Klebsiella pneumoniae endogenous endophthalmitis is a uniformly blinding infection. The authors report that the K1 capsule of invasive K. pneumoniae significantly contributes to disruption of retinal function, intraocular growth to a high density, and persistence despite immune cell recruitment.
Abstract
Purpose.
Endogenous endophthalmitis secondary to Klebsiella pneumoniae liver abscess is a blinding infection that is being reported more frequently in the literature. The K1 capsule and magA contribute to virulence of systemic infection in mice; however, little is known about the role of magA in secondary ocular infections.
Methods.
To assess the role of K. pneumoniae capsule in endophthalmitis, the authors induced experimental endophthalmitis by direct inoculation of 100 colony-forming unit wild-type, magA-deficient, or magA-complemented K. pneumoniae into the posterior segments of mouse eyes. Eyes were analyzed by quantitation of viable bacteria, retinal function, and inflammatory cell influx as well as by histology.
Results.
Wild-type K1 K. pneumoniae caused significant ocular disease. At the end point of 24 hours postinfection, eyes infected with wild-type K. pneumoniae retained significantly less retinal A-wave function than eyes infected with an isogenic magA-mutant strain. B-wave function retention was also greater in eyes infected with the magA mutant than with wild-type K. pneumoniae. Additionally, intraocular growth of the magA-deficient strain was less than it was in the wild-type strain. The amount of myeloperoxidase elicited was also significantly higher for wild-type–infected eyes at 24 hours.
Conclusions.
These results indicate that in the eye, the K1 capsule of invasive K. pneumoniae significantly contributes to the ability of the bacteria to disrupt retinal function, to grow to high density, and to persist despite immune cell recruitment.
Klebsiella pneumoniae has become an important pathogen in recent years. Invasive strains causing pyogenic liver abscesses and other soft tissue abscesses have been reported with increasing frequency. K. pneumoniae was recognized as the etiologic agent of community-acquired pyogenic liver abscess in the Far East1 and has surpassed Escherichia coli as the primary etiologic agent of Gram-negative pyogenic liver abscesses in Taiwan.2 Recently, infections have been reported in the United States, Europe, Middle East, and Australia.3–7 Of importance was the discovery of the mucoviscosity-associated gene A (magA and later designated wzy_K1) in screens of invasive liver isolates.8 In general, magA-containing strains produce copious amounts of polysaccharide capsule and are resistant to serum killing and phagocytosis. The gene encoding MagA is a marker specific to the K1 serotype and is designated as the capsule polymerase, presumably responsible for creating higher order polymers from precursor lipidated polymers.9 Although not all invasive hypermucoviscous (HMV) K. pneumoniae encode MagA, a significant proportion of invasive strains do, making magA an attractive therapeutic target. MagA contributed significantly to the LD50 in a mouse peritoneal infection model. The LD50 for magA-positive strains was <100 colony-forming units (CFUs) and was approximately 4 to 5 logs lower than isogenic mutants, which were deficient for magA.8
Invasive Klebsiella are epidemiologically important. Many reports retrospectively summarize the clinical presentation, risk factors, sequelae, and outcomes of patients diagnosed with community-acquired K. pneumoniae liver abscesses. More than half these patients were diabetic. From these soft tissue abscesses, K. pneumoniae can spread to the eye or meninges, causing metastatic endophthalmitis, meningitis, or both. The risk for metastatic spread to the meninges or eye with underlying K. pneumoniae primary liver abscess is approximately 3% to 10%.10,11 When examined alone, approximately two-thirds of patients presenting with K. pneumoniae endogenous endophthalmitis (KPEE) had an underlying liver abscess caused by the same bacterium.12 Despite treatment, most patients with K. pneumoniae endophthalmitis lose useful vision.13–15 The visual prognosis for these patients is uniformly poor.
Because many patients may not seek medical attention for an underlying liver abscess until they experience ocular pain or vision loss, coupled with the poor prognosis of KPEE, it was important to understand the contribution of the HMV phenotype, specifically magA, to disease in the eye. To this end, we used an experimental model of K. pneumoniae endophthalmitis16 to compare infections with wild-type, isogenic magA-deficient, and trans-complemented magA K. pneumoniae strains. The results clearly demonstrate that magA is a virulence factor in experimental endophthalmitis.
