Anti-CD11a therapy, shown in animals to decrease the expression of endotoxin-induced uveitis, was successful in improving visual acuity, reducing macular thickness, and increasing the CD56bright population in uveitis patients with macular edema.
Abstract
Purpose.
To evaluate the safety and efficacy of treating macular edema, secondary to noninfectious uveitis, with a humanized anti-CD11a antibody.
Methods.
Six patients received weekly subcutaneous treatments for 16 weeks according to this open-label, prospective, noncomparative phase I/II trial. Best corrected visual acuity (BCVA) and central macular thickness (CMT) were compared to baseline. Adverse events were recorded and assessed. Blood was sampled to assess the levels of CD56bright regulatory NK cells before initiation and after termination of the study.
Results.
No serious adverse events were reported by the patients. Patients' ages ranged from 22 to 82 years. Mean BCVA improvements were 6.7 ± 6.9 ETDRS letters in the worse eye and 1.7 ± 5.2 letters in the better eye. Mean CMT reductions were 128 ± 105 μm in the worse eye and 57 ± 68 μm in the better eye. Anti-CD11a antibody treatments resulted in an increase in the CD56bright regulatory NK cell population in the peripheral blood of the patients.
Conclusions.
Anti-CD11a treatment improved visual function, reduced macular thickness, and increased the level of CD56bright regulatory NK cells in patients with uveitic macular edema refractory to other immunosuppressive medications. Targeting CD11a may be beneficial in treating other causes of macular edema. (ClinicalTrials.gov number, NCT00280826.)
Cystoid macular edema (CME) is seen in 33% of patients with uveitis and requires local or systemic treatment for resolution.1 Standard systemic immunosuppressive medications can be associated with significant adverse effects.2–4 Management and prevention of the iatrogenic complications of immunosuppressive therapy accounts for most of the medical resources devoted to these individuals. Consequently, an effective treatment with a safer side effect profile is highly desirable.
Efalizumab (Raptiva; Genentech Inc., San Francisco, CA) is a humanized form of a murine IgG1 antibody directed against CD11a, the α-subunit of lymphocyte function–associated antigen-1 (LFA-1).5 LFA-1 expression is increased in memory T-cells, and ICAM-1 is expressed on vascular endothelial cells at sites of inflammation in a variety of T-cell-mediated disorders, including uveitis.6 Both LFA-1 and intercellular adhesion molecule (ICAM)-1 are thought to play important roles in the pathogenesis of autoimmune disorders, and prior studies have shown that interference with adhesion molecule function, including CD11a, decreases histologic and clinical expression of endotoxin-induced uveitis.7,8 In vitro studies have shown that by binding to CD11a, efalizumab can inhibit T-cell activation, T-cell trafficking, and T-cell adhesion without depleting the T cells.9 Efalizumab was approved for use in moderate to severe plaque psoriasis in adults.10–12
Human NK cells have typically been identified as CD56+CD3− lymphocytes, with two subsets of human NK cells identified based on expression levels of cell surface CD56, CD56dim, and CD56bright.13–15 CD56bright regulatory NK cells have been proposed to play a regulatory role in immune responses based on their lower cytotoxic potential, higher secretion of downregulatory cytokines, and unique surface receptor expression profile.14,16–18 Previous studies have demonstrated that a low-dose infusion of recombinant human IL-2 selectively induces the CD56bright regulatory NK subset.19 Studies in the Laboratory of Immunology of the National Eye Institute and other work have revealed that infusion of daclizumab, a humanized IL-2R (CD25)-blocking antibody, also induces upregulation of this subset in both uveitis and multiple sclerosis.20,21 Patients with active uveitis had a significantly lower level of CD56bright regulatory NK cells in their peripheral blood compared with normal donors.20 The expansion of CD56bright cells correlates with decreased ocular and brain inflammation.20,21 Induced CD56bright regulatory NK cells have the ability to secrete large amounts of IL-10, whereas CD56dim NK cells do not, suggesting that the induction of the CD56bright regulatory NK cells may have a beneficial effect on the remission of active uveitis.20
This study was designed to evaluate the safety and potential efficacy of subcutaneous humanized anti-CD11a antibody treatments for macular edema associated with uveitis, while reducing or eliminating standard medications commensurate with the standard of care.
