.6 log cd/m2 in 1-log steps and included a probe at 0 log cd/m2. This step was added in the second run to test the consistency across runs. By combining the two runs, an RvI function in 0.5-log steps was constructed. Between the two runs, 5 minutes or more of additional dark adaptation were inserted. At each intensity step, red stimuli were followed by photopically matched blue stimuli. The ISI increased from 10 to 60 seconds to help ensure recovery to baseline.
Three men and two women were run on two separate days. The mean age of subjects was 26.4 years (range, 19–37 years).
Experiment 3 Methods: Pupil Response versus Stimulus Intensity Functions with Light Adaptation
The design of experiment 3 was similar to that of experiment 2 except for the addition of a 0.78-log cd/m2 blue background to suppress rod activity. This intensity was chosen to have the same scotopic effectiveness as the white background intensity (30 cd/m2) used to suppress rod activity in the ISCEV standard ERG protocol.28 The stimulus intensity range (−1 to 2.6 log cd/m2) was smaller, and the ISIs (10–30 seconds) were shorter than those in experiment 2 because of the quicker recovery of the pupil responses between stimuli. The experiment was performed in one run in 0.5-log steps. Three minutes of blue light adaption was given before any test stimuli were presented, and the blue light background stayed on during the entire light-adapted paradigm.
Three men and two women were run on two separate days. The mean age of subjects was 24 years (range, 19–32 years).
Experiment 4 Methods: Clinical Protocol
Based on experiments 2 and 3, a clinical protocol was designed. The suggested protocol included three conditions: low-intensity (−3 and −2 log cd/m2) red and blue stimuli in the dark (rod condition), high-intensity (2.6 log cd/m2) red and blue stimuli in the dark (melanopsin condition), and high-intensity (2.6 log cd/m2) red and blue stimuli with rod-suppressing blue background (cone condition). The rationale for each condition will be discussed. After 10-minute dark adaptation, the three conditions were run in order. Photopically matched red stimuli were followed by blue stimuli. Each stimulus at every intensity level was presented twice, and the protocol was repeated at least twice.
Four men and 4 women with normal vision repeated the identical test on two separate days. The mean age of subjects was 30.3 years (range, 19–68 years). Monocular recordings were obtained from right eyes. The procedure for the patients was identical to that used to test the control subjects except that the more severely affected eye was tested.
Results
Experiment 1: Stimulus Duration Comparison
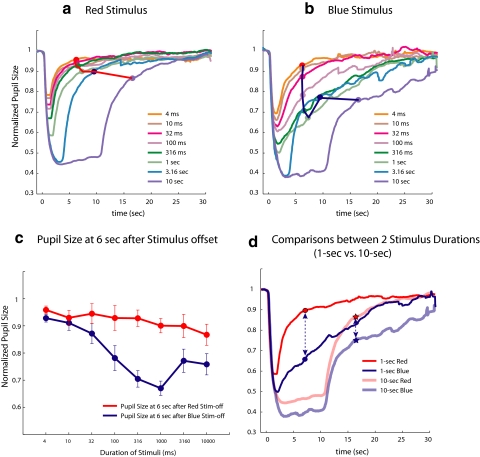
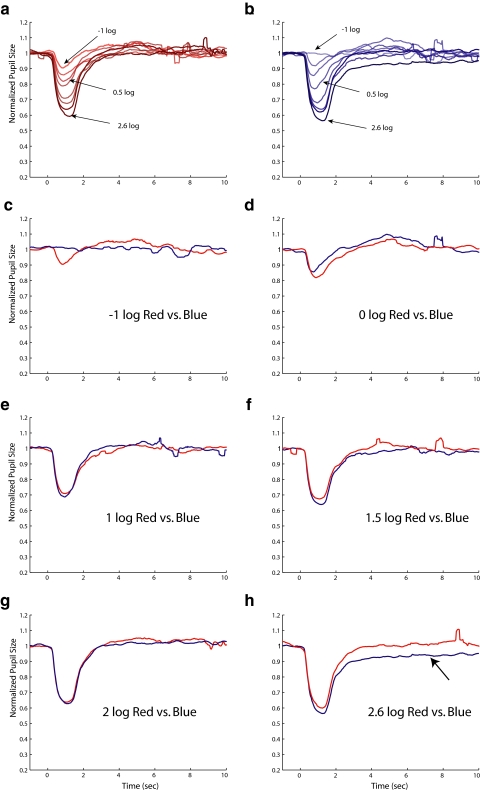
After 10-minute dark adaptation, the subjects' pupils were dilated (mean, 6.6 ± 1.35 mm). Figures 1a and 1b show the average normalized PLR amplitude for the four control subjects. Peak amplitude of the PLR increased as the stimuli became longer for both the red (Fig. 1a) and the blue (Fig. 1b) stimuli. PLRs at 6 seconds after stimulus offset are marked as circles and connected. Figure 1c shows these data at 6 seconds after the stimulus offset with SE bars. After the offset of the red stimuli, the pupil quickly returned to baseline (Fig. 1a), and the sustained pupil contraction was relatively small at 6 seconds and only modestly became more sustained after offset with increasing stimulus duration (Fig. 1c). However, for the blue stimuli, the sustained pupil contraction at 6 seconds after offset was larger than the photopically matched red light stimuli. This sustained pupil contraction after blue light offset did not change monotonically with increased stimulus duration. The sustained pupil contraction at 6 seconds after blue light offset increased for stimulus durations up to 1 second and then decreased for longer stimuli. The difference between the sustained pupil contraction after red and blue light offset is illustrated in Figure 1d, where the responses to the 1-second (saturated colors) and 10-second (desaturated colors) stimuli are shown. Pupil sizes at 6 seconds after stimulus offsets are marked with circles (1-second duration stimulus) and stars (10-second duration stimulus), and the dashed vertical lines show the difference between sustained contractions to the two stimuli of the same duration. Note that this difference is larger for the 1-second stimuli than for the 10-second stimuli. Based on the work of others,11,29,30 the difference between the sustained pupil contractions after light offset following red and blue stimuli should be attributed to melanopsin. Thus, as we seek to maximize our ability to record melanopsin-driven responses, a stimulus duration of 1 second was used in subsequent experiments.
