This study demonstrates that visual signals from the fovea do not necessarily contribute to alterations in peripheral refraction observed in monkeys with experimentally induced axial myopia and reinforces the idea that peripheral vision dominates refractive development.
Abstract
Purpose.
The purpose of this study was to determine whether visual signals from the fovea contribute to the changes in the pattern of peripheral refractions associated with form deprivation myopia in monkeys.
Methods.
Monocular form-deprivation was produced in 18 rhesus monkeys by securing diffusers in front of their treated eyes between 22 ± 2 and 155 ± 17 days of age. In eight of these form-deprived monkeys, the fovea and most of the perifovea of the treated eye were ablated by laser photocoagulation at the start of the diffuser-rearing period. Each eye's refractive status was measured by retinoscopy along the pupillary axis and at 15° intervals along the horizontal meridian to eccentricities of 45°. Control data were obtained from 12 normal monkeys and five monkeys that had monocular foveal ablations and were subsequently reared with unrestricted vision.
Results.
Foveal ablation, by itself, did not produce systematic alterations in either the central or peripheral refractive errors of the treated eyes. In addition, foveal ablation did not alter the patterns of peripheral refractions in monkeys with form-deprivation myopia. The patterns of peripheral refractive errors in the two groups of form-deprived monkeys, either with or without foveal ablation, were qualitatively similar (treated eyes: F = 0.31, P = 0.74; anisometropia: F = 0.61, P = 0.59), but significantly different from those found in the normal monkeys (F = 8.46 and 9.38 respectively, P < 0.05).
Conclusions.
Central retinal signals do not contribute in an essential way to the alterations in eye shape that occur during the development of vision-induced axial myopia.
The eye's spherical equivalent refractive error can vary substantially with eccentricity.1–9 The exact pattern of peripheral refractions is important because peripheral refractive errors may contribute to the development of common central refractive errors and may potentially serve as predictive indicators for those individuals who are at risk for developing myopia.1,10–13 Moreover, in laboratory animals, peripheral visual signals, in isolation, can mediate many aspects of vision-dependent refractive development and when there are conflicting visual signals in the central and peripheral retina, the peripheral retinal signals can dominate axial growth and central refractive development.14–16 Because of the potential significance of peripheral refractive errors, it is important to understand the factors that influence the pattern of peripheral refractions and the shape of the posterior globe, the primary determinant of the pattern of peripheral refractions.
Visual experience can influence the pattern of peripheral refractions. In particular, because ocular growth and refractive development are mediated in large part by local-acting, vision-dependent mechanisms, experimentally imposed hemi-field form deprivation or optical defocus can produce localized alterations in the shape of the posterior globe and the pattern of peripheral refractions.17–21 However, visual manipulations that affect the entire field of view can also alter the pattern of peripheral refractions. For example, full-field form deprivation and full-field hyperopic defocus produce central axial myopia and relative peripheral hyperopia, at least along the horizontal meridian.21,22 The relative peripheral hyperopia is due to the fact that as the central axial length increases, the eye becomes less oblate in shape, i.e., the increases in vitreous chamber depth are smaller in the periphery than in the central retina. In this respect, the patterns of peripheral refractive errors in monkeys with experimentally-induced myopia are very similar to those in humans with common forms of myopia.5,22–25
Why full-field manipulations alter the shape of the eye and the pattern of peripheral refractions is not well understood. It has been argued that the changes in eye shape could be determined by mechanical factors. Extraocular mechanical factors,24,26–29 genetically determined regional variations in the mechanical properties of the sclera,30–32 and mechanical factors associated with the normal oblate geometry of the eye (Genest R, et al. IOVS 2010;51:ARVO E-Abstract 1193) have been hypothesized to influence eye shape during ocular growth. It is also possible that there are eccentricity-dependent variations in the number, density, and/or effectiveness of the local, vision-dependent regulatory mechanisms.4,10,23,24 These eccentricity-dependent variations could reflect changes in the density or number of critical retinal neurons or a range of potential components in the local ocular cascade that transforms visual signals into biochemical signals that alter the size of the eye.33,34 In addition, eccentricity-dependent variations in the strength or effectiveness of visual signals could also influence ocular growth and lead to alterations in ocular shape. For example, because the resolution of retinal neurons generally decreases with eccentricity in primate eyes, the impact of a given amount of optical defocus or a specific degree of form deprivation effectively decreases with eccentricity. In essence, the strength of full-field hyperopic defocus or form deprivation as stimuli for ocular elongation is greatest in the central retina and decreases with eccentricity.
