Intraperitoneal and intravitreal injections of endotoxin induce comparable cytokine responses in mouse eyes, but only local injection results in substantial cellular infiltration. This paradox probably results from desensitization of circulating neutrophils when endotoxin is injected systemically.
Abstract
Purpose.
A marked cellular infiltrate has been observed when endotoxin (lipopolysaccharide [LPS]) is injected into the mouse eye, but systemically injected LPS does not produce a comparable effect. Several hypotheses were tested to reconcile this discordance.
Methods.
BALB/c mice were injected intravitreally (ivt) or intraperitoneally (ip) with Escherichia coli LPS. Uveitis was assessed by traditional and intravital microscopy. Cytokine levels in the eye, plasma, or spleen were measured by single or multiplex ELISA assays.
Results.
The eye's higher sensitivity was confirmed to local LPS exposure, as 250 ng ivt LPS produced a brisk leukocytic infiltrate whereas ip injection of 100 μg LPS did not. The hypothesis was tested that the lack of a cellular infiltrate after ip LPS is explained by less induction of cytokines in the eye, but surprisingly, ip LPS resulted in comparable cytokine levels to ivt LPS. The hypothesis was disproved that the eye's sensitivity to local LPS is due to lack of expression of intracellular inhibitors of LPS such as A20, IRAK-M, or SARM. Finally, the hypothesis that systemic LPS inhibits diapedesis was tested by injection of LPS ip and ivt simultaneously, a strategy that did not significantly reduce leukocyte rolling or sticking in iris vessels but blocked the cellular infiltrate normally seen with ivt LPS.
Conclusions.
Systemic and local LPS exposures produce discordant effects within the murine eye. The hypothesis that systemic LPS desensitizes leukocytes to the stimuli responsible for transmigration offers a plausible explanation for this discordance.
Uveitis, which is inflammation of the uvea of the eye, is a clinical condition that often arises in young adulthood. Although it has a point prevalence of only about 1 per 1000,1 the early onset, in comparison to diseases like diabetic retinopathy and macular degeneration, makes uveitis roughly comparable to diabetes in terms of years of visual loss within the population.
Uveitis can be induced in laboratory animals through a variety of paradigms, just as uveitis clinically is a heterogeneous collection of diseases.2 Endotoxin-induced uveitis (EIU) in rats was first described in 19803 and remains one of the most frequently studied animal models of acute inflammatory uveitis. The systemic injection of endotoxin, also known as lipopolysaccharide (LPS), in most strains of rats produces a brisk, but transient, leukocyte infiltrate in the eye, especially in the anterior uveal tract. Mice are also susceptible to LPS-induced eye inflammation, but we and others have observed only a very scant cellular infiltrate in the anterior uveal tract after LPS is injected systemically.4 If, however, the LPS is injected into the vitreous of a mouse eye, a marked cellular infiltration occurs.5
The differences in the murine eye's responsiveness to local versus systemic LPS exposure remain unexplained. We tested three hypotheses to investigate these differing responses: (1) locally injected LPS induces a greater inflammatory response than systemic LPS, because of increased cytokine synthesis; (2) locally injected LPS fails to induce one or more of the known negative regulators of LPS, such as A20, SARM, or IRAK-M; and (3) systemic LPS desensitizes circulating leukocytes to chemotactic stimuli, such that the leukocytes fail to enter the eye.
Methods
Mice
These studies were performed with female BALB/c mice (Jackson Laboratories, Bar Harbor, ME) between the ages of 8 and 10 weeks. Animals were fed standard laboratory chow and water ad libitum. The use of the animals was reviewed and approved by the OHSU Institutional Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of EIU
Escherichia coli 0111:B4 LPS (InvivoGen; San Diego, CA) was dissolved in pyrogen-free phosphate-buffered saline (PBS). Mice were administered either an intraperitoneal (ip) injection of 25 or 100 μg LPS in 200 μL or an intravitreal (ivt) injection of 250 ng LPS in 2 to 4 μL. The ivt injections were performed as previously described,5,6 in animals under 1.7% isoflurane anesthesia, with a 30-gauge needle, taking special care to avoid touching the lens. Control mice were injected with PBS alone.