Figure 1.
Average PLRs from five normal subjects. (a) PLRs to red stimuli of eight different durations, coded by color. The pupil size at 6 seconds after stimulus offset is marked as filled dots, connected by solid line. (b) Same as (a) for blue stimuli. (c) Pupil size at 6 seconds as a function of stimulus duration with ±1 SE bars. (d) PLRs to 1-second (saturated red and blue tracings) compared to 10-second (desaturated red and blue tracings) stimuli. The filled circles are marked at the same points as in (a) and (b), with dots for the pupil tracings elicited by 1-second stimuli and with stars for the pupil tracings elicited by 10-second stimuli. The stimulus intensity used in this experiment was 2.5 log cd/m2.
Experiment 2: Pupil Response versus Stimulus Intensity Functions in the Dark
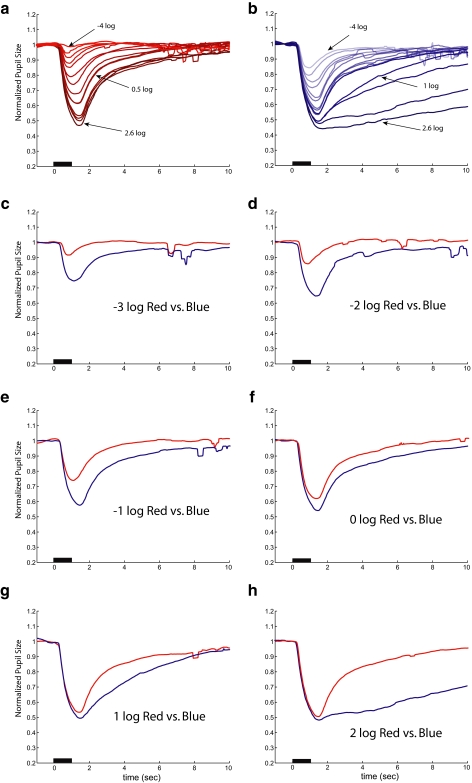
After 10-minute dark adaptation, the subjects' pupils were dilated (mean, 7.0 ± 0.50 mm). Figure 2 shows the normalized PLRs to the 1-second stimuli averaged for the seven control subjects. The PLR is shown from 1 second before (baseline) to 10 seconds after the 1-second stimulus (shown as black bar on x-axis). Figures 2a and 2b show the responses for the red and blue stimuli from −4 to 2.6 log cd/m2. The PLRs to red stimuli show a monotonic increase, a saturation of peak amplitudes at high intensities, and a fast recovery (Fig. 2a), whereas the PLRs to blue stimuli are characterized by the emergence of a sustained response after the 1-second stimuli for intensities of 1 log cd/m2 or greater (Fig. 2b). There were few to no PLRs to the −4 log red stimulus. Figures 2c to 2h show the comparisons of the PLRs to photopically matched red and blue stimuli. The blue stimuli induced larger PLRs in the low-intensity range from −4 to −1 log cd/m2 (Figs. 2c–e), but the peak amplitudes for red and blue stimuli are similar at the highest intensities (Figs. 2g, 2h). However, at these highest intensities, the PLR response to the blue stimulus is clearly more prolonged (sustained) than it is to the red stimulus after stimulus offset.
Figure 2.
Average PLRs from seven normal subjects to stimuli of different intensities in the dark. (a) PLRs to red stimuli at 14 intensity levels. (b) PLRs to blue stimuli at 14 intensity levels. (c–h) Pairs of PLRs, from (a) and (b), to photopically matched red and blue stimuli. Black bars: 1-second stimulus presentation.
