This study reveals that Suppressor of cytokine signaling-1 mitigates uveitis by inhibiting the infiltration of inflammatory cells into the retina and conferring protection to retinal cells by inhibiting activities of proapoptotic cytokines.
Abstract
Purpose.
Suppressors of cytokine signaling (SOCS) proteins regulate the intensity and duration of cytokine signals and defective expression of SOCS1 and SOCS3 has been reported in a number of human diseases. The purpose of this study was to investigate the role of SOCS1 in intraocular inflammatory diseases (uveitis) and whether SOCS1 expression is defective in patients with ocular inflammatory diseases.
Methods.
Blood from patients with scleritis or healthy human volunteers was analyzed for SOCS expression by RNase protection assay and RT-PCR. The authors generated SOCS1 transgenic rats and mice (SOCS1-Tg), induced experimental autoimmune uveoretinitis (EAU) by active immunization with interphotoreceptor retinal binding protein or adoptive transfer of uveitogenic T cells, and investigated effects of SOCS1 overexpression on EAU. SOCS1-mediated protection of retinal cells from apoptosis was assessed by annexin V staining.
Results.
Induction of cytokine-induced SH2 protein was comparable between patients and volunteers, whereas 80% of lymphocytes from patients with scleritis failed to induce SOCS1 in response to IL-2. Compared with wild-type littermates, SOCS1-Tg rats/mice developed less severe EAU. Constitutive overexpression of SOCS1 in retina inhibited expression of chemokines (CCL17, CCL20, CXCL9, CXCL10), reduced Th17/Th1 expansion, and inhibited recruitment of inflammatory cells into the retina. The authors also show that SOCS1 protected retinal cells from staurosporine as well as H2O2-induced apoptosis.
Conclusions.
Defective expression of SOCS1 in patients with scleritis, taken together with SOCS1-mediated protection of neuroretinal cells from apoptosis, suggest that SOCS1 has neuroprotective function in the retina, implying that administration of SOCS1 mimetic peptides may be useful in treating uveitis or scleritis.
Ocular inflammatory diseases are one of the major causes of severe visual handicap and include diverse diseases such as scleritis and uveitis.1 Scleritis is a sight-threatening inflammatory ocular disease that affects the sclera (the fibrous outer wall of the eye) and is often associated with systemic diseases such as Wegener's granulomatosis and rheumatoid arthritis.2 Uveitis (intraocular inflammatory diseases) includes sight-threatening diseases such as Behçet's disease, birdshot retinochoroidopathy, and ocular sarcoidosis.3 Several features of human uveitis are similar to those observed in the animal model of T-cell–mediated uveoretinitis and experimental autoimmune uveoretinitis (EAU).4,5 Ocular inflammatory diseases are characterized by the production of a plethora of cytokines and the extent of pathology depends, to a large degree, on ability of the host to limit destructive effects of proinflammatory cytokine by endogenous feedback regulators of cytokine activity.6–8 Proinflammatory cytokines implicated in the pathogenesis of EAU include interferon gamma (IFN-γ), interleukin-1 (IL-1), IL-6, IL-12, IL-17, and IL-23. On the other hand, cytokines including IL-10, IL-27, and IL-35 mitigate the inflammatory responses induced by autoreactive T cells that mediate CNS inflammatory diseases.9–11
Most of these proinflammatory cytokines mediate their biological activities through the activation of Janus (JAK) kinases and STAT (signal transducers and activators of transcription) family of transcription factors.12,13 On cytokine binding to its cognate receptor, receptor-associated JAKs are activated by transphosphorylation, providing phosphotyrosine-docking sites that recruit and activate specific STAT proteins.13 Activated STATs translocate into the nucleus, where they bind to specific DNA sequences and activate gene expression.14 Because cytokines exert profound effects on numerous cell types and cellular processes, cytokine signals are under stringent control; the inability to regulate the duration and intensity of cytokine signaling is the cause of a number of chronic inflammatory and autoimmune diseases. Suppressor of cytokine signaling (SOCS) proteins are induced by these cytokines and function as components of a negative feedback loop that regulate initiation, intensity, duration, and quality of cytokine responses.15,16 Defective regulation of SOCS1 and SOCS3 expression is implicated in many diseases, including allergy, autoimmune diseases, diabetes, obesity, metabolic syndrome, and cancer.17 SOCS1 is a crucial negative regulator of IFN-γ–, IL-4–, IL-6–, and TNF-α–mediated immune responses and expression of chemokines that mediate trafficking of inflammatory cells into the retina.18–21 In a previous report we showed that SOCS1 expression is markedly induced in retinal cells during ocular inflammation.22 On the basis of this we hypothesize that the induction of SOCS1 expression may protect retinal cells from cytotoxic effects of proinflammatory cytokines during ocular inflammation.
Uveitis and scleritis are characterized by elevated levels of IFN-γ and other cytokines2 in the eye and in this study we investigated whether these potentially blinding inflammatory diseases derive in part from defects in SOCS1 expression. We show that the majority of T cells in blood of patients with scleritis are unable to induce expression of SOCS1 in response to cytokine stimulation, suggesting that these patients may be defective in mounting effective negative feedback mechanisms needed to curtail IFN-γ–mediated inflammatory responses. To further investigate the potential role of SOCS1 in uveitis we generated transgenic rats and mice with targeted overexpression of SOCS1 in the retina. We show that SOCS1 mitigates uveitis, suggesting that SOCS1 mimetics may be used to protect retinal cells from pathogenic effects of proinflammatory cytokines during ocular inflammation.