To gain insights into the factors that influence the changes in eye shape during development of central axial myopia, we sought to determine whether visual signals from the central retina contribute to the characteristic changes in ocular shape associated with central myopia. Specifically we examined the effects of foveal ablation on the pattern of peripheral refractions in normal and form-deprived monkeys.
Materials and Methods
Subjects
The subjects were 35 infant rhesus monkeys (Macaca mulatta) that were obtained at 2 to 3 weeks of age and housed in our primate nursery that was maintained on a 12-hour light/12-hour dark lighting cycle (see Reference 35 for husbandry details). All rearing and experimental procedures were reviewed and approved by the University of Houston's Institutional Animal Care and Use Committee and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Data are presented for four subject groups. Two groups were reared with monocular form deprivation. For one of the form-deprived groups, which included eight monkeys, the visual signals from the central retina of the deprived eye were eliminated via laser photoablation at the start of the diffuser-rearing period (FA+FD monkeys). The retinas of the 10 monkeys in the other form deprivation group remained intact throughout the treatment period (FD monkeys). The FD monkeys provided comparison data for the effects of form deprivation on the pattern of peripheral refractions. For both form-deprived subject groups, the fellow eyes served as untreated controls. Control data for the laser photoablation procedures were obtained from a group of five monkeys that underwent monocular central retinal photoablation at 22 ± 4 days of age and subsequently reared with unrestricted vision (FA monkeys). The fellow eyes of the FA monkeys were untreated. Control data for normal peripheral refractions were obtained from 12 infant monkeys that were reared with unrestricted vision and without any treatment for either eye (normal monkeys).
Monocular form-deprivation was produced by securing a diffuser spectacle lens in front of the treated eyes (“LP” Bangerter Occlusion Foils attached to zero-powered lenses; Fresnel Prism and Lens Co., Prairie, MN) and a clear zero-powered lens in front of the fellow eyes (see References 15 and 16 for details). The diffuser-rearing procedures were initiated at 22 ± 2 days of age and the monkeys wore the diffusers continuously until 155 ± 17 days of age. For the FA+FD and FA monkeys, the foveas and most of the perifoveas (the central 10–12°) of their treated eyes were photocoagulated using either an argon or a frequency-doubled YAG (Yttrium Aluminium Garnet) laser (see Reference 15 for the details of the laser procedure). With both lasers, the foveal burns were overlapped to ensure complete photoablation of the fovea.
The data for the central refractive errors for the FA, FA+FD, and FD monkeys have also been previously reported.15,22,36 The central and peripheral refractive errors for six of the normal monkeys have also been previously reported.22 The rearing and measurement regimens for the six new normal monkeys were identical with those for the six normal monkeys that were studied previously, and thus the data for all 12 normal monkeys were combined as a single group.
Ocular Biometry
Ocular biometric measurements were performed at ages corresponding to the start of the diffuser-rearing period and periodically throughout the treatment period. Our basic methods have been described in detail elsewhere.20,36,37 In brief, to make the ocular measurements, the monkeys were anesthetized with an intramuscular injection of ketamine hydrochloride (15–20 mg/kg) and acepromazine maleate (0.15–0.2 mg/kg) and cycloplegia was produced with topically-administered 1% tropicamide. The refractive status of each eye was determined independently by two experienced investigators by streak retinoscopy and then averaged38 and specified as spherical-equivalent, spectacle-plane refractive corrections. Refractive errors were measured along the pupillary axis (the “central” refraction) and at 15° intervals along the horizontal meridian out to eccentricities of 45°.36 Throughout the report, eccentricities are specified with respect to the visual field.
Ocular axial dimensions were measured by A-scan ultrasonography implemented with either a 7 (Image 2000; Mentor, Norwell, MA) or 12 MHz transducer (OTI Scan 1000; OTI Ophthalmic Technologies, Inc, Ontario, Canada). Corneal curvature was measured with a hand-held keratometer (Alcon Auto-keratometer; Alcon Systems Inc., St. Louis, MO) and/or a videotopographer (EyeSys 2000; EyeSys Technologies Inc., Houston, TX). Both instruments provided repeatable and comparable measures of central corneal curvature in infant monkeys.37 The videotopographer also provided measures of the shape of the peripheral cornea.