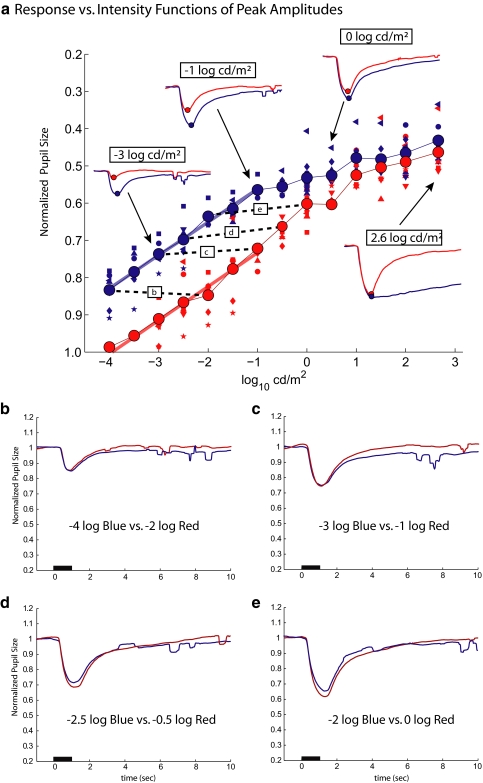
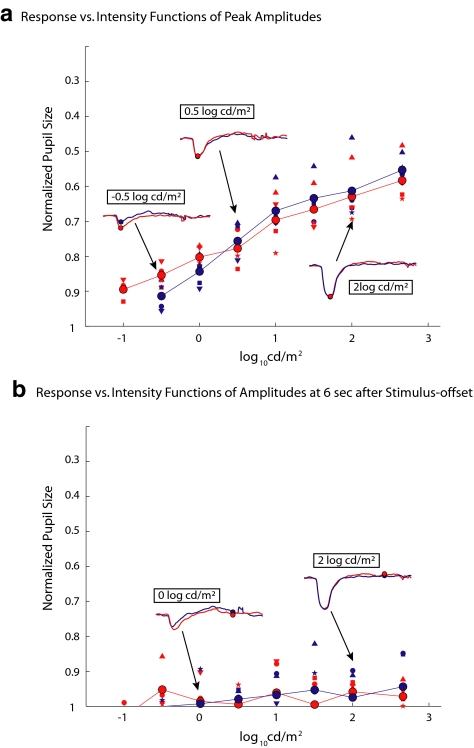
Figure 3a shows the RvI functions of normalized peak PLR as a function of log stimulus intensity. Note that the y-axis (normalized pupil size at peak contraction amplitude) is reversed so that larger responses (smaller pupil at peak contraction) are higher on the axis. The small symbols represent the data for individual subjects, and the large filled circles are the mean values of the seven subjects. The thin lines connect these mean values. The RvI function for the blue light changes almost linearly on the semi-log plot from −4 to approximately −1 log cd/m2 but then appears to saturate before increasing slowly for lights above 0 log cd/m2. The thick blue line is the linear regression line for the blue data between −4 to −1 log cd/m2. This regression line for the blue responses was shifted horizontally to determine the line of best fit to the responses to red stimuli between −4 and −1 log cd/m2. The separation between blue and red lines was 1.94 log units. Insets show the corresponding averaged PLRs for stimulus light intensities of −3, −1, 0, and 2.6 log cd/m2 of the photopically matched red and blue stimulus pairs. Peaks are marked as colored circles in each inset.
Figure 3.
(a) Peak normalized pupil size versus stimulus intensity is shown for seven normal subjects (small symbols) and their mean values (large symbols connected by the thin lines). The blue thick line is the best fitting linear regression line for the data between −4 to −1 log cd/m2, and the red thick line is this same line horizontally shifted for best fit to the red data. The insets show the PLRs to photopically matched red and blue stimuli at the intensity levels indicated. Note that y-axes are reversed so that larger responses are at the top. Dashed lines: pairs of stimuli used in the lower panels, with the corresponding panels indicated in the boxes. (b–e) Pairs of PLRs to red and blue stimuli separated by 1.94 log units, showing that they are nearly equal in contraction amplitude.
According to this regression analysis, over the lower range of intensities, when blue and red lights are photopically equated (cd/m2) but presented under conditions of dark adaptation, the red stimuli must be 1.94 log units more intense than the blue stimuli to obtain the same peak response. Figures 3b to 3e confirm this. The PLRs in response to the pair of light stimuli with nearly a 2-log unit separation are approximately the same. The dashed lines in Figure 3a indicate the pairs of stimuli used in the lower panels, with the corresponding panel indicated in the boxes. The approximately 2-log unit separation is close to the 2.3 log units for equal rod (scotopic) effectiveness according to the calibration for scotopic cd/m2. Thus, over the lower range of intensities, the PLRs to the blue stimuli are undoubtedly rod mediated. Although we cannot rule out a small cone contribution to the PLRs to the red stimuli, the rods probably dominate in the low range of intensity as well. In any case, to assess the rod contribution to the PLR in a clinical protocol, blue lights lower than −1.0 log cd/m2 under dark-adapted conditions can be used.