Methods
This nonrandomized, prospective, open-label pilot study to treat patients with CME secondary to noninfectious intermediate and/or posterior uveitis was conducted at the National Eye Institute between October 2006 and July 2008 under an Investigational New Drug (IND) application. The study protocol was reviewed and approved by the Institutional Review Board of the National Institutes of Health, and all procedures conformed to the Declaration of Helsinki. Informed consent was obtained from all patients.
Inclusion and Exclusion Criteria
Inclusion criteria included a diagnosis of sight-threatening intermediate, posterior, or panuveitis of at least 3 months' duration as the source of persistent CME in one or both eyes that required immunosuppressive medications to treat and control (with at least 20 mg per day of prednisone [or equivalent] or any combination of two or more immunosuppressive medications); that exhibited intolerance to the indicated systemic medications required; or, that, even though the uveitis was controlled, required discontinuation of present medications because of potential or actual unacceptable side effects. No specific activity level of uveitis was a criterion for inclusion and only the presence, independent of duration, of CME was a criterion. Best corrected visual acuity had to be at least 20/200 or better in one eye. Laboratory and renal and liver function test results had to be normal or to show mild abnormalities, no worse than those defined by the Common Toxicity Criteria.22 Participants could not be pregnant or lactating and those of reproductive potential who were sexually active agreed to use acceptable birth control methods throughout the course of the study.
Exclusion criteria included participants under the age of 18 years; those receiving other treatments with an ICAM- or LFA-1-directed monoclonal antibody or other investigational agents that would interfere with the ability to evaluate the safety, efficacy, and pharmacokinetics of anti-CD11a; and those with significant active infections, a history of cancer (other than a nonmelanoma skin cancer), diagnosed within the past 5 years, or a history of neurologic diseases. Participants were recruited from the National Eye Institute (NEI) uveitis clinic, and all were screened under the uveitis screening protocol, to establish eligibility.
Medication Dose Regimen and Administration
Patients who qualified for the study received weekly subcutaneous treatments of anti-CD11a, with the first dose being a test dose of 0.7 mg/kg and subsequent doses of 1 mg/kg (not to exceed 200 mg per dose), for a total treatment duration of 16 weeks. Participants returned to the eye clinic weekly for administration of the medication. Treatment was regarded as successful if any of the following occurred: a decrease in CMT as measured by optical coherence tomography (OCT), improvement in visual acuity, or at least a 50% decrease in use of standard immunosuppressive medications.
Ophthalmic and Medical Evaluation
All patients underwent baseline medical and ophthalmic examinations that included best corrected visual acuity measurements using the standardized ETDRS refraction protocol at 4 m, intraocular pressure by applanation tonometry, slit lamp biomicroscopic examination, dilated funduscopic examination, fluorescein angiography, and fundus photography. Imaging (Stratus OCT-3; Carl Zeiss Meditec, Dublin, CA) was performed, and mean central macula thickness (CMT; area A1 with a 1-mm diameter) was recorded at interval visits. Medical examinations included a physical examination with laboratory measurements, such as a complete blood count with differential, an electrolyte panel, renal function testing, and liver function testing. Tabulations of adverse events (AEs), concomitant medications, and laboratory investigations were recorded and reviewed at each visit. Meeting the safety endpoint (i.e., ≥15 letter loss of visual acuity) or having a serious AE directly attributed to anti-CD11a therapy would lead to termination of therapy.
Primary and Secondary Outcome Assessment
The primary outcome assessed was the safety of anti-CD11a in uveitis patients. Secondary outcomes included the effects of treatment on CME via changes in CMT using OCT and changes in visual acuity (ETDRS) from baseline to termination. Other outcomes assessed were the ability to reduce concomitant immunosuppression and the levels of CD56bright regulatory cells from baseline to termination.
Safety outcomes were recorded by observing the nature, severity, and frequency of systemic toxicities, AEs, and infections throughout the study. Safety assessments were made at each visit, with a review of the previous visit interval. Each participant was instructed to report any apparent AEs between scheduled visits and returned for additional evaluations and appropriate treatment between scheduled visits if necessary. Adverse medical events were evaluated and treated by the Internal Medicine Service when clinically indicated, and appropriate consultations were obtained if necessary. After assessment by the Internal Medicine Service, an evaluation of the possible relatedness of the AE to the administration of anti-CD11a was made and the findings recorded by the investigators.