Methods
Animals
Eight- to 10-week-old C57BL/6J mice were acquired from the Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with an intramuscular injection of ketamine (85 mg/kg) and xylazine (14 mg/kg). Eyes were topically anesthetized with 0.5% proparacaine before infection. All procedures were carried out in strict accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, institutional guidelines, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Bacteria
K. pneumoniae clinical isolate NTUHK2044 and the isogenic ΔmagA mutant are described in Fang et al.8 For trans-complementation of magA, a 1.7-kb fragment containing magA was amplified by PCR from strain NTUHK2044 using primers magAAgeI(-121)-5′GAGACCGGTAACCGTTACGAACTTGAACGAGC and magABamHI(+1591) 5′GATCGGATCCTCCAAGGAAGGTGTTGAAATGCC, which annealed at −121 and +1591 nucleotides from the start codon and contained AgeI and BamHI restriction sites, respectively. Plasmid pBR328 was digested with AgeI and BamHI, and the larger 3.9-kb fragment was gel purified. The 3.9-kb fragment was blunted with T4 polymerase. After digesting the 1.7-kb magA-containing fragment with AgeI and BamHI, the fragment was ligated to the 3.9-kb fragment from pBR328 to generate pBR328Δ::magA, which was introduced into NTUHK2044ΔmagA and selected on chloramphenicol to generate NTUHK2044ΔmagA pBR328Δ::magA or “magA complement.” The presence of magA transcript was verified by semiquantitative RT-PCR. Briefly, 2 μg RNA was reverse transcribed using a reverse transcription system (GoScript; Promega, Madison, WI) and the cDNA was amplified using primers specific to magA [magA+1115 (forward) 5′CAGATCTGGGCCAGTCCGAAAGTGAACGA and magA+1753 (reverse) 5′GAGAGGATCCGCAATGGCCATTTGCGTTAG] and the 16S rRNA gene [16S (forward) 5′GCGGTAATACGGAGGGTGC and 16S (reverse) 3′CACATCCGACTTGACAGACC] Klebsiella. A strain containing only the empty vector (pBR328Δ) was constructed by self-ligating the 3.9-kb fragment of pBR328, previously generated, after using T4 polymerase to fill in 5′ overhangs.
Plasmid stability of the magA complement was analyzed both in vitro and in vivo. For in vitro stability, bacteria were passaged overnight twice without selection. Total and chloramphenicol-resistant colonies were counted for each passage. For in vivo stability, approximately 100 CFUs of the magA complement strain were used to intraocularly infect mice, as described here. After 18 hours, eyes were removed and homogenized in PBS. Total and chloramphenicol-resistant (ComR) colonies were counted. Data are shown as the mean of at least three replicate samples ± SD. Two-tailed, two-sample t-tests assuming equal variance were used to statistically compare groups.
Quantification of Capsular Polysaccharide
Bacteria were grown overnight in Luria-Bertani broth. Capsular polysaccharide was quantified by a uronic acid assay as previously reported,17 with adjustments made for smaller volumes. Briefly, cultures were mixed with one-fifth volumes of 1% Zwittergent 3–14 in 100 mM citric acid (pH 2). After incubation at 50°C, debris was removed by centrifugation, and 125 μL supernatant was precipitated with 0.5 mL ethanol. Precipitate was dissolved in 100 μL H2O and vigorously mixed with 600 μL of 20 mM sodium borate in concentrated H2SO4. Samples were boiled for 5 minutes and cooled in an ice H2O bath before the addition 10 μL of 0.15% 3-phenylphenol in 0.5% NaOH. Absorbance at 520 nm was measured, and a standard curve of glucuronic acid was used to calculate the concentration of uronic acids. Data are reported as the mean micrograms per 109 CFUs ± SD of triplicate samples.
Transmission Electron Microscopy
Eighteen-hour cultures were washed in PBS and fixed in 5% gluteraldehyde/4% paraformaldehyde in PBS. Cells were washed three times with PBS and once with distilled water, spotted on glow discharged 300 Mesh Cu Formvar-coated grids, stained for 10 seconds with 2% uranyl acetate, washed once with water, and allowed to dry at ambient conditions. Images were taken on a transmission electron microscope (H-7600; Hitachi, Tokyo, Japan) with a beam voltage of 80 kV.