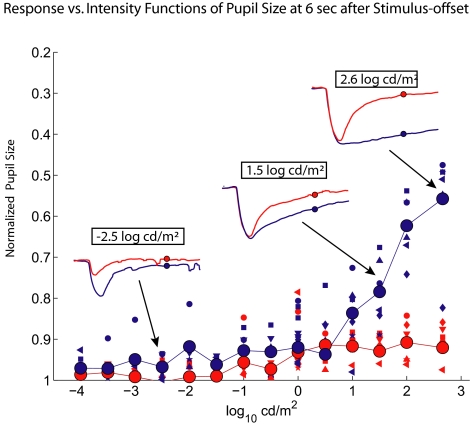
Based on previous work, the sustained pupil responses after the offset of blue stimuli are attributed to melanopsin in the ipRGC. Figure 4 shows the RvI function for the pupil size at 6 seconds after stimulus offset. Recall that the response at 6 seconds after stimulus offset was taken as a measure of the sustained response in Figure 1. In the lower intensity range (< 0 log cd/m2), neither the red nor the blue stimulus caused a significant sustained response. However, when the intensity of the blue stimulus was greater than 0.5 log cd/m2, the PLRs began to show the sustained responses to light offset, and the amplitude of this sustained response increased over the rest of the intensity range used. In contrast, there were few or no sustained responses to the red stimuli. For our clinical protocol, to assess the melanopsin contribution, we measure the sustained response to the 1-second, 2.6-log cd/m2 blue stimulus offset.
Figure 4.
RvI functions of pupil size at 6 seconds after stimulus offset in the dark. The data are presented as in Figure 3a. Note that the sustained pupil response to stimulus offset starts to occur after 1-second blue light offset of 0.5 log cd/m2 but did not significantly occur after red light offset.
Experiment 3: Pupil Response versus Stimulus Intensity Functions with Light Adaptation
To study cone contribution to the PLR, rod contribution was suppressed with a blue background, as described in Methods. After 2 minutes of light adaptation, the mean of the subjects' resting pupil diameter was 5.16 ± 0.27 mm. Figures 5a and 5b show the normalized PLRs plotted as in Figures 2a and 2b. Unlike the dark-adapted data, the responses to the red stimuli are equal to, or larger than, the responses to the photopically matched blue flashes (Figs. 5c–h). Although it is not important for our purposes here, the relatively greater effectiveness of the red stimuli at the lowest intensities may indicate the dominance of the long-wave sensitivity (red) cones because the blue background will have a smaller effect on this receptor type than it will on the middle-wave sensitivity (green) cones. More important, there is no indication of a rod contribution here, although there is a suggestion of a small, sustained melanopsin contribution to the 2.6-log cd/m2 blue stimulus offset (Fig. 5h, arrow).
Figure 5.
Average PLRs from five normal subjects to stimuli with various intensities on a blue background. The data are presented as in Figure 2. Unlike the dark-adapted data, the responses to the red stimuli are equal to, or larger than, the responses to the photopically matched blue flashes. There is a suggestion of a small sustained melanopsin contribution only to the brightest, 2.6-log cd/m2 blue stimulus offset (h, arrow)
Figure 6a shows the RvI functions of peak PLR versus stimulus intensity in the same manner as in Figure 3a, but for the blue background condition. Unlike what can be seen in Figure 3a, the two RvI functions for 1-second duration red and blue light stimuli are similar, except for the small difference at the low-intensity levels (< 0 log cd/m2), as described. The similar RvI functions for photopically matched blue and red stimuli argue for the peak response being dominated by the cones for both stimuli presented on a blue background. A red stimulus on the blue background should provide a good clinical test for a cone contribution to the pupillary light reflex.
Figure 6.
(a) Peak normalized pupil size versus stimulus intensity on a blue background is shown for five normal subjects (small symbols) and their mean values (large symbols connected by the thin lines). (b) RvI functions of pupil size at 6 seconds after stimulus offset on a blue background. Note that under blue background conditions, there is no significant sustained pupil response after light offset in control subjects, in contrast to the no background condition. Data are presented as in Figures 3a and 4.
Figure 6b shows RvI functions for the pupil size at 6 seconds after stimulus offset, plotted as in Figure 4. Unlike what can be seen in Figure 4, there was little evidence of sustained responses by melanopsin in either the red or the blue condition, except perhaps at the highest blue intensity (Fig. 5h, arrow). This result suggests that the blue background effectively suppressed melanopsin activity and rod activity. For possible mechanisms, see Discussion.
Experiment 4: Clinical Protocol
Control Subjects.
Based on the results from experiments 2 and 3, a clinical protocol was designed. This protocol had three conditions, as follows: rod condition, −3 and −2 log cd/m2 blue stimuli in the dark; melanopsin condition, 2.6 log cd/m2 blue stimuli in the dark; cone condition, 2.6 log cd/m2 red stimuli on the blue background. Although it was not strictly necessary to assess the contributions of the rods, cones, and melanopsin, photopically equated red stimuli were also included in the rod and melanopsin conditions, and a photopically equated blue stimulus was included in the cone condition.