Materials and Methods
Normal Donors and Patients
Blood samples were obtained from five patients with scleritis and five healthy human volunteers after Institutional Review Board (IRB) approval and consent as required by National Institutes of Health (NIH) IRB-approved patient protocols and adhered to the Declaration of Helsinki. Patient 1 had HLA-B27–associated sclerouveitis disease, without systemic involvement. Patient 2 had inactive panuveitis, sarcoidosis, and active scleritis. Patient 3 had idiopathic scleritis. Patient 4 had HLA-B27–associated sclerauveitis, with radiographic evidence of subclinical ankylosing spondylitis. Patient 5 had Cogan's Syndrome with keratoscleritis. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using lymphocyte separation media. PBMC (1 × 106 cells/mL) was stimulated for 4 days with human recombinant IL-2 (rIL-2) at a 100 U/mL final concentration in 96-well flat-bottom or 24-well tissue-culture plates (Costar, Cambridge, MA). Under this stimulation condition, trypan blue staining of the cultures indicated no change in cell number or viability (95%) over the course of the experiments. RNA was isolated immediately after purification of the freshly isolated PBMCs or T cells. Control human retina RNA used for RNase protection assay (RPA) analysis was obtained from a healthy 87-year-old male human volunteer that died from injuries sustained in an automobile accident.
Mice
C57BL/6 mice (6–8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). SOCS1−/−/STAT1−/− and STAT1−/− mice have previously been described.23,24 Animal care and use were in compliance with NIH guidelines.
Generation of SOCS Transgenic Rats and Mice
The DNA fragment p-opsin-SOCS1, used for generating the SOCS1-expressing transgenic (Tg) rats and mice, is shown later in Figure 2. The construct, comprised of the 639 nucleotide mouse SOCS1 cDNA, fused to a 270 basepair (bp) SV40 polyadenylation sequence at its 3′ end and targeted expression of the 212 amino acid SOCS1 protein in retina photoreceptor cells, was driven by a 526-bp opsin promoter element25 at its amino terminus. This fragment (1μg/mL) encoding mouse SOCS1 cDNA was injected into the pronucleus of single-cell mouse embryos to generate the SOCS1-Tg mice as previously described.26–28 For generating SOCS1-Tg rat, 60-day-old female Fischer rats were injected (intraperitoneally [IP]) with luteinizing hormone releasing hormone antagonist. Superovulation was subsequently induced by IP injection of 10 IU pregnant mare serum chorionic gonadotrophin (Sigma) and transgenic rats were generated as described.29 Screening of tail DNA to identify transgene-positive mice or rats was by PCR with the following PCR primer pair: (5′-GCC GTG GGT CGC GAG AAC CTG-3′ and 5′-CAG ACA TGA TAA GAT ACA TTG ATG AG-3′). Western blotting was used to identify animals with targeted expression of SOCS1 in the retina. The transgenic Fischer rats were crossed with wild-type (WT) Lewis rats for seven generations to derive the SOCS1-Tg strain on Lewis background. Animal care was in compliance with NIH guidelines and adhered to the ARVO Animal Statement.
Figure 2.
Generation of transgenic rats and mice with targeted overexpression of SOCS1 in the retina. (A) Derivation of a 1500-bp fragment consisting of an opsin promoter element fused to murine SOCS1 cDNA and an enhancerless–promoterless SV40 polyadenylation sequence. The chimeric cDNA fragment was microinjected into the pronuclei of one-cell mouse or rat embryos. Western blot analysis of whole cell extracts isolated from lens or retina of WT or SOCS1 transgenic (SOCS1-Tg) rat (B) or retina of WT and SOCS1-Tg mice (C). (D) Retinal cells from WT or SOCS1-Tg mice were stimulated with IFN-γ and pSTAT1 expression was detected by Western blot analysis.
Induction of Experimental Autoimmune Uveitis
Six- to 8-week-old SOCS1-Tg or WT (litter mates) mice were immunized with an SC injection of 150 μg interphotoreceptor retinal binding protein (IRBP) and 300 μg of human IRBP peptide (1–20) in 0.2 mL emulsion (1:1 vol/vol) with complete Freund's adjuvant (CFA) containing Mycobacterium tuberculosis strain H37RA (2.5 mg/mL). SOCS1-Tg or WT (litter mates) rats were immunized with 50 μg IRBP in CFA containing M. tuberculosis strain H37RA (2.5 mg/mL) as described.30 The animals also received Bordetella pertussis toxin (0.3 μg/mouse) concurrent with immunization. Clinical disease was established by histology and fundoscopy as described.31,32
Histologic Analysis
WT or SOCS1-Tg rat or mouse eyes were harvested, fixed in 4% glutaraldehyde for 30 minutes, and transferred to 10% buffered formalin. After adequate fixation, specimens were dehydrated through graded alcohols and embedded in methacrylate. Serial vertical sections through the papillary–optic nerve plane were cut and stained with hematoxylin and eosin as described.26 Photographs of representative sections were taken on a photomicroscope (Carl Zeiss AG, Oberkochen, Germany).
Isolation and Stimulation of Retinal Cells
Retinal cells were isolated as previously described.22 Briefly, the retina was cut into small pieces and digested in collagenase/DNase digestion buffer at 37°C for 3 hours, with intermittent tituration with a 10-mL pipette. Digestion reaction was quenched with a 10-fold volume of 10% fetal bovine serum and cells were washed twice with complete medium.
Flow Cytometry and Intracellular Cytokine Analysis
Mouse PBMCs were isolated by density gradient centrifugation using a polysaccharide plus buffered sodium chloride solution (Ficoll-Hypaque; GE Healthcare Life Biosciences, Piscataway, NJ). CD4+ T cells were isolated from mouse blood or lymph nodes (LNs) using T-cell enrichment columns and CD4+ cells magnetically labeled with CD4 (MicroBeads; Miltenyi Biotec, Bergisch-Gladbach, Germany). Cells were analyzed directly without prior activation with the autoantigen IRBP. To estimate the levels of antigen-specific CD4+ T cells in blood of WT or SOCS1-Tg mice with EAU we used the very sensitive antigen-specific CD154 assay.33 For intracellular cytokine analysis, PBMC, T cells, or retina cells were stimulated for 5 hours with phorbol 12-myristate acetate (20 ng/mL)/ionomycin (1 μM) in the presence of a protein transporter inhibitor as recommended (GolgiStop; BD Pharmingen, San Diego, CA). Intracellular cytokine staining was performed using appropriate equipment (Cytofix/Cytoperm kit; BD Biosciences, San Jose, CA). Cell analysis and cell sorting using labeled anti-CD3, -CD4, -CD8, IL-17, IFN-γ mAbs and corresponding isotype control antibodies (Pharmingen) were performed on a flow cytometer (FACSCalibur; BD Biosciences).