Experimental K. pneumoniae Endophthalmitis
Approximately 100 CFUs of mid-logarithmic-phase bacteria in a volume of 0.5 μL were injected into the mid-vitreous of C57BL/6J mice as previously described.16 Eyes were analyzed by scotopic electroretinography,16,18 bacterial quantitation,19 histology,18 and myeloperoxidase (MPO) abundance (Hycult Biotech, Uden, The Netherlands). Three replicate samples per time point were tested at 0, 3, and 6 hours (mean ± SEM), and six replicate samples per time point were tested for all other time points (mean ± SEM).
Statistical Analysis
For in vitro and in vivo analyses, two-tailed, two-sample t-tests assuming equal variance were used to statistically compare groups. P ≤ 0.05 was considered significant.
Results
Capsular Polysaccharide
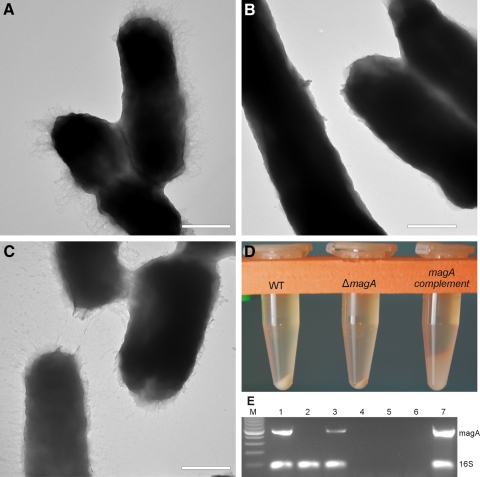
The capsule of K. pneumoniae was visualized by transmission electron microscopy. Wild-type NTUHK2044 had a fibrous halo corresponding to the HMV phenotype (Fig. 1A), similar to that described previously.8 This halo was absent in the ΔmagA mutant (Fig. 1B). However, the magA-complemented strain was similar in appearance to the wild-type strain, with extracellular material visible (Fig. 1C). After establishing that the wild-type and magA complement strains did not grow differently in vitro (data not shown), capsule production was assessed qualitatively by sedimentation. When equal CFUs were sedimented at 10,000g, the ΔmagA mutant cells packed well and formed a solid pellet (Fig. 1D). However, neither the wild-type nor the magA-complemented strain formed tight pellets, and the magA complement had a pellet more than twice the volume of the wild-type (Fig. 1D). The amount of capsule, as a function of uronic acid content, was quantified. There was no statistical difference in the amount of uronic acid detected in overnight cultures of the wild-type and magA-complement strains (7.25 ± 0.35 μg/109 CFU vs. 6.88 ± 0.68 μg/ 109CFU [P = 0.44]), indicating the complement restored wild-type levels of capsule production. The amount of uronic acid produced by the ΔmagA strain was below the limit of detection for the assay. To verify the deletion and complementation of magA, we used semiquantitative RT-PCR to detect magA expression. The presence of the magA transcript was verified in wild-type and complemented strains but was not detected in the mutant strain (Fig. 1E). These data suggested that the complemented strain produced capsule equal to that of the wild-type K. pneumoniae and that the capsule from these strains inhibited tight packing of cells similar to what has been described previously.19
Figure 1.
K. pneumoniae capsule. Transmission electron microscopy visualization of capsule production from NTUHK2044 (A), ΔmagA (B), and magA complement (C). Sedimentation of overnight cultures at 10,000g for 10 minutes (D). Semiquantitative RT-PCR detecting magA and 16S rRNA (E). Lanes 1–3, reverse transcriptase; lanes 4–6, no reverse transcriptase; lane M, 100-bp DNA ladder; lanes 1 and 4, NTUHK2044; lanes 2 and 5, ΔmagA; lanes 3 and 6, magA complement; lane 7, NTUHK2044 genomic DNA control.
To ensure that none of the strains produced hemolytic or proteolytic toxins, cell-free supernatant from overnight cultures in LB were spotted onto agar plates containing either sheep erythrocytes or casein and were incubated overnight at 37°C. As expected, no zone of clearing was observed for any strains (data not shown).