Leukocyte Counts and Flow Cytometric Analysis
Total white blood cell counts were obtained with a hemocytometer. The effect on circulating neutrophil numbers was quantified by flow cytometry using the neutrophil-specific marker Ly-6G conjugated to phycoerythrin (PE) and its PE-conjugated isotype control (BD Pharmingen, San Jose, CA), as previously described.7
Intravital Microscopy
Intravital microscopy was used to measure the ongoing in vivo leukocyte response within the iris vasculature and extravascular tissue at the indicated times after ivt or ip LPS injection. At the time of videomicroscopy, mice were injected ip with rhodamine 6G (35 mg/kg, Sigma-Aldrich, St. Louis, MO) to label circulating leukocytes. After anesthesia with 1.7% isoflurane in oxygen, 10-second black-and-white videos from three independent regions of the iris vasculature were captured with a video camera (Kappa Scientific, Gleichen, Germany) on an epifluorescence microscope (Orthoplan; Leica, Wetzlar, Germany). Measurements of the diameter and length of each vessel segment or iris tissue and counts of rolling, adhering, and infiltrating leukocytes were made off-line with ImageJ software as previously described (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).8,9
Histopathology
The mice were euthanatized and the eyes harvested between 4 and 24 hours after LPS or saline injections. The eyes were formalin-fixed overnight and embedded in paraffin. Seven-micrometer sections were cut through the pupillary–optic nerve axis at four different depths and then stained with hematoxylin and eosin (H&E). The number of infiltrating leukocytes within the anterior and posterior chambers, but not the vitreous chamber, in four sections at each of the four different levels was determined by a masked observer.
Cytokine Analysis
Protein was purified from whole eyes, dissected eye tissues (pools from eight mice) or spleen in lysis buffer containing 20 mM Tris-HCl (pH 7.6), 100 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, supplemented with 0.1 mg/mL phenylmethylsulfonyl fluoride and a protease cocktail inhibitor (Complete Mini; Roche, Mannheim, Germany), as previously published.7 Plasma was obtained by centrifuging approximately 1 mL of blood collected in tubes with 50 μL of 10 USP units/mL heparin flushing solution (Hepflush-10; APP Pharmaceutical, Schaumburg, IL). Protein concentrations were measured with BCA reactions (Pierce-Endogen, Rockford, IL). Cytokine levels were measured by standard ELISAs per the manufacturer's instructions (R&D Systems, Minneapolis, MN), except for the experiment in Figure 2, in which a multiplex ELISA was used (Luminex; Millipore, Billerica, MA). In the case of the ELISAs, particulates were first removed by centrifugation in filter tubes (0.22 μm; Millipore) and the ELISA data were analyzed on computer (BeadView Multiplex Analysis Software, ver. 1.0; Upstate Cell Signaling Solutions).
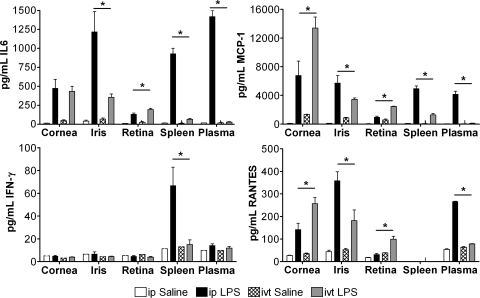
Figure 2.
Differential induction of cytokines by ip or ivt LPS. Mice were injected with ip 100 μg LPS or saline. The indicated cytokines were measured by multiplex ELISA in dissected eye tissues (cornea, iris, and retina) along with spleen and plasma at 4 hours after injection. Tissues dissected from eight mice (16 eyes) were pooled, and three independent pools were assayed for each data point. Values are the mean ± SEM, *P < 0.05 compared with saline treatment.
Immunoblot Analysis
Immunoblot analysis was performed as previously published10 on equal amounts of protein extracted as described above. Samples were separated on a 12% polyacrylamide gel (Bio-Rad, Hercules, CA), transferred onto polyvinylidene difluoride (Millipore) membrane, and blocked with PBS containing 5% nonfat milk and 0.1% Tween (Sigma-Aldrich). Primary antibodies to A20 (rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA), SARM (rabbit polyclonal; Abcam, Cambridge, MA), IRAK-M (rabbit polyclonal; Prosci, Poway, CA), and β-actin (clone AC-15; Sigma-Aldrich) were used. The near-infrared (NIR) signal was detected using NIR fluorophore-labeled secondary antibodies (goat anti-rabbit IgG with IRDye 680 and goat anti-mouse IgG with IRDye 800CW; LI-COR, Lincoln, NE), which were detected with a scanner and software (Odyssey; LI-COR).