Statistics
Repeated measures ANOVAs (SuperANOVA; Abacus Concepts, Inc., Berkeley, CA) and multiple comparisons were used to detect if there were differences in refractive errors as a function of eccentricity between eyes and between subject groups (i.e., differences in the patterns of peripheral refraction). Probability values were adjusted with the Geisser-Greenhouse correction to compensate for the violations of the assumption of sphericity.39 When no significant eccentricity-dependent differences were found, power analyses were performed (G* power 3.1.2; Franz Faul, University of Kiel, Germany)40 to calculate the minimum effect sizes that the repeated measures ANOVAs were likely to detect. Specifically, for comparisons between the normal and FA monkeys, interocular and between group eccentricity-dependent differences of 0.75 diopters (D) could be detected with powers greater than 0.9. Primarily because of greater intersubject variability, similar comparisons between the FA+FD and FD monkeys could detect eccentricity-dependent differences of 2.0 D with powers greater than 0.8. The comparisons between normal monkeys and the fellow eyes of FA+FD or FD monkeys manifested powers greater than 0.9 for detecting eccentricity-dependent differences of 1.0 D (FA+FD monkeys) and 1.50 D (FD monkeys), respectively. Orthogonal regression analysis was performed to compare the relationship between the central ametropias and the relative peripheral refractive errors between subject groups. Paired t-tests and two-sample t-tests were used to compare individual central refractive errors and ocular dimensions between eyes (e.g., treated versus fellow) and between groups, respectively. When data are reported as means, the variability of the data are expressed as standard deviations.
Results
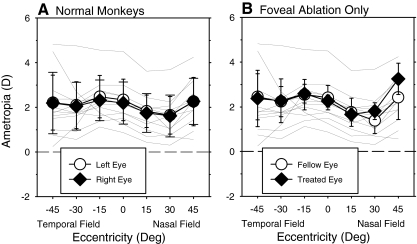
At ages corresponding to the end of the diffuser-rearing period (152 ± 12 days), the normal monkeys exhibited similar amounts of moderate hyperopia along the pupillary axes in both eyes (Fig. 1A, right eye average = +2.20 ± 0.94 D; left eye average = +2.33 ± 0.93 D); the average absolute degree of central anisometropia (right eye − left eye) was 0.22 ± 0.19 D. As previously reported,36 the peripheral refractive errors were generally less hyperopic than the central refraction, particularly in the nasal visual field, and the patterns of peripheral refractions in the two eyes were very similar (F = 0.21, P = 0.82).
Figure 1.
Mean (SD) spherical-equivalent, spectacle plane, refractive corrections plotted as a function of visual field eccentricity along the horizontal meridian for the treated (or right, filled symbols) and fellow eyes (or left, open symbols) of the normal monkeys and the monkeys that had foveal ablations in the treated eye and allowed unrestricted visual experience (FA monkeys). The thin solid lines illustrate the data for the right eyes of individual normal monkeys. Error bars represent standard deviations.
Foveal ablation did not produce systematic alterations in either the central or peripheral refractive errors of the treated eyes. The average respective central refractive errors were +2.26 ± 0.27 D and +2.41 ± 0.54 D for the treated and fellow eyes of the FA monkeys, which were not significantly different from the central refractions for the normal monkeys (treated eyes: T = −0.22, P = 0.83; fellow eyes: T = −0.59, P = 0.57). Moreover, there were no statistically significant differences in the patterns of peripheral refractions between the treated eyes of the FA monkeys and those for the eyes of normal monkeys (F = 1.48, P = 0.25). There were significant eccentricity-dependent differences in the patterns of peripheral refractions between the treated and fellow control eyes of the FA monkeys (F = 4.76, P = 0.02), which post hoc tests revealed were due primarily to the fact that the treated eyes were significantly more hyperopic than their fellow eyes at the 45° nasal field eccentricity (P = 0.001). However, the central and peripheral refractions for all treated eyes and the degree of anisometropia at every eccentricity for each FA monkey were within the range of values for the normal monkeys (see Fig. 2).
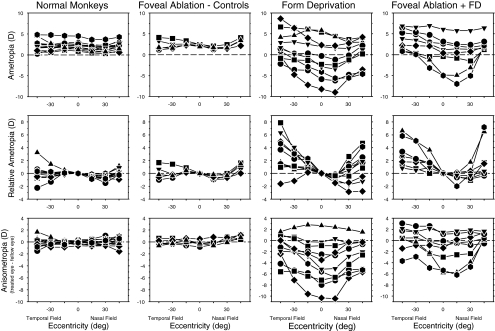
Figure 2.