Intraocular Growth
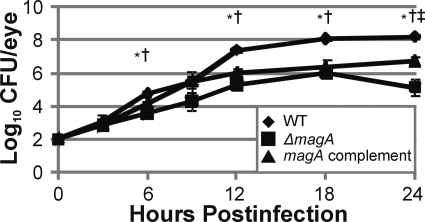
The growth of K. pneumoniae wild-type NTUHK2044, the ΔmagA mutant, and the magA complement were compared in the experimental endophthalmitis model. There was no significant difference in CFUs recovered from eyes infected with any strain at 0, 3, and 9 hours after infection. At 12 hours postinfection, the wild-type strain grew to a higher density than the ΔmagA mutant, reaching 7.35 log10 CFU/eye compared with only 5.28 log10 CFU/eye for mice infected with the ΔmagA strain (P = 6.13 × 10−8; Fig. 2). This difference was maintained until 24 hours, when the end point values for intraocular growth were 8.18 and 5.14 log10 CFU/eye for wild-type and ΔmagA strains, respectively (P = 1.87 × 10−4; Fig. 2). These results indicated that the wild-type strain grew to a greater density in the eye and maintained populations approximately 3 logs higher than the ΔmagA strain. When the magA complement strain was tested, the growth curve was similar to that of the wild-type and ΔmagA strains until 12 hours. At this time, the complement strain reached 6.04 log10 CFU/eye, which was significantly lower than wild-type (P = 1.97 × 10−5; Fig. 2). The growth of the complement strain remained lower than wild-type at both 18 and 24 hours (P = 1.32 × 10−3 and P = 6.28 × 10−3, respectively; Fig. 2) but was greater than the ΔmagA strain at 24 hours (P = 0.0075).
Figure 2.
Intraocular growth of K. pneumoniae during experimental endophthalmitis. Eyes were injected with 100 CFU of wild-type, ΔmagA, or magA complement K. pneumoniae and were enucleated at the indicated time points, homogenized, and plated on BHI agar for bacterial quantification. *P ≤ 0.05 for wild-type versus ΔmagA. †P ≤ 0.05 for wild-type versus magA complement. ‡P ≤ 0.05 for ΔmagA versus magA complement. Two-tailed, two-sample t-tests assuming equal variance were used to statistically compare groups.
To ensure plasmid stability in the magA complement, bacteria were grown in LB with selection, back-diluted into LB without antibiotics, and grown overnight at 37°C. Total CFUs and the number of CFUs resistant to chloramphenicol were not different after the first or second overnight passage (passage 1, 9.38 ± 0.12 total log10 CFU/mL vs. 9.33 ± 0.12 ComR log10 CFU/mL [P = 0.26]; passage 2, 9.22 ± 0.19 total log10 CFU/mL vs. 9.24 ± 0.17 ComR log10 CFU/mL [P = 0.88]). The growth of ΔmagA containing the empty vector (pBR238Δ) in vitro was not significantly different from that of the ΔmagA strain and did not restore the HMV phenotype (data not shown); thus, we did not expect to gain further insight into the effect of MagA by comparing these two strains in vivo. In addition, eyes were infected with the magA strain, and, after 18 hours, the numbers of total and chloramphenicol-resistant colonies were not statistically different (7.05 ± 0.50 total log10 CFU/eye vs. 7.10 ± 0.45 ComR CFU/eye [P = 0.90]). Colonies that were resistant to chloramphenicol retained their HMV phenotype after passage in vitro and in vivo, indicating that the magA complement plasmid is stable without selection both in vitro and in vivo.