RNase Protection Assay
RNase protection assay (RPA) was performed with RNA (10 μg), [32P]UTP-radiolabeled RNA probes, and a human SOCS RPA kit (BD Biosciences, San Diego, CA) as recommended by the manufacturer.
Quantitative and Semiquantitative PCR Analyses
Total RNA was extracted from PBMCs, LN, and retinal cells using an RNA/DNA/protein extraction solution according to the procedures recommended by the manufacturer (TRIzol reagent; Life Technologies, Gaithersburg, MD). All RNA samples were digested with RNase-free DNase 1 (Life Technologies) for 30 minutes, purified by phenol/chloroform extractions, and precipitated in 0.4 M LiCl. RNA (10 μg), a commercial synthesis system (SuperScript III Reverse Transcriptase; Life Technologies, Gaithersburg, MD), and oligo(dT)12–18 primer were used for first-strand synthesis as previously described.34 Samples were subjected to hot-start RT-PCR with gene-specific primers and a DNA sequence context amplifier (AmpliTaq Gold DNA Polymerase; Applied Biosystems, Foster City, CA). First-strand synthesis containing each mRNA sample, but no reverse transcriptase, was performed to control for possible DNA contamination of mRNAs used as target for PCR amplification; failure to obtain RT-PCR products with any of the PCR amplimers confirmed the absence of contaminating DNA templates. PCR-amplified fragments were fractionated on agarose gels. All cDNA preparations used were suitable substrates for PCR amplification on the basis of efficient amplification of a ß-actin sequence. Real-time PCR was performed on a fast real-time PCR system (ABI 7500) and PCR parameters were as recommended by the manufacturer (TaqMan Universal PCR Kit; Applied Biosystems). Primers and probes for SOCS1 and SOCS3 were purchased from Applied Biosystems.
Western Blot Analyses
Preparation of whole cell lysates was as described.35 Blots were probed with polyclonal pSTAT1, SOCS1, or β-actin–specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Preimmune serum was used in parallel as controls and signals were detected with horseradish peroxidase–conjugated secondary F(ab′)2 Abs (Zymed Laboratories, San Francisco, CA) using an electrogenerated chemiluminescence system (ECL Western Blotting System; Amersham, Arlington Heights, IL).
Apoptosis Assay
Retinal cells were isolated from WT or SOCS1-Tg mice. Dead cells were removed by density-gradient centrifugation (Ficoll-Hypaque) and the viable cells (1 × 106) were plated and rested overnight in Dulbecco's modified Eagle's (complete) medium. The cultures were then treated with staurosporine (2 μM) for 6 hours or H2O2 (100 μM) for 1 hour and cells undergoing apoptosis were detected by fluorescence-activated cell sorting (FACS), using the equipment (Annexin V Apoptosis Detection Kit I; BD Biosciences) as described.36
Statistical Analysis
Experiments were repeated at least twice, and usually three or more times. For EAU, disease severity for each animal was calculated as an average of both eyes. Statistical analyses were performed by nonparametric statistics Mann–Whitney U test. Probability values of P ≤ 0.05 were considered statistically significant. The data, whenever applicable, were presented as mean + SD, unless otherwise specified.
Results
Capacity to Induce SOCS1 Expression Is Compromised in Patients with Scleritis
To determine whether the persistent inflammation in the eyes of patients with chronic ocular inflammatory disease derive from defects in SOCS expression, we analyzed induction of SOCS expression by PBMC of patients with scleritis using PBMC of healthy human subjects serving as controls. Ocular inflammatory activity of the patients was graded as previously described37 and clinical and demographic information of each patient with scleritis is shown (Fig. 1A). Although our scale grades only the diffuse type, some patients with diffuse characteristics also had clinical nodules present. Therefore our patient cohort presented a mixed nodular and diffuse scleritis. To establish the pattern of SOCS induction by normal human PBMC and retina, we analyzed RNA not only from stimulated or unstimulated PBMCs, but also from human retina for SOCS expression by the RPA. We show that SOCS3 was constitutively expressed in human PBMCs, whereas SOCS1 and CIS (cytokine-induced SH2 protein) were expressed at very low levels (Fig. 1B). However, after stimulation by IL-2, transcription of SOCS1 and CIS was appreciably induced, consistent with previous reports showing that IL-2 induces the upregulation of SOCS1 and CIS gene expression in normal human PBMCs.38,39 On the other hand, expression of SOCS3 was slightly downregulated in response to IL-2 stimulation (Fig. 1B); real-time quantitative 5′-nuclease fluorogenic RT-PCR assay (qPCR) confirmed that IL-2 induced the downregulation of SOCS3 expression by CD4+ T cells in the blood of patients and healthy subjects (Fig. 1C). The latter results are in line with a previous report indicating that the SOCS3 mRNA level in lymphocytes is inversely correlated with the amount of IL-2 secretion.35 In contrast to the robust induction of SOCS1 transcription by PBMCs of healthy volunteers, PBMCs from four of five patients with scleritis were unable to upregulate SOCS1 in response to IL-2 stimulation (Fig. 1C). The defect was specific, in that the T cells in PBMCs from each patient exhibited a normal pattern of CIS gene transcription, indicating that the response of patient PBMCs to cytokine signaling was not affected by the immunosuppressive therapy (Fig. 1C). The T-cell growth factor IL-2 also activates transcription of interferon regulatory factor 1 (IRF-1) gene, a transcription factor that plays a pivotal role in T-cell activation and effector functions.40 We show here that IRF-1 mRNA levels were persistently elevated in the blood of patients with scleritis compared with that of normal volunteers (Fig. 1D), suggesting that the inability to induce SOCS1 expression during inflammation may contribute to the persistent expression of IRF-1 and Th1 cytokines observed in chronic ocular inflammatory diseases. The higher levels of IRF-1 could also have derived from other mononuclear cells such as monocyte and macrophages that express proinflammatory cytokines that may also contribute to the development of scleritis.