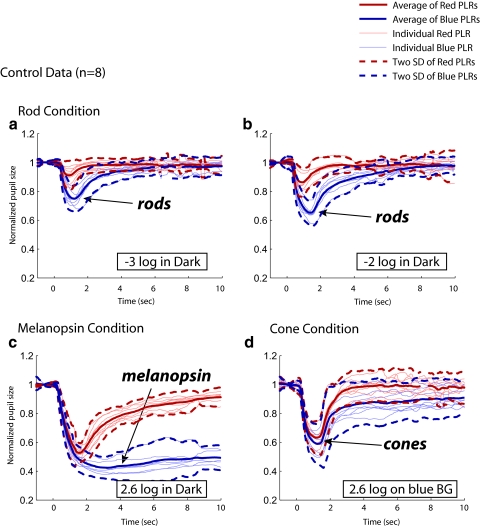
Figure 7 shows the normalized PLRs for all three conditions and for eight control subjects. The thin lines represent the individual PLRs (average across two daily sessions), and the thick lines are averaged across all eight subjects. The dashed lines show ±2 SD. Reproducibility in the eight control subjects was fairly good in all three conditions.
Figure 7.
PLRs for eight control subjects (thin lines) for the three conditions of the clinical protocol. The bold solid and dashed lines show the mean ±2 SD for the eight control subjects. (a, b) Rod condition: −3 and −2 log cd/m2 blue light stimuli in the dark. (c) Melanopsin condition: 2.6 log cd/m2 blue light stimuli in the dark. (d) Cone condition: 2.6 log cd/m2 red light stimuli on a blue background. Though not strictly necessary to assess the contributions of the rods, cones, and melanopsin, photopically equated red stimuli were also included for the rod and melanopsin conditions, and a photopically equated blue stimulus was included for the cone condition. The gaps seen in some of the records are due to the removal of extreme eye blinks.
Retinitis Pigmentosa.
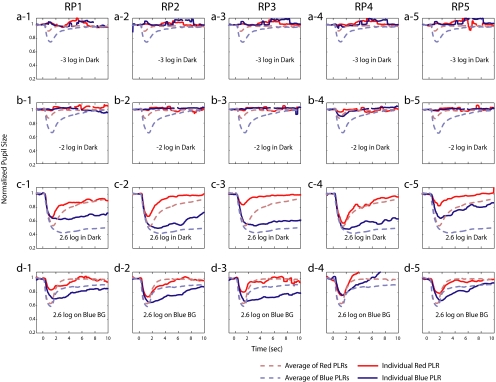
Five patients with severe RP were tested using the suggested clinical protocol. All patients had light perception or better vision, although the ERG signals were nonrecordable (Table 1). Figure 8 shows the results. Each column represents the results from one patient. Rod conditions are shown in the first and second rows (Figs. 8a, 8b), and melanopsin and cone conditions are shown in the third (Fig. 8c) and fourth (Fig. 8d) rows. The solid lines show each patient's PLRs to red and blue stimuli, and the dashed lines show the average of control subjects.
Figure 8.
PLRs from five patients with RP (columns RP1–5) for the clinical protocol. Solid lines: patients' PLRs; dashed lines: average across eight controls subjects. Rows (a) to (d) correspond to the conditions in Figure 7. (a, b) Rod condition. (c) Melanopsin condition. (d) Cone condition. The extreme eye blinks were removed as in Figure 7.
All patients' pupils dilated to some extent but did not reach the average dark-adapted level (∼7 mm) of the control subjects. The low-intensity stimuli of the rod condition in the dark did not induce measurable PLRs from the RP patients (Fig. 8, rows a and b). Only one patient showed a measurable response, and this was to the more intense −2-log cd/m2 blue stimulus (RP4; Fig. 8b-4). However, all patients showed a sizable sustained response to the intense blue stimulus of the melanopsin condition, (Fig. 8c). In fact, the response was nearly equal to the mean of the controls for three of the patients. For the cone condition (Fig. 8d), all RP patients showed PLRs to red stimuli, indicating some residual cone function, although these responses, in general, were smaller than for the controls. As discussed, the red stimulus was probably too intense to evaluate the full extent of the loss of cone function. Interestingly, the blue stimulus under conditions of light adaptation induced a sustained response from all the patients, and this response was larger than that of the controls subject. In other words, the blue background did not seem to suppress the melanopsin-driven responses from RP patients to the same extent seen for the control subjects (cone condition; Fig. 8, row d).
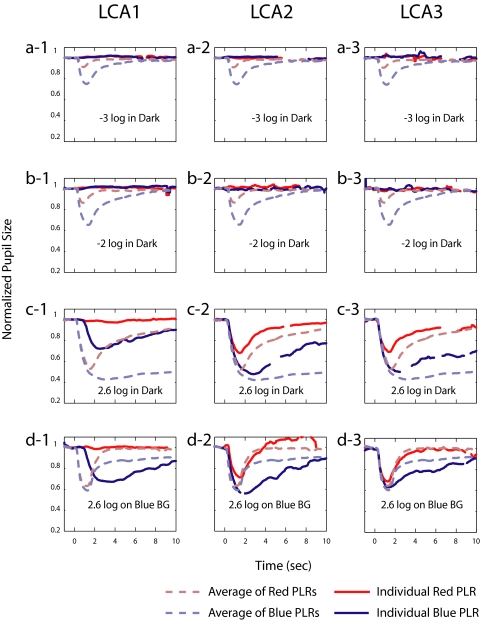
Leber's Congenital Amaurosis.