Statistical Analysis
Data are reported as the mean ± SEM. Mean differences were compared and significant differences were determined by Student's t-test or ANOVA, as appropriate. Differences are considered statistically significant when P < 0.05.
Results
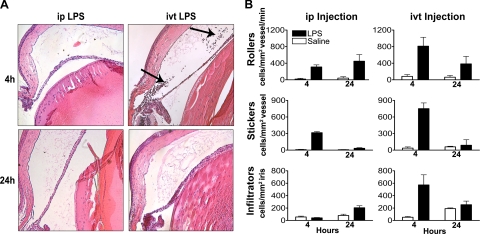
In pilot experiments, we were unable to find a dose of ip-injected LPS that produced a robust uveitis. In one set of mice, an optimal dose of 100 μg LPS resulted in only 3.6 ± 1.6 cells/section (n = 5) in the anterior and posterior chambers at 4 hours after injection and 12.8 ± 0.9 cells/section (n = 6) at 24 hours after injection. In contrast, ivt injection of another set of mice with 250 ng of LPS resulted in 341 ± 112 cells/section (n = 4) at 4 hours, which decreased to 24.4 ± 14.5 cells/section (n = 6) at 24 hours. Because these studies were not done simultaneously, we elected not to compare the number of cells by a statistical test, but the observations are consistent with what we and others have previously reported.4,11,12 It is not unusual to observe that a small dose of LPS injected into the eye produces 100 times the number of cells within the eye compared to that induced by a relatively large dose injected systemically. Representative histologic sections of eyes that display these differences are shown in Figure 1A.
Figure 1.
IP injection of LPS results in fewer infiltrating leukocytes in the iris than an ivt LPS injection but induces comparable numbers of rolling and adherent cells. Mice were administered ip injections of 100 μg LPS or saline. A separate group of mice received ivt injections of 250 ng LPS or saline. (A) The representative images of hematoxylin and eosin–stained sections of eyes obtained 4 and 24 hours after injections depict the lack of leukocytes within the aqueous humor of ip LPS-treated mice compared to ivt LPS-treated mice wherein numerous leukocytes are present within the aqueous humor and near the ciliary body (arrows). Original magnification, ×200. (B) Intravital videomicroscopy was performed at 4 and 24 hours to count rolling and adhering intravascular leukocytes and infiltrating extravascular leukocytes. Values are the mean ± SEM of two experiments, *P < 0.05 compared to saline treatment; n = 16 mice mice/treatment.
Intravital microscopy allows us to quantify leukocyte behavior in iris vessels and stroma in real time, thereby providing a more sensitive means than traditional histologic assessments to quantify cellular responses. As shown in Figure 1B, the ivt injection of LPS resulted in a significant increase in leukocyte rolling and adherence within the vasculature of the iris by 4 hours. A local injection of saline into the eye caused a small, transient increase in intravascular rolling or sticking, but few cells transmigrated into the iris stroma, and the increased adherence was not detectable 24 hours after the saline injection. Systemically administered LPS resulted in increased leukocyte rolling and adherence within the iris vasculature, comparable to that of ivt LPS, albeit some differences in kinetics were observed. Interestingly, in contrast to ivt LPS, ip LPS did not induce a cellular infiltrate that differed from that induced by saline after 4 hours and the number of infiltrating cells after 24 hours was approximately one third of the infiltration induced by ivt LPS at its peak (i.e., 4 hours; Fig. 1B).
A simple explanation for this difference could be that there is a greater induction of inflammatory cytokines within the eye when the LPS is injected locally. Consequently, we injected LPS or saline, either ivt or ip, and measured four cytokines (IL-6, MCP-1, IFNγ, and RANTES) in the cornea, iris/ciliary body, retina, spleen, and plasma (Fig. 2). Not surprisingly, we found that ip LPS (100 μg) induced IL-6, MCP-1, RANTES, and IFNγ production in the plasma and/or spleen, whereas the locally injected LPS (250 ng) did not have this effect. IFNγ was not detected in eyes after either ip or ivt LPS injections. The other three cytokines, IL-6, MCP-1, and RANTES, were readily detected in cornea and iris/ciliary body after either injection, whereas expression in the retina was somewhat less. The amounts produced by the iris/ciliary body were comparable to the amounts produced by the spleen or detected in the plasma. Finally, the levels of these three cytokines in the iris after ip LPS were significantly greater than the levels after ivt LPS with the quantities of LPS that were studied. Since these same doses produced a discordant response in the extent of a cellular infiltrate, we cannot invoke a failure to induce cytokines as a mechanism by which ip LPS fails to cause a marked leukocytic response in the eye.