Figure 1.
Induction of SOCS1 expression is defective in patients with scleritis. (A) Five healthy human volunteers and five patients with scleritis requiring immunosuppressive therapy were studied. Disease grade and immunosuppressive therapy of the patients are indicated. (B) Analysis of RNA from stimulated or unstimulated PBMC and from human retina for SOCS expression by the RNase protection assay. L32 and glyceraldehyde-3-phosphate dehydrogenase, RNA loading controls. (C) Normal human PBMC was stimulated with human rIL-2 and cDNA prepared from the stimulated or nonstimulated cells were analyzed for SOCS1, SOCS3, or CIS expression by real-time RT-PCR. (D) PBMCs from healthy human volunteers and patients with scleritis were analyzed for IRF-1 expression by real-time RT-PCR.
Generation of Transgenic Rat/Mouse with Targeted Overexpression of SOCS1 in the Retina
Ethical considerations and NIH IRB guidelines precluded obtaining retinal tissue biopsy to determine whether SOCS expression is also defective in ocular tissues of patients with scleritis. To further investigate the role of SOCS1 in the retina and intraocular inflammatory diseases, we generated transgenic mice and rats with overexpression of SOCS1 in the retina. The chimeric construct microinjected into the pronuclei of one-cell mouse or rat embryos is a 1500-bp fragment consisting of an opsin promoter element fused to the murine SOCS1 cDNA and an enhancerless–promoterless SV40 polyadenylation sequence (Fig. 2A). The zygotes were transferred into pseudopregnant female C57BL/6 mice or Fischer rats and F9 homozygous transgenic strains were established by brother/sister mating as described.27,28 Western blot analysis of whole cell extracts isolated from ocular tissues of WT and SOCS1 transgenic (SOCS1-Tg) rats (Fig. 2B) or mice (Fig. 2C) established that SOCS1 was successfully targeted to the retina. To examine effects of overexpressing SOCS1 on IFN-γ signaling in the retina, we stimulated retinal cells from WT or SOCS1-Tg mice with IFN-γ. Robust activation of STAT1 (pSTAT1) by WT but not SOCS1-Tg retinal cells provided suggestive evidence that constitutive expression of SOCS1 inhibited activation of the STAT1 pathway in the retina (Fig. 2D).
SOCS1 Protects Mice and Rats from Developing Severe Uveitis
EAU is a well-characterized model of human autoimmune uveitis. Because retinal pathology in EAU results in part from the cytotoxic effects of proinflammatory cytokines secreted by autoreactive T cells and macrophages, the EAU model is ideally suited for use in investigating whether SOCS1-mediated inhibition of cytokine signaling can mitigate retinal pathology during uveitis. We induced EAU in age-matched WT and SOCS1-Tg mice or rats by immunization with IRBP in CFA. The development and severity of EAU was assessed by fundoscopy and/or ocular histologic examination of enucleated eyes. Histologic analysis of the eyes 14 days after immunization with IRBP revealed a more severe uveitis in WT mice (Fig. 3A) or WT rats (Fig. 3B) compared with SOCS1-Tg animals. This was indicated by the massive infiltration of inflammatory cells into the WT retina and vitreous, whereas less inflammatory cells infiltrated the retina and vitreous of SOCS1-Tg mice (Fig. 3A) or SOCS1-Tg rats (Fig. 3B). The presence of numerous retinal folds (indicated by blue arrows) and loss of photoreceptors are hallmarks of severe uveitis; moreover, observation of these clinical features coincided with higher EAU scores in IRBP-immunized WT mice (Fig. 3C) or rats (Fig. 3D). Fundoscopic images of the diseased eyes further revealed substantial vascular changes, including several engorged retinal vessels with severe cuffing (arrowheads) in the WT EAU mouse eye (Fig. 3A, top panels). We further showed that the response of WT and SOCS1-Tg T cells to IRBP was similar (Fig. 3E), suggesting that attenuated disease in SOCS1-Tg mice cannot be attributed solely to inability to respond to the ocular autoantigen, IRBP.
Figure 3.
SOCS1-Tg mice and rats develop less severe EAU. (A) EAU was induced in mice (A) or rats (B) by immunization with IRBP in CFA and disease progression was analyzed by fundoscopy. Fundus images were taken from normal or EAU mice using an otoendoscopic imaging system and assessment of severity of the inflammatory disease was based on changes at the optic nerve disc and retinal vessels or tissues as described.32 Eyes were harvested 14 days postimmunization and histologic sections through the retina were stained with hemotoxylin and eosin stain (A, B). Arrows indicate the presence of inflammatory cells in vitreous (V); blue arrows indicate the retinal folds; red star, retinal detachment. EAU scores of WT and SOCS1-Tg mice (C) and rats (D) were determined by histopathologic analysis as described.31 (E) Lymph node cells of IRBP-immunized mice were stimulated with IRBP for 3 days and proliferative response of the cells was assessed. Results are presented as counts per minute (cpm) and indicate mean values of five replicate cultures.