Three patients with LCA were tested using the clinical protocol. All patients had only light perception; they could not detect hand movement. Two of them, who were brother and sister (LCA2 and LCA3), did show dilation after 10-minute dark adaptation (> 6 mm) and constriction after light adaptation (< 5 mm), but the other subject (LCA1) did not show any pupillary change to the adapting light.
Figure 9 displays the results in the same manner as shown in Figure 8. For all three patients, there was no detectable PLR to the rod condition stimuli (Figs. 9a, 9b). Responses of LCA2 and LCA3 to the melanopsin and cone conditions were similar to the results from the RP patients. There were sizable PLRs to intense red stimuli, indicating a cone contribution, and a sustained response to the blue stimulus offset, suggesting a melanopsin contribution. As in the RP patients, the blue background did not suppress the sustained responses to bright blue light from these two LCA patients (Figs. 9c-2, 9c-3, 9d-2, 9d-3). However, the results from LCA1 were distinguishable from those of the other two LCA patients. This patient showed a significant PLR to intense blue stimuli (Figs. 9c-1, 9d-1), though the photopically matched red stimuli did not elicit a PLR. Moreover, there was a qualitative difference compared with the PLR from the control subjects. The onset of PLR to blue light in Figures 9c-1 and 9d-1 occurred at 1267 ms (in the dark) and 1150 ms (on background). These times were much slower than the onset time of 383 ms (in the dark) for the average response of the control subjects. However, the time to peak was almost the same as for the controls in the dark (2500 ms) but was considerably slower in the background condition (3118 ms) (Figs. 9c-1 vs. 9d-1). It appears that LCA1 has only a melanopsin contribution to the PLR and that its onset is significantly delayed, as expected from electrophysiology.10
Figure 9.
PLRs from three patients with LCA (columns) for the clinical protocol. Data are presented as in Figure 8.
Discussion
This study had two related goals: first, to determine conditions under which the rod, cone, and melanopsin contributions to the human PLRs to brief stimuli can be isolated; second, to determine the optimal conditions for assessing the health of the rod, cone, and melanopsin pathways, with a more abbreviated protocol for clinical testing of patients.
Conditions Favoring Rod, Cone, and Melanopsin Contributions to the PLR
First, based on experiment 1, a 1-second test light is the optimal stimulus duration for assessing the sustained melanopsin contribution to pupil contraction after light offset. Since Dacey at el.10 reported the slow and sustained depolarization of ipRGCs in response to long-duration light stimuli, most human PLR studies concerning melanopsin focused on stimuli with relatively long durations (>10 seconds).11,14,25,31 In comparing the results of these studies to each other and to those reported here, it is worth noting that in our study the PLR was measured in the same eye that received the light stimulation (closed-loop paradigm). Although some of the previous studies used this approach, others stimulated one eye, which was dilated, and recorded from the other (open-looped paradigm). With the closed-loop, but not the open-loop, approach, retinal illumination varies during stimulus presentation as the pupil changes shape. In any case, the results of experiment 1 suggest that with the closed-loop paradigm used here, the 1-second short-wavelength stimulus is the optimal duration for maximizing the sustained melanopsin-mediated response after blue light offset compared with red light offset. Further, the shorter 1-second light stimulus has the advantage of decreasing the time needed between stimulus presentations for the pupil to return to baseline. In addition, it decreases the discomfort to the subject caused by a bright blue stimulus in the dark. Thus, given that the melanopsin contribution was the most important—and likely to be the most difficult—to isolate, subsequent work used 1-second stimuli.
Based on the results from experiment 2, we concluded that the dark-adapted PLRs to the low-intensity blue stimuli were largely, if not entirely, controlled by the rod system. The dark-adapted red and blue RvI functions were parallel and separated by 1.94 log units, close to the estimated relative scotopic sensitivity of ∼2.3 log determined by the Diagnosys software, based on the CIE curves and the LED spectral distributions and confirmed with our psychophysically measured dark-adapted thresholds. Although the PLR to red probably has a small cone contribution, the responses to blue should be rod driven under our conditions of dark adaptation and dim stimulus intensity (−3 and −2 log cd/m2). Second, consistent with this interpretation, the pairs of PLRs to approximately scotopically matched red and blue stimuli were nearly identical. This result is consistent with other studies that showed the dominating contribution of rod over cone in a short-duration stimulus presentation.15,25 On the other hand, we do not know how the rod and cone signals combined at higher intensity. Above 1 log cd/m2 in the dark, the photopically matched red and blue stimuli produced about the same peak amplitude. However, this cannot be simply interpreted as the cone's domination in this range because the PLR response is near saturation and both rods and cones are probably contributing. For a complete understanding of the contribution of the rods and cones to the PLR at brighter light intensity without blue background adaptation, more work is needed. In any case, for our purposes here, we need only conclude that the rods dominate the responses to the low-intensity blue stimuli. The clinical data presented in experiment 4 support this conclusion.