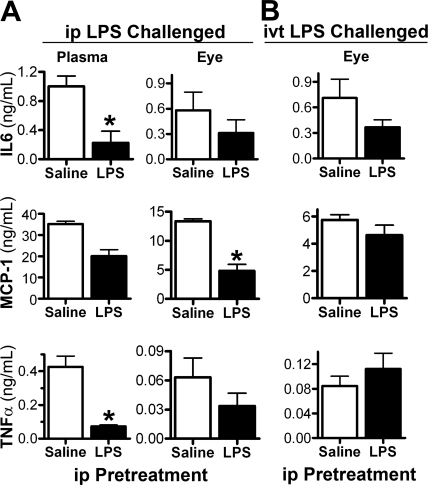
A well-known characteristic of LPS is that repeated exposure produces a pharmacologic tachyphylaxis or tolerance (meaning refractory state to subsequent LPS exposure). Mice were given a low-dose (25 μg) ip injection of either LPS or saline, and 72 hours later, all mice received a 100-μg ip LPS injection. As shown in Figure 3A (left), LPS pretreatment resulted in a reduced production of IL-6, MCP-1, or TNFα in the plasma in response to second LPS challenge (all have P ≤ 0.01), which would be indicative of systemic tolerance Similarly, two ip injections of LPS revealed some tolerance within the eye, as judged by reduced ocular cytokine levels in comparison to those from mice pretreated with saline (P < 0.001 for MCP-1; the reduction was not statistically significant for IL-6 or TNFα). In contrast, an ip injection of LPS did not consistently reduce the ocular cytokine production that resulted from an ivt injection of LPS 72 hours later (Fig. 3B). No evidence of tolerance was seen in the levels of MCP-1 or TNFα; although there was a nonsignificant trend toward reduced IL-6 levels. Figure 3A also shows that the level of TNFα induced in the eye by a single ip injection of LPS (i.e., from the saline pretreated mice) for this dosage and time point was relatively low—only 15% (P < 0.0001) of that detected in plasma—whereas the ocular MCP-1 and IL-6 levels were 37% (P = 0.002) and 58% (P = 0.15), respectively, of the level in plasma. Somewhat surprising is that, despite the robust cellular response to ivt LPS, very little TNFα was produced in the eye, and its levels were comparable to ip LPS (Fig. 3A).
Figure 3.
Responses to ivt LPS are less sensitive to tolerance from ip LPS pretreatment. All mice received an ip injection of either 25 μg LPS or saline. After 72 hours, they were challenged with either an ip 100 μg LPS injection or an ivt 250 ng LPS injection. Cytokine levels in eye tissue and plasma were measured by ELISA at 4 hours after challenge. Values are the mean ± SEM; *P < 0.05 compared with saline treatment; n = 10–12 mice/treatment. Results are representative of two experiments.
The activation of TLR4 by LPS induces a variety of intracellular negative regulators that dampen the inflammatory response. These intracellular signals contribute to endotoxin tolerance.13 Three previously established negative regulators are A20,14 SARM, and IRAK-M. We reasoned that a failure to induce any of these inhibitors by local LPS could account for a greater inflammatory response. As shown in Figure 4, all three of these inhibitors can be detected by immunoblotting in eye tissue, and each is induced by locally injected LPS (Fig. 4A). Interestingly, we find that locally injected LPS is comparable or even superior compared with ip LPS (Fig. 4B) in inducing these important regulatory molecules.
Figure 4.