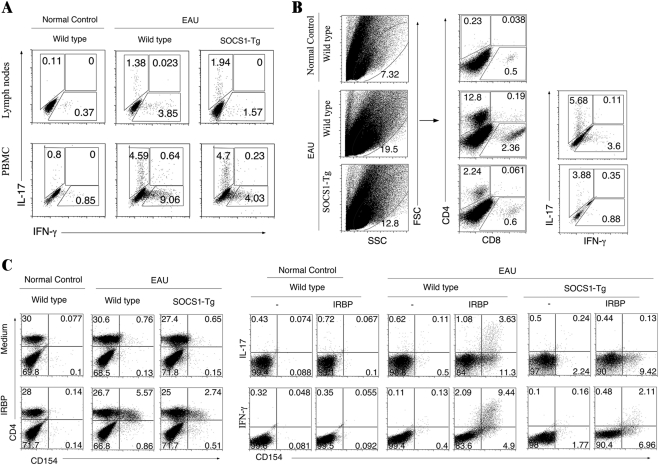
Attenuation of EAU in SOCS1-Tg Mice Correlates with Decrease in Th1 and Th17 Cells
During EAU in mice, both Th1 and Th17 cells are expanded and recruited into the retina and they have been implicated in the etiology of uveitis.9,41,42 Freshly isolated cells from the LN, blood, and retina of WT and SOCS1-Tg mice were therefore analyzed to determine whether the difference in EAU severity between WT and SOCS1-Tg mice could have derived from the amounts of Th17 and Th1 cells produced during EAU. In line with published reports,9,41,42 onset of EAU pathology was temporally correlated with increase of Th17 and Th1 in blood and LNs (Fig. 4A). However, compared with WT mice the percentage of Th1 cells in blood and LNs of IRBP-immunized SOCS1-Tg mice was substantially reduced. We also show that there was a more than 5-fold increase in the percentage of CD4+ T cells infiltrating the retina of WT compared with SOCS1-Tg mice immunized with IRBP (Fig. 4B). Intracellular cytokine analysis further reveal a marked increase in IFN-γ– and IL-17–expressing T cells in WT retina as indicated by 3-fold and 1.4-fold increases of these CD4+ T cells, respectively (Fig. 4B). Because nonspecific T cells are also expanded as a result of adjuvant (CFA) it was necessary to determine the relative abundance of IRBP-specific uveitogenic T cells in these peripheral tissues. We therefore used the very sensitive and specific antigen-induced CD154 expression assay33,43 to detect and quantify the relative amounts of IRBP-specific T cells in the blood of these mice. Cells were isolated from PBMCs of day 21 IRBP-immunized WT or SOCS1-Tg mice, restimulated in vitro with IRBP and the presence of IRBP-responsive T cells, as indicated by induction of CD154 expression, was assessed. We show that 5.57% of CD4+ T cells in blood of WT mice with EAU were IRBP-specific compared with 2.74% in SOCS1-Tg blood (Fig. 4C). Furthermore, 9.44% and 3.63% of these cells produced IFN-γ and IL-17, respectively. By contrast, only 2.11% of the CD4+ T cells in SOCS1-Tg blood produced IFN-γ (Fig. 4C), and these results are consistent with recruitment of fewer numbers of Th17 and Th1 cells into the retina.
Figure 4.
SOCS1 inhibits recruitment of inflammatory cells into the retina. (A) Freshly isolated PBMC or LN cells from WT or SOCS1-Tg mice at early stage of EAU (day 14 postimmunization) were analyzed by the intracellular cytokine assay. CD4+ T cells were gated for IL-17– or IFN-γ–expressing cells. (B) CD4+ T cells present in the retina of WT or SOCS1-Tg mice with EAU were detected and quantified by FACS. Plots were gated on CD4+ or CD8+ T cells. Numbers in quadrants indicate percentage of CD4+ T cells expressing IL-17 and/or IFN-γ. (C) CD4+ T cells isolated from blood of normal unimmunized control, WT, or SOCS1-Tg mice with EAU (day 21 postimmunization) were stimulated with IRBP for 12 hours and then assayed for intracellular cytokine expression. Numbers in quadrants indicate percentage of IFN-γ– and/or IL-17–expressing CD154-positive T cells. Results are representative of three independent experiments.
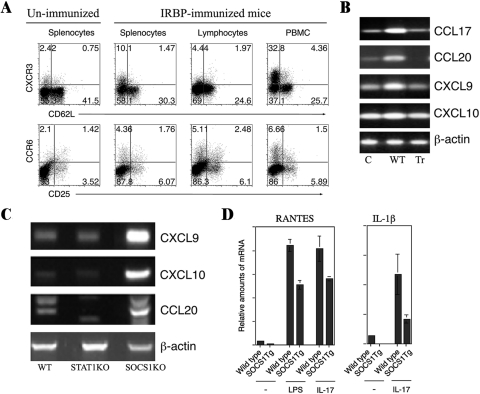
SOCS1 Inhibits Recruitment of Inflammatory Cells into the Retina
Uveitis and scleritis are characterized by aberrant infiltration of massive numbers of leukocytes into the uvea, vitreous, retina, or sclera. In this study, we analyzed the expression of chemokine receptors that promote recruitment of Th1 and Th17 cells into the retina during EAU. Analysis of T cells in the spleen, LN, and blood revealed that the expression of Th1- and Th17-specific chemokine receptors, CXCR3 and CCR6, is upregulated and correlates with recruitment of lymphocytes into the retina during EAU (Fig. 5A). To examine molecular mechanisms that may underlie the reduced recruitment of Th1 and Th17 cells into the retina of SOCS1-Tg mice during EAU, we isolated retina from WT and SOCS1-Tg mice with EAU and analyzed expression of chemokines that mediate chemotaxis of Th1 and Th17 cells into sites of inflammation. We showed that WT retinal cells expressed relatively high levels of the chemokines, CCL17 (TARC), CCL20 (LARC), CXCL9 (MIG), and CXCL10 (IP-10), the ligands of Th1 and Th17 chemokine receptors (CXCR3, CCR4, CCR6). By contrast, the SOCS1-Tg retinal cells did not upregulate expression of these chemotactic molecules (Fig. 5B). We further showed that SOCS1-deficient retinal cells express elevated levels of CXCL9, CXCL10, and CCL20 (Fig. 5C), providing further evidence that expression of these chemokines was negatively regulated by SOCS1. RANTES (Regulated upon Activation, Normal T-cell Expressed, and Secreted [CCL5]) is an 8-kDa chemokine that plays a role in recruiting eosinophils, basophils, and T cells into inflammatory sites, and IL-1β is a proinflammatory cytokine that mediates a variety of cellular processes including differentiation, cell proliferation, and apoptosis. To further characterize the role of SOCS1 in mediating the recruitment of leukocytes into the retina during inflammation, we stimulated WT and SOCS1-Tg retinal cells with lipopolysaccharide (LPS) or IL-17, and examined whether SOCS1 regulates the expression of RANTES and IL-1β. We found that WT retinal cells substantially induced the expression of RANTES and IL-1β, whereas expression of both proteins by the SOCS1-Tg retinal cells was substantially reduced (Fig. 5D). Taken together, these results suggest that SOCS1 negatively regulates the expression of chemokines and other proinflammatory molecules and may contribute to the mechanism that mitigates intraocular inflammation by limiting recruitment of inflammatory cells into the retina.