Only high-intensity blue stimuli in the dark were able to produce sustained pupil responses. We assume, largely based on previous work, that this sustained response is melanopsin driven.10,11,30 For example, Gamlin et al.11 showed that this sustained PLR to lights presented in the dark had the action spectrum of melanopsin in humans and was still present in monkeys whose rod and cone receptor outputs were chemically blocked. The relatively large sustained responses we observe in patients with reduced rod and cone responses support the assumption that these sustained responses are melanopsin driven. Patient LCA1 represents an extreme example. This patient had no sign of a cone or rod contribution to the PLR but had a large, delayed, sustained (melanopsin) PLR, similar to that seen in the monkey when the receptor output is blocked.11 In our control subjects, this presumed melanopsin response was evident to the blue stimuli above 0.5 log cd/m2. Given the spectral absorption curve for melanopsin, PLR to 2.6 log cd/m2 red is essentially free of a melanopsin contribution; thus, we can take the difference between the responses to the red and blue lights of 2.6 log cd/m2 as the melanopsin response (Fig. 7b, vertical dashed line).
To isolate a cone-driven PLR to red stimuli, a 0.78-log cd/m2 blue background was introduced. This background not only suppressed the rod contribution, it also appeared to suppress most of the melanopsin contribution to the 1-second stimulus. It is not clear how much of the suppressive effect of the background on the melanopsin-driven response is due to a passive response compression mechanism,32 to the bistable nature of melanopsin,29,33 to the involvement of an inhibitory signal, perhaps from S-cones,10 or some yet to be determined mechanism. In any case, the identical PLRs to photopically matched red and blue stimuli allow us to safely conclude that the PLRs to red stimuli on the blue background are cone driven.
Clinical Protocol
Based on the results from experiments 1, 2, and 3, we developed a clinical protocol. We evaluate this protocol and suggest some changes based on the results of experiment 4.
Rod Condition.
Two pairs of photopically matched red and blue stimuli in the low-intensity range (−3 and −2 log cd/m2) were presented in the dark. To assess rod sensitivity, only responses to blue stimuli are needed. Thus, the red stimuli could be omitted, although they take relatively little time to present. The proposed protocol worked well. Four of the five RP patients and all the LCA patients showed no response to either of the blue stimulus intensities, suggesting that their rod input to the ipRGC was reduced by >3 log units. The dark-adapted threshold for the PLR to blue stimuli is well below −5 log cd/m2 (Fig. 3a). The other RP patient had a small response to the −2 log cd/m2; therefore, for this patient, the loss was >2 log units compared with that of control subjects, whose PLR to −4 log cd/m2 stimulus was sizable. If a quantitative measure of the loss in sensitivity is required, a more complete response-intensity function must be obtained, as, for example, in the Aleman et al.15 study, which documented a range of rod sensitivity losses in patients with LCA.
Cone Condition.
The photopically matched red and blue 2.6-log cd/m2 stimuli were presented on the blue background. To assess cone sensitivity, only the response to the red stimulus is needed. However, the 2.6-log cd/m2 stimulus is too intense to get a good estimate of the loss of cone sensitivity because of the compressive nonlinear RvI relationship of the pupil response. We suggest that if it is important to obtain an estimate of loss of sensitivity of the cone input to the ipRGC, then a lower intensity red flash should be added to the protocol. Pilot work suggests a flash of approximately 1.0 log cd/m2 would be adequate to show a loss of >2 log units in cone sensitivity relative to controls.
Melanopsin Condition.
The photopically matched intense red and blue stimuli (2.6 log cd/m2) were presented in the dark. With these PLRs, the melanopsin contribution can be estimated from the difference between the responses to the red and blue stimuli. This protocol appeared to work well for the patients in experiment 4. Further, the responses to the intense blue stimuli on the blue background also provide an interesting assay for both melanopsin and receptor contributions. In other words, it appears that patients with reduced rod and cone contributions, but with a viable melanopsin contribution, had a relatively larger melanopsin contribution to the intense blue flash on the blue background than did control subjects.
Caveats and Future Challenges
There is considerable individual variance in pupil sizes, even among the control subjects. The normalization procedure used was effective in reducing this variance in the control group. However, most of the patients with severe visual defects had smaller pupils in the dark. It is not known whether this is secondary to a sustained, melanopsin activation, which is more apparent in eyes with photoreceptor loss. This finding in itself may have diagnostic significance and may have to be better characterized in the future. More work is needed to better understand how the baseline level affects the human PLR in particular and to determine the optimal conditions for decreasing variability in general.
Summary
It is possible to isolate rod, cone, and melanopsin contributions to the PLR elicited with a 1-second stimulus. Further, these contributions can be evaluated with a clinical protocol requiring <30 minutes after a 10-minute period of dark adaptation. For some purposes, this protocol can be substantially shortened. For example, suppose that the objective is to determine the viability of the inner retinal neurons in patients who are candidates for visual prostheses or gene therapy. If we assume the health of the ipRGC is a good assay for the health of the other RGCs, then the pair of red and blue intense flashes in the dark can be used to assess melanopsin, and thus ipRGC, integrity. This would take a minute or two after dark adaptation.