In the top row, spherical-equivalent, spectacle plane, refractive corrections are plotted as a function of horizontal visual field eccentricity for the right eyes of individual normal monkeys (left column) and the treated eyes of the monkeys reared with monocular foveal ablation and unrestricted vision (second column, FA monkeys), monocular form deprivation (third column, FD monkeys), and monocular foveal ablation and form deprivation (right-most column, FA+FD monkeys). The relative peripheral refractive errors (peripheral − central refraction) for the right or treated eyes of individual animals are shown in the second row. The interocular differences in refractive error are plotted as a function of horizontal visual field eccentricity for individual animals in the bottom row.
The patterns of peripheral refractive errors, represented in three formats, are shown for each monkey in Figure 2. The right- (normal monkeys) or treated-eye ametropias measured along the horizontal meridian at the end of the treatment period are shown in the top row of plots; the relative peripheral refractive errors for the right and/or treated eyes (peripheral − central refractions) and the interocular differences in refractive errors (treated eye − fellow eye) are illustrated in the middle and bottom rows, respectively. As reflected by the average refractions shown in Figure 1, the peripheral refractive errors in the treated eyes of the FA monkeys were similar to those in normal monkeys (relative ametropia: F = 1.48, P = 0.25; anisometropia: F = 1.82, P = 0.17); for each presentation format, the data for all FA monkeys fell within the range of values for the normal monkeys.
In many respects, the results for the two groups of form-deprived monkeys were qualitatively similar. Form deprivation disrupted refractive development producing a wide range of central refractive errors in the FD (central range = +5.81 to −5.88 D) and FA+FD monkeys (central range = +6.06 to −4.88 D). For nine of the 10 FD monkeys and six of the eight FA+FD monkeys, the central refractive errors of the treated eyes were less hyperopic/more myopic than their fellow eyes (Fig. 2 bottom row). The average degree of central form deprivation myopia in the FA+FD monkeys (−1.76 ± 2.75 D) was smaller than that for the FD monkeys (−4.08 ± 3.94 D), probably reflecting the loss of the normal contribution of the central retina to the form deprivation response. However, these differences were not significant (T = 1.47, P = 0.16). In addition, the patterns of peripheral refractive errors for the FD and FA+FD monkeys were qualitatively similar (treated eyes: F = 0.31, P = 0.74; anisometropia: F = 0.61, P = 0.59). However, in this respect it is important to note that due to the high degree of intersubject variability in the FD and FA+FD groups, our ability to detect small eccentricity-dependent difference in refractive error was limited. On the other hand, the patterns of peripheral refractions in the treated eyes of both groups of form-deprived monkeys were very different from those found in the normal monkeys (FD monkeys: F = 8.46, P = 0.0009; FA+FD monkeys: F = 9.38, P = 0.0007). For example, for the animals that developed form-deprivation myopia, the greatest myopic alterations were typically observed in the central and near nasal visual fields and the degree of myopia decreased with eccentricity (Fig. 2 top and bottom row). As a result, the treated eyes showed relative peripheral hyperopia, particularly in the temporal visual field (Fig. 2 middle row), and the interocular differences in refractive error decreased with eccentricity in both the nasal and temporal visual fields (bottom row).
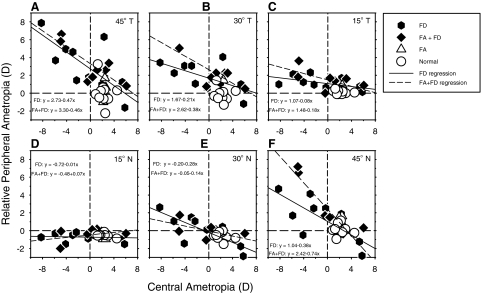
In Figure 3, the relative peripheral refractions for the treated (or right) eyes are plotted as a function of the central ametropia for individual animals. The solid and dashed lines represent the orthogonal regression lines at different eccentricities (panels A–F) for the FD and FA+FD monkeys, respectively. For all eccentricities except the 15° nasal visual field (panel D), the relative peripheral refractions were negatively correlated with the central refractive errors. Specifically, the degree of relative peripheral hyperopia increased with the degree of central myopia. For all eccentricities except the 45° nasal visual field eccentricity (panel F), the slopes of the orthogonal regression lines for the FD and FA+FD monkeys were similar. In particular, the slopes of the functions for the FA+FD monkeys fell within the 95% confidence limits for the regression lines for the FD monkeys and vice versa. Although the orthogonal regression line slope for the 45° nasal field location for the FA+FD monkeys was outside the confidence limits for the FD monkey's function, the slopes of the linear regression lines calculated for these two subject groups were not statistically different (P = 0.09).
Figure 3.