Figure 5.
SOCS1 inhibits expression of proinflammatory molecules that mediate intraocular inflammation. (A) Freshly isolated T cells from spleen, LNs, and PBMCs of WT mice with EAU were analyzed by the intracellular cytokine assay. Numbers in quadrants indicate percentage of CD4+ T cells expressing CXCR3 or CCR6. (B) RNA isolated from the retina of WT or SOCS1-Tg mice immunized with IRBP was analyzed by RT-PCR. (C) RNA isolated from the retina of WT, STAT1-deficient mice (STAT1KO), or SOCS1−/−/STAT1−/− mice (SOCS1KO) was analyzed by RT-PCR. (D) Retinal cells from WT or SOCS1-Tg mice were stimulated with LPS or IL-17. RNA isolated from the cells was analyzed by real-time qPCR.
SOCS1 Protects Mice from Developing Severe Uveitis
We next examined whether reduced severity of EAU in SOCS1-Tg mice was due to fewer proinflammatory activities of SOCS1-Tg T cells or as a result of protective effects conferred onto retinal cells by SOCS1. Cells isolated from draining LN of WT mice with EAU were restimulated in vitro for 3 days with IRBP, transferred (1 × 107 cells/mouse) into age- and sex-matched unimmunized WT or SOCS1-Tg mice and disease development was assessed by fundoscopy. Fundus images acquired 10 days after adoptive cell transfer revealed features characteristic of EAU in the retina of six of the six WT mice examined (Fig. 6A). By contrast, the retinas of four of the SOCS1-Tg mice were normal, whereas two exhibited very mild uveitis (Fig. 6A), suggesting that overexpression of SOCS1 in retinal cells protected SOCS1-Tg mice from developing severe uveitis. The potential role of SOCS1 in promoting the survival of retinal cells was further investigated after treatment of WT and SOCS1-Tg retinal cells with the apoptosis-inducing chemicals, staurosporine, and H2O2. Apoptotic and necrotic cells were assessed by annexin V– and 7-AAD–staining assays, respectively. SOCS1-Tg retinal cells had enhanced survival over WT retinal cells when treated with H2O2 (78.9% vs. 66.9%) or staurosporine (62.3 vs. 47.5%), which provided additional evidence that SOCS1 protects retinal cells (Fig. 6B).
Figure 6.
SOCS1 protects mice from developing severe uveitis. (A) Cells isolated from the spleen of WT mice with EAU were restimulated in vitro for 3 days with IRBP and the cells (1 × 107 cells/mouse) were transferred into naïve WT or SOCS1-Tg mice. Ten days after adoptive cell transfer, disease development was assessed by fundoscopy. (B) Retinal cells from WT or SOCS1-Tg mice were treated with staurosporine or H2O2 and percentages of cells undergoing apoptosis were analyzed by annexin V staining assay. Numbers in quadrants indicate percentage of surviving or necrotic cells or cells undergoing apoptosis.
Discussion
One of our objectives in this study was to investigate whether the aberrant upregulation of cytokine secretion in patients with persistent ocular inflammatory disease derives in part from defects in proteins that mediate negative feedback regulation of activities of proinflammatory cytokine. We found that PBMCs of patients with scleritis could not induce expression of the negative feedback regulatory protein SOCS1. In a previous study, we showed that expression of another member of the SOCS family, SOCS5, is elevated in the blood of patients with uveitis, suggesting that aberrant regulation of SOCS proteins may contribute to pathogenic mechanisms of ocular inflammatory diseases.44 However, ethical concerns that preclude retinal biopsy make it difficult to directly examine the role of SOCS proteins in the retina or whether retinal cells of patients with ocular inflammatory disease are defective in SOCS expression.
In a previous report we showed that expression of SOCS1 undergoes dynamic changes throughout the course of EAU, with the highest level of SOCS1 mRNA detected in the retina at the peak of EAU, whereas disease resolution coincided with decline to basal levels in WT animals.22 SOCS1 gene expression has also been shown to be upregulated in the brain of mice with experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis that shares essential clinical features with EAU.45,46 Although results from several studies suggest that induction of SOCS1, SOCS3, and CIS by inflammatory cells mitigates immune responses by regulating the intensity and duration of cytokine signals,47 until now it has not been clear whether retinal cells also produce SOCS1 and what physiologic role they might have during ocular inflammation. By targeting the overexpression of SOCS1 to the retina of rats and mice and examining the in vivo function of SOCS1 in the well-characterized EAU model, we have shown that the overexpression of SOCS1 protected SOCS1-Tg mice from developing severe EAU (Figs. 3A, 3C). The protective role of SOCS1 was firmly established and confirmed by showing that SOCS1-Tg rats also developed a milder form of EAU compared with that of their WT counterpart (Fig. 3B). Reduced severity of EAU in SOCS1-Tg mice and rats was not due to defects in Ag priming because the WT and SOCS1-Tg T cells were similar in their response to challenge with IRBP (Fig. 3E). Further, we have shown that the peripheral immune response of the SOCS1-Tg mice is intact, given that similar levels of IL-17–producing T cells were detected in the LN and PBMCs of WT and SOCS1-Tg animals at day 14 postimmunization with IRBP/CFA (Fig. 4A). However, at a later stage of EAU (day 21 postimmunization) we observed substantial increases in the percentage of IRBP-specific uveitogenic IL-17– and IFN-γ–expressing T cells in the WT compared with SOCS1-Tg mice (Fig. 4C). Our data suggest that proinflammatory cytokines produced during EAU induced retinal cells to repress the production of chemotactic cytokines (Fig. 5B) and this in part might have contributed to decrease in the numbers of T cells recruited into the retina of SOCS1-Tg mice (Fig. 4B). Although our data suggest that expansion of IFN-γ–producing Th1 and IL-17–expressing Th17 cells is inhibited in SOCS1-Tg mice, the underlying mechanism is still unclear since expression of the SOCS1 transgene is restricted to retinal cells.