Retinal Function
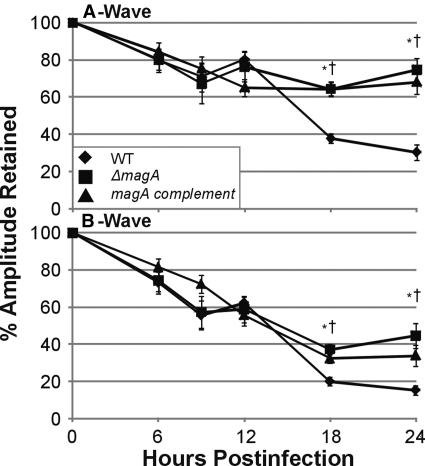
At early time points (6, 9, and 12 hours), the percentage of retained A-wave amplitude declined independently of the phenotype of the strain used because there was no difference between the groups at these time points. At 18 hours, eyes infected with wild-type K. pneumoniae retained only 37.7% A-wave amplitude, whereas eyes infected with the ΔmagA strain retained 64.4% A-wave amplitude (P = 2.92 × 10−6; Fig. 3). At 24 hours, A-wave amplitude of wild-type–infected eyes decreased to 30.3%, whereas eyes infected with the ΔmagA strain retained 74.7% (P = 1.97 × 10−5; Fig. 3). The trend of A-wave retention in eyes infected with the magA complement strain followed that of the ΔmagA strain. By 24 hours, eyes infected with the magA complement strain retained 67.9% A-wave amplitude, which was higher than in wild-type–infected eyes (P = 4.74 × 10−4) but was not different from ΔmagA-infected eyes (P = 0.44).
Figure 3.
Retinal function during experimental K. pneumoniae endophthalmitis. Eyes were injected with 100 CFU of wild-type, ΔmagA, or magA complement K. pneumoniae and were dark adapted at least 6 hours before electroretinography. Infected eyes were compared with the contralateral mock-injected or absolute control eye and were reported at percentage of amplitude of A-wave or B-wave retained. *P ≤ 0.05 for wild-type versus ΔmagA. †P ≤ 0.05 for wild-type versus magA complement. Two-tailed, two-sample t-tests assuming equal variance were used to statistically compare groups.
Results were similar when B-waves were examined. There was a gradual decrease over the first 12 hours in wild-type–infected eyes that was mirrored in the ΔmagA-infected eyes. By 12 hours, the percentage of retained B-wave was 62.3% and 58.8% for wild-type and ΔmagA-infected eyes, respectively (P = 0.66). Over the next 12 hours, the B-wave amplitude of wild-type–infected eyes declined to 15.3%. However, at 18 and 24 hours, eyes infected with the ΔmagA strain had significantly higher B-wave amplitudes (37.2% vs. 20% and 44.6% vs. 15.3%) (P = 8.36 × 10−5 and P = 0.001) than wild-type–infected eyes. The trend of B-wave amplitude loss in magA-complement–infected eyes closely followed that of the eyes infected with ΔmagA K. pneumoniae. At 24 hours there was no difference in retained B-wave amplitude compared with ΔmagA-infected eyes (P = 0.24).
Inflammation
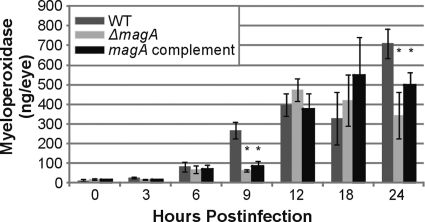
We previously reported that in experimental Bacillus cereus endophthalmitis, polymorphonuclear leukocytes (PMNs) represent the predominant immune cell infiltrating the eye after infection.16 Infiltration of PMNs was measured by an abundance of MPO. There was no significant difference in the amount of MPO detected in eyes infected with wild-type or ΔmagA at 0, 3, 6, 12, or 18 hours (Fig. 4). At 9 hours, eyes infected with wild-type K. pneumoniae contained significantly more MPO (266 ng/eye) than either the ΔmagA (62.5 ng/eye) or the complement (83.8 ng/eye) strains (P = 0.003 and P = 0.01, respectively). By 12 hours, there was at least a 20-fold increase in MPO over baseline levels that was independent of the infecting strain. However, at 24 hours, eyes infected with the wild-type strain contained 709.2 ng/eye MPO compared with only 343.2 ng/eye elicited by the ΔmagA strain (P = 0.031). This difference is consistent with the histologic data at 24 hours, which showed more ocular architectural damage. K. pneumoniae does not produce hemolytic or proteolytic toxins; therefore; much of the histologic pathology may be due to the immune influx in response to bacteria in the vitreous. The abundance of MPO elicited by the magA complement strain generally followed the ΔmagA strain and was not significantly different at any time point analyzed.