Statistical Analysis
Descriptive statistics were obtained for this six–participant pilot study.
Results
Six participants with intermediate and/or posterior or panuveitis with CME were enrolled in the study. The demographic characteristics of the six participants are listed in Table 1. The median age of the participants was 43 years (range, 22–82). Three participants had chronic nongranulomatous idiopathic intermediate uveitis, one participant had pars planitis, one participant had posterior scleritis with intermediate uveitis, and one participant had Vogt-Koyanagi-Harada syndrome. All patients (all eyes) had chronic CME. No patients (no eyes) had received any local therapy for 4 weeks before enrollment.
Table 1.
Demographic Information, Baseline Characteristics, Accompanying Immunosuppression, and Adverse Events of the Study Patients
| Patient | Sex | Race | Age at Enrollment (y) | Diagnosis | Immunosuppressive Medications at Enrollment | Immunosuppressive Medications at Last Follow-Up | Adverse Events (Related to Study Medication)* |
|---|---|---|---|---|---|---|---|
| 001 | M | H | 33 | Chronic NG IU | None | Efalizumab | Transient neutropenia, transient vision loss |
| 002 | F | C | 22 | Chronic NG IU | Pred 20 mg qd | Efalizumab | Transient ocular pain, transient skin rash |
| 003 | M | AA | 31 | Pars Planitis | Cell 1500 mg bid, Pred 15 mg qd, PF qid OU | Efalizumab, Cell 1500 mg bid, Pred 15 mg qd, PF qid OU | Transient lymphocytosis, URI and sore throat, transient headaches |
| 004 | F | C | 60 | Chronic NG IU | Cell 1000 mg bid | Efalizumab; Cell 1000 mg bid; CSA 100 mg bid | Hot flashes |
| 005 | M | A | 28 | VKH | Pred 5 mg qd CSA 100 mg bid AZA 50 mg qd | Efalizumab, Pred 5 mg qd, CSA 50 mg bid | Transient headaches, lymphocytosis, fatigue |
| 006 | F | AA | 82 | Posterior scleritis with IU | Cell 1000 mg bid, Pred 15 mg qd | Efalizumab, Cell 500 mg bid, Pred 15 mg qd | Lymphocytosis, ocular discomfort, dizziness and nausea |
H, Hispanic; C, Caucasian; AA, African American; A, Asian; NG, nongranulomatous; IU, intermediate uveitis; VKH, Vogt-Koyanagi-Harada Syndrome; Pred, Prednisone; Cell, Cellcept (Genentech, S. San Francisco, CA); PF, Prednisolone acetate 1%; CSA, cyclosporine; AZA, azathioprine; bid, twice daily; qd, daily; qid, four times a day.
Adverse events deemed related to study medication.
All patients tolerated the anti-CD11a treatment well and no serious AEs, including serious infections attributable to the study medication, were reported by the patients. AEs were graded based on the possibility and plausibility of their relation to the study medications: not related, remotely related, possibly related, probably related, or related (Table 2). The severity of AEs was graded according to CTCAE 3.0 (Common Terminology Criteria for Adverse Events). Most of the AEs were mild or moderate. All events were transient and resolved spontaneously or with simple treatments. The most consistent AEs were lymphocytosis (three patients) and headaches (two patients), which resolved without sequelae.
Table 2.