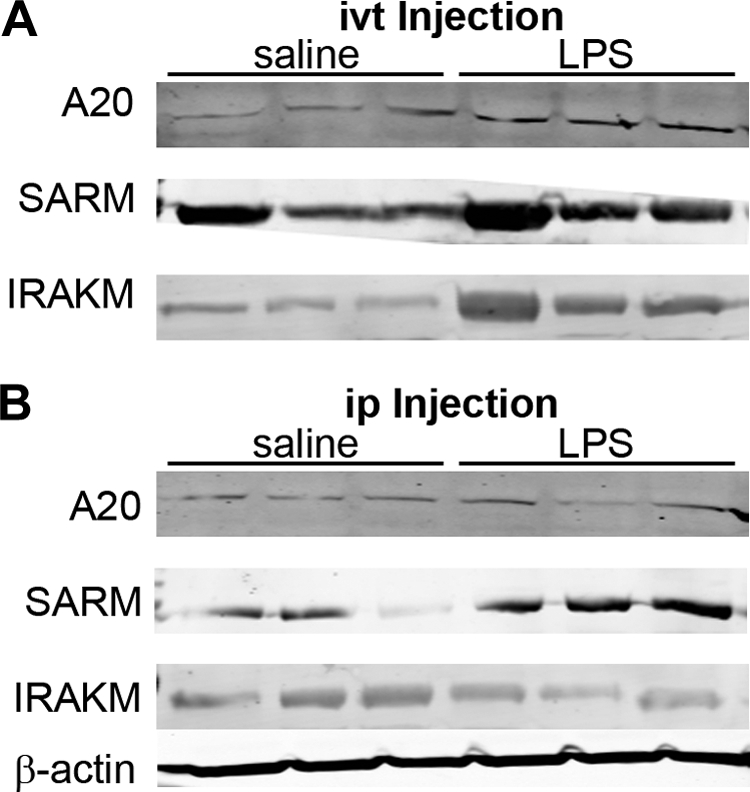
Negative regulators of LPS signaling are induced by ivt and ip injection of LPS. Mice received 100 μg ip LPS, 250 ng ivt LPS, or saline control injections. Expression of the negative regulators A20, SARM, and IRAK-M in eye tissue homogenates at 4 hours after injection was assessed by immunoblot analysis. Data are for three mice per treatment. Results are representative of one of three experiments. β-Actin was also detected as a protein loading control for each blot; one representative panel is shown.
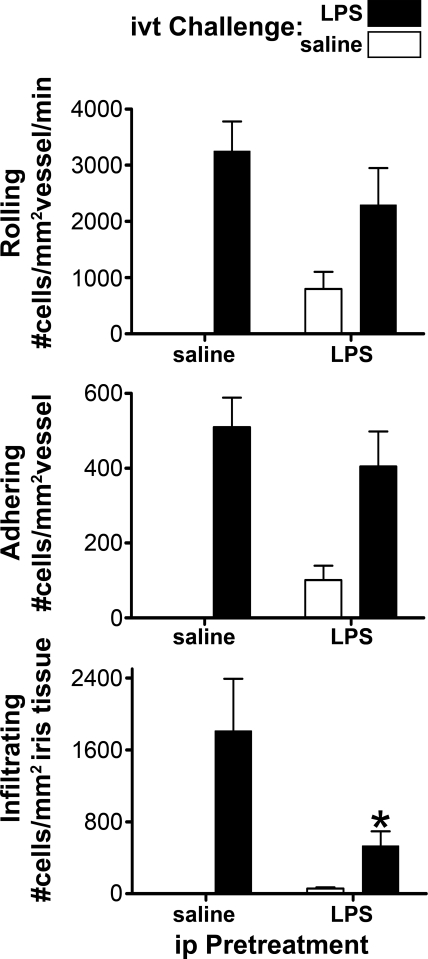
We and others have called attention to the anti-inflammatory effects of LPS. For example, in rabbits, the intravenous injection of LPS results in a transient inability of the rabbit polymorphonuclear leukocyte (PMN) to respond to specific chemotactic stimuli.15 Figure 5 shows that the effect of ip LPS in mice was dominant with regard to leukocyte infiltration of iris tissue. As measured by intravital microscopy, the simultaneous injection of LPS ip and ivt blocked the infiltration of leukocytes that ivt LPS would normally produce 6 hours later. At the time of the intravital microscopy, we found by flow cytometry that the peripheral blood leukocyte count was approximately 50% in the ip LPS-treated mice compared with that in the saline controls. Leukopenia, however, was not a likely explanation for the reduced infiltration, because the level of leukocyte rolling and adhering was not reduced significantly (indicating that there are adequate numbers of circulating leukocytes) and the reduction in infiltrating cells is far greater than the relative reduction in the peripheral white cell count. In a similar experiment, we found no difference in cell infiltration in response to ivt LPS in mice when the ip LPS injection preceded the ivt injection by 24 hours (data not shown). This indicates that the desensitization is transient, and it is consistent with a modest cellular infiltrate detectable in the iris 24 hours after ip LPS.