However, the reduced severity of EAU in SOCS1-Tg mice might derive from the fact that constitutive expression of SOCS1 modified the response of retinal cells to proinflammatory molecules. As revealed by analysis of the response of SOCS1-Tg and WT retinal cells to LPS or the proinflammatory cytokine IL-17, SOCS1 repressed expression of RANTES, a chemokine that plays a role in recruiting eosinophils, basophils, and T cells into inflammatory sites. In addition, expression of the IL-1β that initiates pathogenic inflammatory responses and implicated in apoptosis was downregulated in SOCS1-Tg retinal cells (Fig. 5D). Retinal cells are particularly vulnerable to apoptosis and they constitutively express a variety of proteins such as FAS and FASL that contribute to the mechanism that maintains immune privilege.48 It is therefore of note that after the treatment of retinal cells with the apoptosis-inducing staurosporine and H2O2, we observed increased survival of the SOCS1-Tg retinal cells, suggesting that SOCS1 may confer protection to retinal cells from apoptosis (Fig. 6B). In our adoptive transfer experiment we show that, whereas the transfer of IRBP-specific pathogenic T cells caused retinal pathology in WT mice, these cells were less effective in inducing pathologic changes in SOCS1-Tg mice (Fig. 6A), further underscoring the role of SOCS1 in conferring protection to the neuroretina during ocular inflammation. Although SOCS1 upregulation does confer protection from severe EAU, its overexpression in transgenic mice and rats retinas did confer complete protection, implying that although SOCS1 may play an important role in protection of the retina during inflammation, other immunosuppressive factors are also required.
It is now widely accepted that unrestrained neuroinflammatory responses deriving from excessive secretion of cytokines by cells of the innate and adaptive immune systems contribute to neuronal or photoreceptor cell deficit, preceding neurodegenerative changes in multiple sclerosis, uveitis, Alzheimer's disease, and age-related macular degeneration. In uveitis and scleritis, pathology results from infiltration of the retina by inflammatory cells that secrete copious amounts of IFN-γ and retinal integrity is compromised because most retinal cells are terminally differentiated and do not regenerate after cytokine-induced cytotoxicity. A major goal of most treatment modalities used in human ocular inflammatory diseases is therefore to eliminate inflammatory cells or neutralize activities of the proinflammatory cytokines they produce.1,49 Our analyses of the blood of patients with scleritis and transgenic rodents with targeted expression of the SOCS1 protein in the retina provide valuable insights into the role played by endogenous negative feedback regulators of cytokine signaling in mitigating pathogenic autoimmunity in the eye. Although the retina does not return to its normal architecture after the inflammation subsides, activating these endogenous regulatory proteins curtails the duration of cytokine activities in the retina.
Acknowledgments
The authors thank the staff of the National Eye Institute Genetic Engineering Core Facility for assistance with generation of the transgenic rats and mice.
Footnotes
Supported in part by the National Eye Institute and National Institutes of Health Intramural Research Programs.
Disclosure: C.-R. Yu, None; R.R. Mahdi, None; H.-M. Oh, None; A. Amadi-Obi, None; G. Levy-Clarke, None; J. Burton, None; A. Eseonu, None; Y.-J. Lee, None; C.-C. Chan, None; C.E. Egwuagu, None
References
- 1. Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol. 2002;21:273–289 [DOI] [PubMed] [Google Scholar]
- 2. Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130:469–476 [DOI] [PubMed] [Google Scholar]
- 3. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308 [DOI] [PubMed] [Google Scholar]
- 4. Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495 [PubMed] [Google Scholar]
- 5. Nussenblatt RB. Proctor Lecture. Experimental autoimmune uveitis: mechanisms of disease and clinical therapeutic indications. Invest Ophthalmol Vis Sci. 1991;32:3131–3141 [PubMed] [Google Scholar]
- 6. Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35 [DOI] [PubMed] [Google Scholar]
- 7. Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res. 2004;23:617–637 [DOI] [PubMed] [Google Scholar]
- 8. Dick AD, Cheng YF, Liversidge J, Forrester JV. Immunomodulation of experimental autoimmune uveoretinitis: a model of tolerance induction with retinal antigens. Eye (Lond). 1994;8:52–59 [DOI] [PubMed] [Google Scholar]
- 9. Amadi-Obi A, Yu CR, Liu X, et al. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718 [DOI] [PubMed] [Google Scholar]
- 10. Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635 [DOI] [PubMed] [Google Scholar]
- 13. Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662 [DOI] [PubMed] [Google Scholar]
- 14. Mertens C, Darnell JE., Jr SnapShot: JAK-STAT signaling (Abstract). Cell. 2007;131:612. [DOI] [PubMed] [Google Scholar]
- 15. Naka T, Fujimoto M, Kishimoto T. Negative regulation of cytokine signaling: STAT-induced STAT inhibitor. Trends Biochem Sci. 1999;24:394–398 [DOI] [PubMed] [Google Scholar]
- 16. Hilton DJ, Richardson RT, Alexander WS, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176 [DOI] [PubMed] [Google Scholar]
- 18. Kimura A, Naka T, Nagata S, Kawase I, Kishimoto T. SOCS-1 suppresses TNF-alpha-induced apoptosis through the regulation of Jak activation. Int Immunol. 2004;16:991–999 [DOI] [PubMed] [Google Scholar]