Relative peripheral refractive corrections (peripheral − central refraction) for the right or treated eyes of individual monkeys plotted as a function of the central ametropia for each horizontal field eccentricity for the normal monkeys and the monkeys reared with monocular foveal ablation and unrestricted vision (FA monkeys), monocular form deprivation (FD monkeys), and monocular foveal ablation and form deprivation (FA+FD monkeys). The solid and dashed lines represent the orthogonal regression lines for the FD and FA+FD monkeys, respectively.
A key point illustrated in Figure 3 is that the influence of the central ametropia, as reflected in the slopes of the orthogonal regression lines, increased systematically with eccentricity. For example, at 15° in the temporal field, every −1.00 D of central ametropia was associated with less than +0.25 D (+0.08 D for FD, and +0.18 D for FA+FD monkeys) of relative peripheral hyperopia, and the amount of relative peripheral hyperopia increased to about +0.50 D (+0.47 D for FD, and +0.46 D for FA+FD monkeys) for every diopter of relative central myopia at the 45° temporal eccentricity. In the nasal field, the amount of relative peripheral hyperopia associated with every −1.00 D of central myopia was essentially zero at the 15° eccentricity and increased to +0.28 D (FD) and +0.14 D (FA+FD) at the 30° eccentricity, and +0.38 D (FD) and +0.74 D (FA+FD) at the 45° nasal eccentricity.
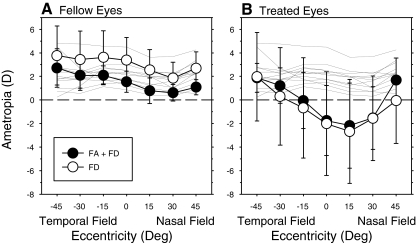
The analysis in Figure 3 indicates that when the treated eyes of FD and FA+FD monkeys have similar central refractions, the peripheral refractions should also be similar. To illustrate this point, Figure 4 shows the average refractive errors plotted as a function of eccentricity for the fellow (A) and deprived eyes (B) of a subset of FD and FA+FD monkeys that had comparable amounts of form deprivation myopia. In particular, Figure 4 summarizes data for the eight FD monkeys and the five FA+FD monkeys that exhibited greater than −0.50 D of central myopic anisometropia (treated eye − fellow eye). The average central ametropias for the treated eyes of these FD and FA+FD monkeys were −2.02 ± 4.40 D and −1.75 ± 2.97 D, respectively (T = −0.13; P = 0.90). For both subgroups of monkeys, the average central and peripheral refractive errors of the fellow eyes (Fig. 4A) were within the normal ranges at all eccentricities. The central refractions of the fellow eyes of the FD monkeys were more hyperopic than the fellows eyes of the FA+FD monkeys (+3.38 ± 1.93 vs. +1.55 ± 0.90 D; T = 2.32, P = 0.043) [Note that there were no differences in the mean central refractions when all the fellow eyes were included in the analysis (+3.24 ± 1.78 vs. +2.14 ± 1.28; T = 1.55, P = 0.14)]. However, there were no between group differences in the pattern of peripheral refractions for the fellow eyes (F = 0.33, P = 0.71), and the functions for both subgroups of form-deprived monkeys were not significantly different from the normal monkeys (FD monkeys: F = 3.14, P = 0.06; FA+FD monkeys: F = 2.80, P = 0.09). In contrast, the refractive errors for the central visual fields of the treated eyes were outside of the normal range for both the FD and FA+FD monkeys (Fig. 4B), and the degree of myopia significantly decreased with eccentricity in both the nasal and temporal fields (FD monkeys: F = 14.06, P = 0.0001; FA+FD monkeys: F = 11.58, P = 0.0001). For both groups of monkeys, there were significant eccentricity-dependent differences in refractive error between their fellow and treated eyes (FD monkeys: F = 10.48, P = 0.001; FA+FD monkeys: F = 6.09, P = 0.03). However, the patterns of peripheral refractions of the treated eyes were not significantly different between the FD and FA+FD monkeys (F = 0.73, P = 0.51).
Figure 4.
Mean (± SD) spherical-equivalent, spectacle plane refractive corrections plotted as a function of horizontal visual field eccentricity for fellow (A) and treated eyes (B) of the monkeys reared with foveal ablation and form deprivation (FA+FD monkeys) and those reared with form deprivation alone (FD monkeys). The thin solid lines illustrate the data for right eyes of individual normal monkeys. Error bars represent standard deviations.