Figure 5.
IP LPS desensitizes the eye to cellular infiltration in response to ivt LPS. Mice were administered ip injections of 25 μg LPS or saline and, at the same time, ivt injections of either 250 ng LPS or saline. All four possible combinations were tested. The intraocular inflammation at 6 hours after injection was assessed by intravital microscopy. Values are the mean ± SEM; *P < 0.05 compared with saline treatment; n = 8 to 14 mice/treatment. Results are representative of two experiments.
Discussion
TLRs and other closely related molecules including the NLR (Nod-like receptor), CLR (C type lectin receptor) and RLR (retinoic acid-like receptor) families constitute the major initial mechanism by which mice and humans can recognize an invading pathogen.16 The innate immune system plays a critical role in the development of autoimmune or immune-mediated diseases. For example, polymorphisms in TLRs or variations in the gene copy number for TLRs affect susceptibility to diseases which are generally attributed to an adaptive immune response such as systemic lupus erythematosus.17 Most mouse models of autoimmunity require an adjuvant, which generally works by activation of a TLR or closely related NLR. In several animal models, the absence of bacterial flora in the gut has a marked ameliorating effect on the disease.18 Bacterial cell walls are easily detectable in the synovium of patients with rheumatoid arthritis,19 and one current theory is that oral flora greatly contribute to the autoimmune response characteristic of this disease.20 Finally, mutations in one of the NLR family members, NOD2, result in autosomal dominant inflammation in both the joint and the uveal tract.21 In light of the importance of the innate immune system in inflammation, even in diseases characterized by an adaptive immune response, it is critical to understand endotoxin-induced uveitis. Endotoxin-induced uveitis has been the prototypical model of acute inflammatory uveitis, yet some fundamental questions, such as why the mouse eye is so sensitive to locally administered LPS but not to systemic LPS exposure, have remained. Our study provides insight into the discordant effects of local versus systemic LPS in the eye.
Khan et al.22 have also taken note of the differing effects of LPS when injected locally versus systemically, although they have not reported on ocular studies. Similar to our observations, they have reported that systemic LPS has a minimal inhibitory effect on rolling or adhesion and a marked effect on extravasation. Their data support an impaired ability of leukocytes to migrate in response to the chemotactic factor, MIP-2, after these cells are exposed to LPS.22 They attribute this to an effect of LPS on the MAP kinase signaling pathway. We have demonstrated in rabbits that intravenously injected LPS results in a marked reduction of the neutrophil response to complement-dependent inflammation in the skin,15 and it causes a downregulation of the receptors for C5a, leukotriene B4, and F-met-leu-phe.23 An inability to migrate in response to chemotactic stimuli could account for the minimization of a cellular infiltrate in the eye after ip LPS but not after ivt LPS.
Like virtually all responses in the body, the response to LPS is marked by checks and balances. Some intracellular molecules, including A20, SARM,24 SOCS,25 and IRAK-M,13 are known to be induced by LPS and in turn act to dampen the inflammatory response. The response to LPS can also be modified at the level of the TLR4 receptor, which may involve the receptor itself or an accessory, cell-surface molecules like CD14 or SIGIRR.26 Cytokines such as IL-10, MCP-1, and IFNβ can also participate in modifying the response to LPS. Our failure to find a reduction in A20, SARM, or IRAK-M does not exclude a potential role for one of these other factors in the eye, which could contribute to the eye's sensitivity to locally administered LPS.
Our study has several additional limitations. It may be that we would have arrived at different conclusions if we had measured different cytokines or a different index of inflammation, such as vascular permeability or prostaglandin synthesis. We did not test our hypotheses in detail in multiple strains of mice or at other time points; however, we have observed similar cell trafficking and cytokine responses after ip and ivt injections of LPS into C57BL/6 mice at 4, 6, and 24 hours after injection (data not shown).