Acknowledgments
The authors thank Stephen H. Tsang, Elona Dhrami-Gavazi, and Neeco Palmer (Department of Ophthalmology, Columbia University, New York, NY) for their help in recruiting and evaluating the patients.
Footnotes
Supported by National Institutes of Health Grant R01-EY-09076; the Department of Veterans Affairs (Rehabilitation, Research and Development Division); National Institutes of Health/National Eye Institute Grant 1R01EY018853-01A2; Department of Defense Grant TATRC VRP09; an unrestricted grant from Research to Prevent Blindness; and the Pomerantz Family Endowed Chair.
Disclosure: J.C. Park, None; A.L. Moura, None; A.S. Raza, None; D.W. Rhee, None; R.H. Kardon, None; D.C. Hood, None
References
- 1. Bergamin O, Kardon RH. Latency of the pupil light reflex: sample rate, stimulus intensity, and variation in normal subjects. Invest Ophthalmol Vis Sci. 2003;44:1546–1554 [DOI] [PubMed] [Google Scholar]
- 2. Bergamin O, Zimmerman MB, Kardon RH. Pupil light reflex in normal and diseased eyes: diagnosis of visual dysfunction using waveform partitioning. Ophthalmology. 2003;110:106–114 [DOI] [PubMed] [Google Scholar]
- 3. Bremner FD, Shallo-Hoffmann J, Riordan-Eva P, Smith SE. Comparing pupil function with visual function in patients with Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 1999;40:2528–2534 [PubMed] [Google Scholar]
- 4. Kardon R, Kawasaki A, Miller NR. Origin of the relative afferent pupillary defect in optic tract lesions. Ophthalmology. 2006;113:1345–1353 [DOI] [PubMed] [Google Scholar]
- 5. Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007;27:195–204 [DOI] [PubMed] [Google Scholar]
- 6. Volpe NJ, Plotkin ES, Maguire MG, Hariprasad R, Galetta SL. Portable pupillography of the swinging flashlight test to detect afferent pupillary defects. Ophthalmology. 2000;107:1913–1921, discussion 1922 [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm B, Ludtke H, Peters T, Schmid R, Wilhelm H, Zrenner E. [Automated swinging flashlight test in patients with optic nerve diseases]. Klin Monatsbl Augenheilkd. 2001;218:21–25 [DOI] [PubMed] [Google Scholar]
- 8. Young RS, Kennish J. Transient and sustained components of the pupil response evoked by achromatic spatial patterns. Vision Res. 1993;33:2239–2252 [DOI] [PubMed] [Google Scholar]
- 9. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073 [DOI] [PubMed] [Google Scholar]
- 10. Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754 [DOI] [PubMed] [Google Scholar]
- 11. Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247 [DOI] [PubMed] [Google Scholar]
- 14. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116:1564–1573 [DOI] [PubMed] [Google Scholar]
- 15. Aleman TS, Jacobson SG, Chico JD, et al. Impairment of the transient pupillary light reflex in Rpe65(-/-) mice and humans with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45:1259–1271 [DOI] [PubMed] [Google Scholar]
- 16. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82:3763–3770 [DOI] [PubMed] [Google Scholar]
- 19. Besch D, Sachs H, Szurman P, et al. Extraocular surgery for implantation of an active subretinal visual prosthesis with external connections: feasibility and outcome in seven patients. Br J Ophthalmol. 2008;92:1361–1368 [DOI] [PubMed] [Google Scholar]
- 20. Chow AY, Bittner AK, Pardue MT. The artificial silicon retina in retinitis pigmentosa patients (an American Ophthalmological Association thesis). Trans Am Ophthalmol Soc. 2010;108:120–154 [PMC free article] [PubMed] [Google Scholar]
- 21. Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172–182 [DOI] [PubMed] [Google Scholar]
- 22. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology. 2011;118:376–381 [DOI] [PubMed] [Google Scholar]
- 23. Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010;51:2764–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52:2287–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDougal DH, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010; 50:72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pianta MJ, Kalloniatis M. Characterisation of dark adaptation in human cone pathways: an application of the equivalent background hypothesis. J Physiol. 2000;528:591–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein M, Birch DG. Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST). Doc Ophthalmol. 2009;119:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77 [DOI] [PubMed] [Google Scholar]
- 29. Mure LS, Cornut PL, Rieux C, et al. Melanopsin bistability: a fly's eye technology in the human retina. PLoS One. 2009;4:e5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young RS, Kimura E. Pupillary correlates of light-evoked melanopsin activity in humans. Vision Res. 2008;48:862–871 [DOI] [PubMed] [Google Scholar]
- 31. Kawasaki A, Herbst K, Sander B, Milea D. Selective wavelength pupillometry in Leber hereditary optic neuropathy. Clin Exp Ophthalmol. 2010;38:322–324 [DOI] [PubMed] [Google Scholar]
- 32. Boynton RM, Whitten DN. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970;170:1423–1426 [DOI] [PubMed] [Google Scholar]
- 33. Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22:411–424 [DOI] [PMC free article] [PubMed] [Google Scholar]