Comparisons between the treated and fellow eyes for the subset of FD and FA+FD monkeys that exhibited greater than −0.50 D of central myopic anisometropia revealed that there were no significant interocular differences in anterior chamber depth or lens thickness for any group of monkeys (T values ranged from −1.47 to 1.42; P values ranged from 0.186 to 0.711). However, the interocular differences in central refractive error observed in both groups of form-deprived monkeys were associated with interocular differences in vitreous chamber depth. Specifically, the average vitreous chamber depths for the treated eyes of the FD (10.88 ± 1.03 mm vs. 9.94 ± 0.69 mm; T = 4.06, P = 0.005) and FA+FD monkeys (10.49 ± 0.72 mm vs. 9.73 ± 0.26 mm; T = 2.93, P = 0.04) were significantly longer than those for their fellow eyes. The average interocular differences in central vitreous chamber depth (treated eye − fellow eye) for the FD (0.94 ± 0.66) and FA+FD monkeys (0.76 ± 0.58 mm) were similar (T = 0.53, P = 0.61) and significantly larger than those exhibited by normal monkeys (FD monkeys: 0.03 ± 0.10 mm; T = 3.91, P = 0.006; FA+FD monkeys: T = 2.80, P = 0.049). On the other hand, in the FA monkeys, foveal ablation by itself did not produce significant interocular differences in central vitreous chamber depth (treated eye = 10.19 ± 0.22 mm, fellow eye = 10.16 ± 0.13 mm, T = 0.47, P = 0.67).
The pattern of peripheral refractions is influenced by the shape of the posterior globe and eccentricity dependent changes in optical power. We have previously reported that the changes in the pattern of peripheral refractions in form-deprived monkeys were closely associated with alterations in the shape of the posterior globe measured via magnetic resonance imaging.22 Although we did not obtain imaging data from the FA+FD monkeys, the alterations in the peripheral refractions of these animals cannot be attributed to alterations in the cornea. The corneal topographies of the treated and fellow eyes of the FA+FD monkeys were similar (average corneal power: OD = 55.09 ± 1.52, OS = 54.77 ± 1.62; T = 1.20, P = 0.30); in particular, the rates of peripheral corneal flattening were not significantly different between the treated and fellow eyes (average Q-values: OD = 0.024 ± 0.197, OS = −0.179 ± 0.464; T = 0.72, P = 0.51). In addition, the changes in radial astigmatism as a function of eccentricity were comparable in the treated and fellow eyes of the FA+FD monkeys (F = 2.27, P = 0.14) and there were no statistically significant differences between the FD and FA+FD monkeys in the amounts of astigmatism as a function of eccentricity (F = 0.61, P = 0.62).
Discussion
Many vision-dependent aspects of central refractive development are not dependent on visual signals from the fovea.14–16,41,42 In particular, visual signals from the fovea are not essential for emmetropization, central axial myopia produced by form deprivation, or the compensating axial growth produced by experimentally imposed refractive errors.14–16 Likewise, the results of this study indicate that visual signals from the fovea do not play a critical role in peripheral refractive error development. Specifically, the similarities in the pattern of peripheral refractions between normal monkeys and the FA monkeys indicate that emmetropization in the periphery is not dependent on central vision. Moreover, the similarities in the patterns of peripheral refractions between the FD and FA+FD monkeys indicate that visual signals from the fovea are not essential for the development of the relative peripheral hyperopia found in animals with central axial myopia. The results from both groups of monkeys with foveal ablations also support the idea that peripheral vision can dominate ocular growth and refractive development and, in particular, extend previous observations on the impact of peripheral vision on axial length and central refraction to eye shape and the pattern of peripheral refractive errors.
While there are nonvisual processes that influence the overall size and shape of the globe, it is likely that the local acting, vision-dependent mechanisms that regulate refractive development10,19–21 were responsible for the close interocular correspondence between the patterns of peripheral refractive errors in the two eyes of the normal and FA monkeys. First, the operating properties of the local acting mechanisms that regulate ocular shape would be expected to be the same in the two eyes of a given animal (e.g., the mechanisms in the two eyes would have similar gains or sensitivities to visual signals). In addition, the nature of the peripheral visual signals would be expected to be very similar in the two eyes. Foveal ablation does not appear to alter the cornea or crystalline lens. In this respect, our keratometric measures confirmed that there were no systematic interocular differences in central corneal power or shape and, while we did not assess crystalline lens curvature, there were no interocular differences in lens thickness or anterior chamber depth in the FA monkeys. In other words, the optical properties of the two eyes were not obviously different. Finally, the overall effective focus of the two eyes across the visual field should have been similar because fixation and accommodation, which are strongly yoked in primates,43,44 were controlled by the fellow non-treated eyes in the FA monkeys. In essence, the interocular agreement in peripheral refraction in the normal and FA monkeys came about because the two eyes experienced very similar visual images across most of the retina.