Adverse Events
| Patient | Description | Infection | Related to Agent | Severity | Treatment Required | Hospitalized | Outcome |
|---|---|---|---|---|---|---|---|
| 001 | Pterygium | No | No | Mild | No | No | Monitored |
| Dry eye | No | No | Mild | ATs | No | Monitored | |
| Neutropenia | No | Possibly | Mild | No | No | Recovered, no residual effects | |
| Decrease in VA | No | Possibly | Moderate | No | No | Recovered, no residual effects | |
| 002 | Pain with eye movements | No | Remotely | Mild | No | No | Recovered, no residual effects |
| Skin rash | No | Remotely | Mild | No | No | Recovered, no residual effects | |
| 003 | Lymphocytosis | Possible | Remotely | Moderate | No | No | Recovered, no residual effects |
| URI | Possible | Remotely | Mild | No | No | Recovered, no residual effects | |
| Sore Throat | Possible | Remotely | Mild | No | No | Recovered, no residual effects | |
| Headache | No | Possibly | Mild | OTC med | No | Recovered, no residual effects | |
| 004 | Hot flashes | No | Possibly | Mild | No | No | Recovered, no residual effects |
| Neck and back pain | No | Not related | Mild | No | No | Chronic disorder, not resolved | |
| Depression | No | Not related | Moderate | Rx med | No | Recovered, no residual effects | |
| Headache | No | Not related | Mild | OTC med | No | Recovered, no residual effects | |
| Ocular Discomfort | No | Not related | Moderate | ATs | No | Recovered, no residual effects | |
| 005 | Skin rash | No | Not related | Moderate | Benedryl | No | Recovered, no residual effects |
| Headache | No | Probably | Mild | OTC med | No | Recovered, no residual effects | |
| Sinusitis | No | Not related | Mild | No | No | Recovered, no residual effects | |
| Lymphocytosis | No | Probably | Mild | No | No | Recovered, no residual effects | |
| Fatigue | No | Possibly | Mild | No | No | Recovered, no residual effects | |
| 006 | Lymphocytosis | No | Possibly | Mild | No | No | Recovered, no residual effects |
| Ocular discomfort | No | Remotely | Mild | No | No | Recovered, no residual effects | |
| Dizziness and nausea | No | Remotely | Mild | No | No | Recovered, no residual effects |
All adverse events associated with the study medication were classified as not related, remotely related, possibly related, probably related or related. All events were transient and resolved spontaneously or with minimum treatment.
All participants showed a reduction in CME, as evidenced by OCT. Mean CMT reductions were 128 ± 105 μm (worse eye range, 48–301) and 57 ± 68 μm (better eye range, 1–19). Those patients with significant edema (>600 μm, three patients in total) had, on average, greater amounts of reduction in CME (average, 186 μm vs. 87 μm; Fig. 1). All patients experienced an improvement in visual acuity; mean BCVA improvement was 6.7 ± 6.9 ETDRS letters (worse eye) and 1.7 ± 5.2 letters (better eye). Those with baseline vision of 20/125 or worse experienced at least one line or more of improvement. Five of the six participants maintained clinical quiescence of their uveitis during the study, whereas one patient required the addition of cyclosporine to achieve quiescence. Three patients were able to reduce their concomitant immunosuppressive medications by 50%, defined as a reduction in the number of their immunosuppressive medications by half or reduction of the dosages of their medications by half.
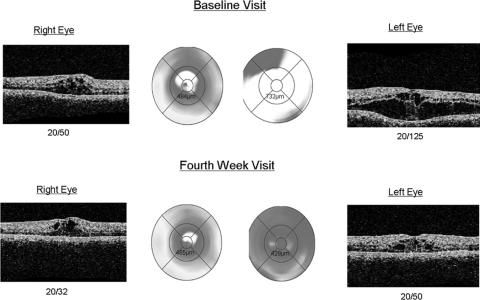
Figure 1.
OCT for patient 2. Comparison of the initial visit (top) with the 4th week visit (bottom) demonstrates considerable improvement, OS ≫ OD, in CME in both eyes, with subsequent improvements in visual acuity and CMT.
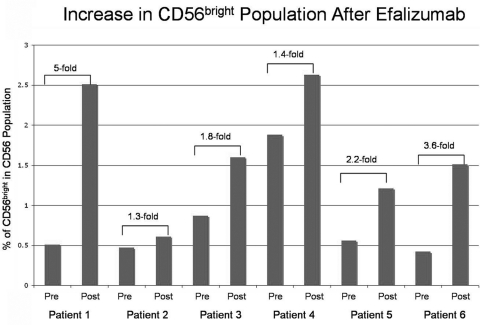
All patients showed an expansion in the CD56bright regulatory NK cell population (range, 1.3–5.5-fold increase; Figs. 2, 3) from baseline. The three patients with the most CME showed the least amount of expansion (average increase, 1.5-fold vs. 3.6-fold). Those participants in which immunosuppression was reduced had an average increase of 2.4-fold, whereas the patient who needed additional medications had only a 1.4-fold increase.