We are intrigued by the hypothesis that the mouse eye makes relatively small quantities of specific cytokines such as TNFα, but we consider this observation to be preliminary, as the reduction was relative, not absolute, and other doses, time points, or strains might yield different results. In unpublished studies, we have also noted that the anti-inflammatory cytokine IL-10 is relatively difficult to detect in the mouse eye, consistent with what has been observed in aqueous humor of patients with uveitis. In fact, a high level of IL-10 in human aqueous humor suggests lymphoma rather than an inflammation.27 Although the unique sensitivity of the eye to LPS remains a mystery, the measurements of IL-10 and TNFα in the mouse eye may be a clue. TNFα is generally considered an inflammatory mediator, but it, too, has anti-inflammatory effects. TNFα can block the development of renal disease in a murine model of lupus28 and can dampen inflammation triggered from brain ischemic injury.29,30 Although the inhibition of TNFα is frequently used to treat diseases ranging from rheumatoid arthritis to psoriasis, the inhibition of TNFα can be complicated by the development of inflammation including uveitis,31 multiple sclerosis, drug-induced lupus,32 or psoriasis.33 The low levels of TNFα in the eye after LPS injection are consistent with the inability of TNFα inhibition to affect endotoxin-induced uveitis as reported by us and others.34,35 TGFβ is abundant in aqueous humor,36 and this protein is known to downregulate the production of IFNγ and TNFα in tissue culture studies.37
Thus, our observations indicate that systemic and locally injected LPS both induce cytokine synthesis in the eye, but more robustly for some cytokines compared to others; that both ip and ivt LPS induce the intracellular regulators of TLR4 activation, A20, SARM, and IRAK-M; and that the failure of ip LPS to induce cellular infiltration in the mouse eye is probably accounted for by a transient inability of endotoxin-exposed mouse leukocytes to infiltrate iris stroma in response to chemotactic stimuli.
Footnotes
Supported by National Institutes of Health Grants EY019604, EY010572, and EY019020 and an unrestricted grant from Research to Prevent Blindness New York. HLR receives career development support from the American College of Rheumatology and Research to Prevent Blindness, the William C. Kuzell Foundation, the William and Mary Bauman Foundation, and the Stan and Madelle Rosenfeld Family Trust.
Disclosure: J.T. Rosenbaum, None; A. Woods, None; J. Kezic, None; S.R. Planck, None; H.L. Rosenzweig, None
References
- 1. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500 [DOI] [PubMed] [Google Scholar]
- 2. Rosenbaum JT, Nozik RA. Uveitis: many diseases, one diagnosis. Am J Med. 1985;79:545–547 [DOI] [PubMed] [Google Scholar]
- 3. Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613 [DOI] [PubMed] [Google Scholar]
- 4. Li Q, Peng B, Whitcup SM, Jang SU, Chan C-C. Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Exp Eye Res 1995;61:629–632 [DOI] [PubMed] [Google Scholar]
- 5. Planck SR, Becker MD, Crespo S, et al. Characterizing extravascular neutrophil migration in vivo in the iris. Inflammation. 2008;31:105–111 [DOI] [PubMed] [Google Scholar]
- 6. Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566 [PubMed] [Google Scholar]
- 7. Rosenzweig HL, Martin TM, Planck SR, et al. Activation of NOD2 in vivo induces IL-1 beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker MD, Nobiling R, Planck SR, Rosenbaum JT. Digital video-imaging of leukocyte migration in the iris: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J Immunol Methods. 2000;240:23–37 [DOI] [PubMed] [Google Scholar]
- 9. Rosenzweig HL, Martin TM, Jann MM, et al. NOD2, the gene responsible for familial granulomatous uveitis, is essential in a mouse model of muramyl dipeptide-induced uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenzweig H, Galster K, Planck S, Rosenbaum J. NOD1 expression in the eye and functional contribution to IL-1{beta} dependent ocular inflammation in mice. Invest Ophthalmol Vis Sci. 2009;50:1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith JR, Subbarao K, Franc DT, Haribabu B, Rosenbaum JT. Susceptibility to endotoxin induced uveitis is not reduced in mice deficient in BLT1, the high affinity leukotriene B4 receptor. Br J Ophthalmol. 2004;88:273–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Planck SR, Han YB, Park JM, O'Rourke L, Gutierrez-Ramos J-C, Rosenbaum JT. The effect of genetic deficiency of adhesion molecules on the course of endotoxin-induced uveitis. Curr Eye Res. 1998;17:941–946 [DOI] [PubMed] [Google Scholar]