In monkeys with central axial myopia produced by either form deprivation or hyperopic defocus, the accompanying alterations in the pattern of peripheral refractions are strongly correlated with changes in the shape of the posterior globe.16,20–22 Specifically, the relative peripheral hyperopia associated with central axial myopia produced by form deprivation primarily reflects the fact that the vitreous chamber becomes less oblate/more prolate in shape during vision-induced central axial elongation.16,20–22 Given the quantitative and qualitative similarities in the peripheral refractions of the myopic eyes in the FD and FA+FD monkeys, it is likely that the alterations in peripheral refractive errors in the FA+FD monkeys are also due to prolate changes in the posterior globe. In this respect, the results indicate that central retinal signals do not contribute in an essential way to the alterations in eye shape that occur during the development of vision-induced axial myopia. On the other hand, visual signals from the peripheral retina, in isolation, are capable of altering the overall shape of the eye and the pattern of peripheral refraction. The fact that foveal ablation did not alter the pattern of peripheral refractions associated with form-deprivation myopia suggests that several factors are unlikely to contribute significantly to the eccentricity-dependent differences in axial elongation associated with central myopia. For example, it is unlikely that the observed changes in shape came about because the central retina was affected more by the diffuser lenses than the peripheral retina. In other words, the changes in shape do not simply reflect an eccentricity-dependent decrease in the strength of the visual signal for growth, or conversely an eccentricity-dependent increase in the strength of visual signals to slow growth. It is also unlikely that the observed shape changes simply reflect eccentricity dependent differences in the density of some critical population of retinal neurons.
Although there is still much to learn about the factors that influence the changes in eye shape and the pattern of peripheral refractions that occur during the development of central myopia, the results of this study serve as another example of the potential significance of peripheral vision in the regulation of eye growth and refractive development. Previous studies have shown that peripheral vision can directly alter central axial elongation rates resulting in anomalous central ametropia. This study shows that peripheral vision can, via its influence on peripheral refraction and presumable eye shape, put the eye at risk for the development of central ametropias. In this respect, the results emphasize the importance of taking into account peripheral vision when assessing the effects of vision on refraction. These results also highlight the need to investigate whether potential peripheral optical treatment strategies for controlling myopia progression produce unwanted alterations in the pattern of peripheral refractive errors.
Acknowledgments
The authors thank Dr. Ying-sheng Hu for assistance with the statistical analyses.
Footnotes
Supported by National Institutes of Health Grants EY-03611, EY-07551, and RR-17205; funds from the Vision CRC and the UH Foundation. JH was supported by an Ezell Fellowship from the American Optometric Foundation.
Disclosure: J. Huang, None; L.-F. Hung, None; E.L. Smith III, P
References
- 1. Stone RA, Flitcroft DI. Ocular shape and myopia. Ann Acad Med Singapore. 2004;33:7–15 [PubMed] [Google Scholar]
- 2. Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981;58:691–695 [DOI] [PubMed] [Google Scholar]
- 3. Millodot M, Lamont A. Letter: refraction of the periphery of the eye. J Opt Soc Am. 1974;64:110–111 [DOI] [PubMed] [Google Scholar]
- 4. Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A Opt Image Sci Vis. 2002;19:2363–2373 [DOI] [PubMed] [Google Scholar]
- 5. Atchison DA, Pritchard N, Schmid KL, Scott DH, Jones CE, Pope JM. Shape of the retinal surface in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2005;46:2698–2707 [DOI] [PubMed] [Google Scholar]
- 6. Ferree CE, Rand G. Interpretation of refractive conditions in the peripheral field of vision. Arch Ophthalmol. 1933;9:925–938 [Google Scholar]
- 7. Ferree CE, Rand G, Hardy C. Refraction for the peripheral field of vision. Arch Ophthalmol. 1931;5:717–731 [Google Scholar]
- 8. Ferree CE, Rand G, Hardy C. Refractive asymmetry in the temporal and nasal halves of the visual field. Am J Opthalmol. 1932;15:513–522 [Google Scholar]
- 9. Rempt F, Hoogerheide J, Hoogenboom WP. Peripheral retinoscopy and the skiagram. Ophthalmologica. 1971;162:1–10 [DOI] [PubMed] [Google Scholar]