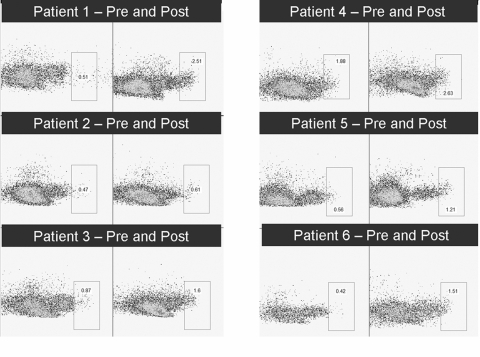
Figure 2.
Flow cytometry of CD56bright cells before and after anti-CD11a treatment of the six participants. All posttreatment analyses demonstrated an increase in the CD56bright cell population.
Figure 3.
Cell counts showed a 1.3- to 5-fold increase in CD56bright cells in the six participants.
Discussion
Humanized anti-CD11a antibody (efalizumab) has been approved by the FDA for the treatment of adults with moderate to severe chronic plaque psoriasis at the time this study was conducted. It had shown efficacy in the treatment of psoriasis, a T-cell-mediated disease. This study is the first to use anti-CD11a in uveitis patients and the first of all biological agents targeted against adhesion molecules to be used in the treatment of uveitis and/or associated macular edema in humans.
Our group had previously shown that interference with adhesion molecule functioning, including CD11a, positively altered the course of disease in animals with experimentally induced uveitis.7 Binding to CD11a on lymphocytes blocked the interaction between LFA-1 and ICAM-1 and, as a result, interrupted lymphocyte migration and inflammation. In this study, the addition of anti-CD11a not only controlled ocular inflammation and aided in the resolution of macular edema, but also allowed patients to reduce the amount of current immunosuppression, reflecting circumstances seen in the laboratory.
Vitreous levels of ICAM-1 have been found to be higher in patients with diabetic macular edema when compared with those in nondiabetic or diabetic patients without retinopathy.23 Dexamethasone has been shown to downregulate ICAM-1 expression in diabetic rats, correlating with its effect on leukocyte accumulation and retinal vascular permeability.24 We propose that targeting CD11a leads to a decrease in T-cell trafficking and adhesion, which is likely to lead to less permeability, less accumulation of macular fluid, and a chance for resorption; hence, patients would have improvement in macular edema and vision.
CD56bright regulatory NK cells have been proposed to play a regulatory role in immune responses.14,16–18 They have the ability to secrete large amounts of IL-10, an anti-inflammatory cytokine.20 We have previously shown that patients with active uveitis have a significantly lower level of CD56bright regulatory NK cells than do normal donors.20 In this pilot study, all patients demonstrated an expansion of CD56bright NK cells, consistent with their clinical picture and previous studies conducted at NEI. Of interest, those with more edema had less expansion. This may represent the difficulty in controlling their disease when compared with those less active. Little is known about the molecular mechanisms of daclizumab (anti-IL2R, or anti-CD25)-induced expansion of the peripheral CD56bright NK cell population. Our new observation that an anti-CD11a-blocking antibody, can also induce the expansion of the peripheral CD56bright NK cell population suggests that our previous observation of induction of CD56bright cells by the humanized anti-CD25 antibody daclizumab may be independent of blockage of the IL-2 receptor. Whether the induction of CD56bright NK cells is a general phenomenon of humanized antibody therapy is of particular interest. Further studies on other humanized monoclonal antibodies may help to shed light on the nature of this expansion of CD56bright cells.
All our patients tolerated the medication well and did not have any serious side effects. Side effects experienced were those similarly seen with conventional immunosuppressive therapies. Unfortunately, since the completion of this study, efalizumab (Raptiva; Genentech) has been taken off the market due to safety concerns. Three consecutive cases of progressive multifocal leukoencephalopathy (PML) were reported in long-term users of the medication. PML has been reported to occur with other immunosuppressive agents, such as mycophenolate mofetil (Cellcept; Genentech, South San Francisco, CA), that are commonly used for uveitis, but to date no PML has been reported in patients using these agents for uveitis.25 The events associated with efalizumab were typically seen in older patients who had used it for more than a year. One could postulate that since adhesion molecules play an important role in leukocyte recruitment and trafficking,7–12 agents targeting adhesion molecules would be more likely to cause immune function abnormalities that it can normally control. However, not much is known about risk factors associated with PML, and, once diagnosed, the prognosis is poor, making the decision to use efalizumab more difficult. Another monoclonal antibody against an adhesion molecule, natalizumab, has been approved by the FDA for the treatment of multiple sclerosis and, due to its association with PML, strict prescribing criteria have been assigned.