- 19. Morita Y, Naka T, Kawazoe Y, et al. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor alpha-induced cell death in fibroblasts. Proc Natl Acad Sci USA. 2000;97:5405–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naka T, Narazaki M, Hirata M, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929 [DOI] [PubMed] [Google Scholar]
- 21. Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921 [DOI] [PubMed] [Google Scholar]
- 22. Takase H, Yu CR, Liu X, Fujimoto C, Gery I, Egwuagu CE. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–127 [DOI] [PubMed] [Google Scholar]
- 23. Yu CR, Mahdi RM, Liu X, et al. SOCS1 regulates CCR7 expression and migration of CD4+ T cells into peripheral tissues. J Immunol. 2008;181:1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naka T, Tsutsui H, Fujimoto M, et al. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4 signaling in vivo. Immunity. 2001;14:535–545 [DOI] [PubMed] [Google Scholar]
- 25. Ham DI, Kim SJ, Chen J, et al. Central immunotolerance in transgenic mice expressing a foreign antigen under control of the rhodopsin promoter. Invest Ophthalmol Vis Sci. 2004;45:857–862 [DOI] [PubMed] [Google Scholar]
- 26. Egwuagu CE, Sztein J, Chan CC, Mahdi R, Nussenblatt RB, Chepelinsky AB. gamma Interferon expression disrupts lens and retinal differentiation in transgenic mice. Dev Biol. 1994;166:557–568 [DOI] [PubMed] [Google Scholar]
- 27. Egwuagu CE, Sztein J, Chan CC, et al. Ectopic expression of gamma interferon in the eyes of transgenic mice induces ocular pathology and MHC class II gene expression. Invest Ophthalmol Vis Sci. 1994;35:332–341 [PubMed] [Google Scholar]
- 28. Egwuagu CE, Sztein J, Mahdi RM, et al. IFN-gamma increases the severity and accelerates the onset of experimental autoimmune uveitis in transgenic rats. J Immunol. 1999;162:510–517 [PubMed] [Google Scholar]
- 29. Egwuagu CE, Mahdi RM, Chan CC, et al. Expression of interferon-gamma in the lens exacerbates anterior uveitis and induces retinal degenerative changes in transgenic Lewis rats. Clin Immunol. 1999;91:196–205 [DOI] [PubMed] [Google Scholar]
- 30. Egwuagu CE, Mahdi RM, Nussenblatt RB, Gery I, Caspi RR. Evidence for selective accumulation of V beta 8+ T lymphocytes in experimental autoimmune uveoretinitis induced with two different retinal antigens. J Immunol. 1993;151:1627–1636 [PubMed] [Google Scholar]
- 31. Chan CC, Caspi RR, Ni M, et al. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990;3:247–255 [DOI] [PubMed] [Google Scholar]
- 32. Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008;87:319–326 [DOI] [PubMed] [Google Scholar]
- 33. Frentsch M, Arbach O, Kirchhoff D, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124 [DOI] [PubMed] [Google Scholar]
- 34. Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187 [DOI] [PubMed] [Google Scholar]
- 35. Li W, Nagineni CN, Efiok B, Chepelinsky AB, Egwuagu CE. Interferon regulatory transcription factors are constitutively expressed and spatially regulated in the mouse lens. Dev Biol. 1999;210:44–55 [DOI] [PubMed] [Google Scholar]
- 36. Liu X, Mameza MG, Lee YS, et al. Suppressors of cytokine-signaling proteins induce insulin resistance in the retina and promote survival of retinal cells. Diabetes. 2008;57:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sen HN, Sangave AA, Goldstein DA, et al. A standardized grading system for scleritis. Ophthalmology. 2011;118:768–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood. 2001;97:221–226 [DOI] [PubMed] [Google Scholar]
- 39. Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387 [DOI] [PubMed] [Google Scholar]
- 40. Schwarz LA, Stevens AM, Hrachovy JA, Yu-Lee LY. Interferon regulatory factor-1 is inducible by prolactin, interleukin-2 and concanavalin A in T cells. Mol Cell Endocrinol. 1992;86:103–110 [DOI] [PubMed] [Google Scholar]
- 41. Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirchhoff D, Frentsch M, Leclerk P, et al. Identification and isolation of murine antigen-reactive T cells according to CD154 expression. Eur J Immunol. 2007;37:2370–2377 [DOI] [PubMed] [Google Scholar]
- 44. Egwuagu CE, Yu CR, Li Z, Nussenblatt RB. SOCS5 mRNA levels in peripheral blood mononuclear cells (PBMC): a potential bio-marker for monitoring response of uveitis patients to Daclizumab therapy. J Autoimmun. 2005;24:39–46 [DOI] [PubMed] [Google Scholar]
- 45. Maier J, Kincaid C, Pagenstecher A, Campbell IL. Regulation of signal transducer and activator of transcription and suppressor of cytokine-signaling gene expression in the brain of mice with astrocyte-targeted production of interleukin-12 or experimental autoimmune encephalomyelitis. Am J Pathol. 2002;160:271–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stark JL, Lyons JA, Cross AH. Interferon-gamma produced by encephalitogenic cells induces suppressors of cytokine signaling in primary murine astrocytes. J Neuroimmunol. 2004;151:195–200 [DOI] [PubMed] [Google Scholar]
- 47. Yu CR, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem. 2003;278:29752–29759 [DOI] [PubMed] [Google Scholar]
- 48. Ferguson TA, Griffith TS. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev. 2006;213:228–238 [DOI] [PubMed] [Google Scholar]
- 49. Nussenblatt RB, Fortin E, Schiffman R, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci USA. 1999;96:7462–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]