- 10. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468 [DOI] [PubMed] [Google Scholar]
- 11. Hoogerheide J, Rempt F, Hoogenboom WP. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–215 [DOI] [PubMed] [Google Scholar]
- 12. Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmid G. Retinal steepness vs. myopic shift in children. Optom Vis Sci. 2004;12S:23. [DOI] [PubMed] [Google Scholar]
- 14. Smith EL, 3rd, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith EL, 3rd, Ramamirtham R, Qiao-Grider Y, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668 [DOI] [PubMed] [Google Scholar]
- 18. Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Invest Ophthalmol Vis Sci. 1984;25:652–659 [PubMed] [Google Scholar]
- 19. Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77 [DOI] [PubMed] [Google Scholar]
- 20. Smith EL, 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith EL, 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang J, Hung LF, Ramamirtham R, et al. Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2009;50:4033–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Logan NS, Gilmartin B, Wildsoet CF, Dunne MC. Posterior retinal contour in adult human anisomyopia. Invest Ophthalmol Vis Sci. 2004;45:2152–2162 [DOI] [PubMed] [Google Scholar]
- 24. Atchison DA, Jones CE, Schmid KL, et al. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004;45:3380–3386 [DOI] [PubMed] [Google Scholar]
- 25. Singh KD, Logan NS, Gilmartin B. Three-dimensional modeling of the human eye based on magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2006;47:2272–2279 [DOI] [PubMed] [Google Scholar]
- 26. Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030 [PubMed] [Google Scholar]
- 27. Greene PR. Mechanical considerations in myopia: relative effects of accommodation, convergence, intraocular pressure, and the extraocular muscles. Am J Optom Physiol Opt. 1980;57:902–914 [PubMed] [Google Scholar]
- 28. van Alphen GW. Choroidal stress and emmetropization. Vision Res. 1986;26:723–734 [DOI] [PubMed] [Google Scholar]
- 29. Walker TW, Mutti DO. The effect of accommodation on ocular shape. Optom Vis Sci. 2002;79:424–430 [DOI] [PubMed] [Google Scholar]
- 30. Feldkaemper MP, Burkhardt E, Schaeffel F. Localization and regulation of glucagon receptors in the chick eye and preproglucagon and glucagon receptor expression in the mouse eye. Exp Eye Res. 2004;79:321–329 [DOI] [PubMed] [Google Scholar]
- 31. Schippert R, Brand C, Schaeffel F, Feldkaemper MP. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res. 2006;82:710–719 [DOI] [PubMed] [Google Scholar]
- 32. Mathis U, Schaeffel F. Transforming growth factor-beta in the chicken fundal layers: an immunohistochemical study. Exp Eye Res. 2010;90:780–790 [DOI] [PubMed] [Google Scholar]
- 33. Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41:2050–2058 [PubMed] [Google Scholar]
- 34. McBrien NA, Cottriall CL, Annies R. Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Vis Neurosci. 2001;18:571–580 [DOI] [PubMed] [Google Scholar]
- 35. Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435 [DOI] [PubMed] [Google Scholar]
- 36. Hung LF, Ramamirtham R, Huang J, Qiao-Grider Y, Smith EL., 3rd Peripheral refraction in normal infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2008;49:3747–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kee CS, Hung LF, Qiao Y, Habib A, Smith EL., 3rd Prevalence of astigmatism in infant monkeys. Vision Res. 2002;42:1349–1359 [DOI] [PubMed] [Google Scholar]
- 38. Harris WF. Algebra of sphero-cylinders and refractive errors, and their means, variance, and standard deviation. Am J Optom Physiol Opt. 1988;65:794–802 [DOI] [PubMed] [Google Scholar]
- 39. Keselman HJ, Algina J, Kowalchuk RK. The analysis of repeated measures designs: a review. Br J Math Stat Psychol. 2001;54:1–20 [DOI] [PubMed] [Google Scholar]
- 40. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160 [DOI] [PubMed] [Google Scholar]
- 41. Artal P, Derrington AM, Colombo E. Refraction, aliasing, and the absence of motion reversals in peripheral vision. Vision Res. 1995;35:939–947 [DOI] [PubMed] [Google Scholar]
- 42. Wang YZ, Thibos LN, Bradley A. Effects of refractive error on detection acuity and resolution acuity in peripheral vision. Invest Ophthalmol Vis Sci. 1997;38:2134–2143 [PubMed] [Google Scholar]
- 43. Campbell FW. Correlation of accommodation between the two eyes. J Opt Soc Am. 1960;50:738. [DOI] [PubMed] [Google Scholar]
- 44. Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optom Vis Sci. 2009;86:E31–E39 [DOI] [PMC free article] [PubMed] [Google Scholar]