In summary, anti-CD11a therapy was found to be safe and well-tolerated by the patients in this short-term pilot study. Anti-CD11a treatment demonstrated improvement in visual function and macular thickness. Uveitis was controlled and current immunosuppressive regimens were reduced. Larger studies are needed to continue to assess the safety, tolerability, and efficacy of treatments targeting adhesion molecules for macular edema associated with uveitis or other disorders. With more adhesion molecules under investigation and their promising effects on the rise, these agents may eventually find their place in the field of autoimmune disorders with modification in dosing or application.
Footnotes
Presented at the American Academy of Ophthalmology, Atlanta, Georgia, August 2008.
Supported by the National Eye Institute's intramural research program.
Disclosure: L.J. Faia, None; H.N. Sen, None; Z. Li, None; S. Yeh, None; K.J. Wroblewski, None; R.B. Nussenblatt, None
References
- 1. Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113:1446–1449 [DOI] [PubMed] [Google Scholar]
- 2. Jabs DA, Rosenbaum JT. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2001;131(5):679. [DOI] [PubMed] [Google Scholar]
- 3. Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513 [DOI] [PubMed] [Google Scholar]
- 4. Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008;146(6):802–812, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raptiva package insert. South San Francisco, CA: Genentech, Inc.; 2009 [Google Scholar]
- 6. Gottlieb AB, Krueger JG, Wittkowski K, Dendrick R, Walicke PA, Garovoy M. Psoriasis as a model for T-cell-mediated disease: immunobiologic and clinical effects of treatment with multiple doses of efalizumab, an anti-CD11a antibody. Arch Dermatol. 2002;138(5):591–600 [DOI] [PubMed] [Google Scholar]
- 7. Whitcup SM, DeBarge LR, Caspi RR, Harning R, Nussenblatt RB, Chan CC. Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune uveitis. Clin Immunol Immunopathol. 1993;67(2):143–150 [DOI] [PubMed] [Google Scholar]
- 8. Whitcup SM, Hikita N, Shirao M, et al. Monoclonal antibodies against CD54 (ICAM-1) and CD11a (LFA-1) prevent and inhibit endotoxin-induced uveitis. Exp Eye Res. 1995;60(6):597–601 [DOI] [PubMed] [Google Scholar]
- 9. Leonardi CL. Efalizumab: an overview. J Am Acad Dermatol. 2003;49(2 suppl):S98–S104 [DOI] [PubMed] [Google Scholar]
- 10. Efalizumab (Raptiva) for treatment of psoriasis. Med Lett Drugs Ther. 2003;45(1171):97–98 [PubMed] [Google Scholar]
- 11. Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003;290(23):3073–3080 [DOI] [PubMed] [Google Scholar]
- 12. Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349(21):2004–2013 [DOI] [PubMed] [Google Scholar]
- 13. Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438 [PubMed] [Google Scholar]
- 14. Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–4486 [PubMed] [Google Scholar]
- 15. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640 [DOI] [PubMed] [Google Scholar]
- 16. Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191 [PubMed] [Google Scholar]
- 17. Campbell JJ, Qin S, Unutmaz D, et al. Unique subpopulations of CD56_NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482 [DOI] [PubMed] [Google Scholar]
- 18. Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151 [DOI] [PubMed] [Google Scholar]
- 19. Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin-2. J Clin Invest. 1993;91:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174(9):5187–5191 [DOI] [PubMed] [Google Scholar]
- 21. Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56 (bright) natural killer cells mediate immunomodulatory effects of IL-2R alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;11;103(15):5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Common Toxicity Criteria. Frederick, MD: National Cancer Institute, the National Institutes of Health; 2009. http://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_mapping_docs [Google Scholar]
- 23. Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79 [DOI] [PubMed] [Google Scholar]
- 24. Wang K, Wang Y, Gao L, Li X, Li M, Guo J. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull. 2008;31(8):1541–1546 [DOI] [PubMed] [Google Scholar]
- 25. Cellcept package insert. South San Francisco, CA: Genentech, Inc.; 2010 [Google Scholar]