- 13. van 't Veer C, van den Pangaart PS, van Zoelen MA, et al. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007;179:7110–7120 [DOI] [PubMed] [Google Scholar]
- 14. Xiong Y, Qiu F, Piao W, Song C, Wahl LM, Medvedev AE. Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-{beta}-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated Factor 6, and I{kappa}B kinase {gamma} and increases A20 expression. J Biol Chem. 2011;286:7905–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenbaum JT, Hartiala KT, Webster RO, Howes EL, Jr, Goldstein IM. Antiinflammatory effects of endotoxin: inhibition of rabbit polymorphonuclear leukocyte responses to complement (C5) derived peptides in vivo and in vitro. Am J Pathol. 1983;113:291–299 [PMC free article] [PubMed] [Google Scholar]
- 16. Akira S. TLR signaling. Curr Topics Microbiol Immunol. 2006;311:1–16 [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Ortiz H, Velazquez-Cruz R, Espinosa-Rosales F, Jimenez-Morales S, Baca V, Orozco L. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69:1861–1865 [DOI] [PubMed] [Google Scholar]
- 18. Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Heijden IM, Wilbrink B, Tchetverikov I, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arth Rheum. 2000;43:593–598 [DOI] [PubMed] [Google Scholar]
- 20. Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol. 2010;6:727–730 [DOI] [PubMed] [Google Scholar]
- 21. Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20 [DOI] [PubMed] [Google Scholar]
- 22. Khan AI, Heit B, Andonegui G, Colarusso P, Kubes P. Lipopolysaccharide: a p38 MAPK-dependent disrupter of neutrophil chemotaxis. Microcirculation. 2005;12:421–432 [DOI] [PubMed] [Google Scholar]
- 23. Goldman DW, Enkel H, Gifford LA, Chenoweth DE, Rosenbaum JT. Lipopolysaccharide modulates receptors for leukotriene B4, C5a, and formyl-methionyl-leucyl-phenylalanine on rabbit polymorphonuclear leukocytes. J Immunol. 1986;137:1971–1976 [PubMed] [Google Scholar]
- 24. Sheedy FJ, O'Neill LAJ. The troll in Toll: Mal and Tram as bridges for TLR2 and TLR4 signaling. J Leukoc Biol. 2007;82:196–203 [DOI] [PubMed] [Google Scholar]
- 25. Strengell M, Lehtonen A, Matikainen S, Julkunen I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79:1279–1285 [DOI] [PubMed] [Google Scholar]
- 26. Chen X, Zhao Y, Wu X, Qian G. Enhanced expression of single immunoglobulin Il-1 receptor-related molecule ameliorates lipopolysaccharide-induced acute lung injury in mice. Shock. 2010;35:198–204 [DOI] [PubMed] [Google Scholar]
- 27. Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48:3253–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacob CO, McDevitt HO. Tumor necrosis factor-a in murine autoimmune “lupus” nephritis. Nature. 1988;331:356–357 [DOI] [PubMed] [Google Scholar]
- 29. Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490 [DOI] [PubMed] [Google Scholar]
- 30. Rosenzweig HL, Minami M, Lessov NS, et al. Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–1674 [DOI] [PubMed] [Google Scholar]
- 31. Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis?—a registry-based study. Arthritis Rheum. 2007;56:3248–3252 [DOI] [PubMed] [Google Scholar]
- 32. Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005;123:903–912 [DOI] [PubMed] [Google Scholar]
- 33. Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996–1001 [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum JT, Han YB, Park JM, Kennedy M, Planck SR. Tumor necrosis factor alpha is not essential in endotoxin induced eye inflammation: studies in cytokine receptor deficient mice. J Rheumatol. 1998;25:2408–2416 [PubMed] [Google Scholar]
- 35. Kasner L, Chan C-C, Whitcup SM, Gery I. The paradoxical effect of tumor necrosis factor alpha (TNFà) in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1993;34:2911–2917 [PubMed] [Google Scholar]
- 36. Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor- b as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211 [PubMed] [Google Scholar]
- 37. Espevik T, Figari IS, Shalaby MR, et al. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]