Figure 4.
Inflammation during experimental K. pneumoniae endophthalmitis. Eyes were injected with 100 CFU of wild-type, ΔmagA, or magA complement K. pneumoniae and were enucleated at the indicated time points postinfection, homogenized in PBS, and stored at −80°C before assay for myeloperoxidase by ELISA. *P ≤ 0.05 versus wild-type. Two-tailed, two-sample t-tests assuming equal variance were used to statistically compare groups.
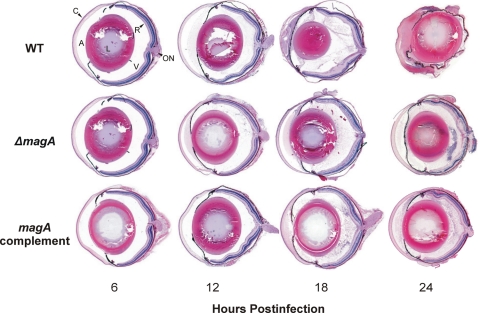
Representative histologic sections of experimental K. pneumoniae endophthalmitis are shown in Figure 5. Eyes infected with any strain were indistinguishable from control eyes at 6 hours because all retinal layers were intact and the vitreous was clear. At 12 hours, eyes infected with the ΔmagA strain exhibited mild optic nerve inflammation and minimal fibrin deposition, whereas eyes infected with the wild-type strain exhibited marked fibrin deposition and cellular infiltration in both the anterior and the posterior chambers. At 18 hours, there was significant inflammation in eyes infected with the wild-type strain, with anterior chamber fibrin deposition, separation of the corneal stroma from the epithelium, and separation of ganglion cell and inner plexiform layers in the retina. There was only mild disruption of the retinal ganglion cell layer in ΔmagA-infected eyes at 18 hours. At 18 hours, there was also cellular infiltration into the vitreous of infected eyes regardless of the infecting strain. The amount of inflammation in wild-type–infected eyes was significant at 24 hours and resulted in phthisis. Eyes infected with the ΔmagA- or magA-complemented strains remained intact.
Figure 5.
Histology during experimental K. pneumoniae endophthalmitis. Eyes were injected with 100 CFU of wild-type, ΔmagA, or magA complement K. pneumoniae and were enucleated at the indicated time points postinfection, processed for histology, and stained with hematoxylin and eosin. Eyes at 6 hours for all strains were indistinguishable from mock-injected or absolute control eyes at any time point. Images are magnified approximately 10-fold. A, aqueous humor; C, cornea; L, lens; ON, optic nerve; R, retina; V, vitreous.
Discussion
Endogenous bacterial endophthalmitis is a potentially blinding disease, especially when caused by Gram-negative pathogens. In the human population, endogenous K. pneumoniae endophthalmitis is a frequent sequela of an underlying infection, and endophthalmitis caused by K. pneumoniae is typically of endogenous origin. However, there are no well-characterized models of endogenous bacterial endophthalmitis. We therefore chose to directly inoculate eyes with K. pneumoniae to minimize complicating factors associated with systemic disease and to evaluate the ability of various strains to cause disease once they gain access to the intraocular environment. The limitation to this model includes the absence of predisposing factors described in the clinical literature that are associated with systemic disease, including diabetes and hepatobiliary disease.20–22 The direct inoculation model facilitated a clear and direct examination of the role of MagA during experimental K. pneumoniae endophthalmitis independent of underlying diseases. This reproducible experimental model has been valuable in evaluating the intraocular virulence of various organisms, including K. pneumoniae.16,18 In experimental models and clinical cases of bacterial endophthalmitis, organisms that cannot be quickly cleared from the eye continue to cause inflammation and to recruit inflammatory cells into the posterior segment that likely contribute to visual loss by obstructing the visual axis, causing neuronal cell death by bystander damage, or both. Further retinal damage can be caused by the presence of bacterial toxins; however, K. pneumoniae does not produce hemolytic or proteolytic toxins.
We previously compared HMV K. pneumoniae with a nonisogenic, non-HMV strain in the experimental mouse endophthalmitis model.18 Here, we report on the ability of isogenic strains of K. pneumoniae with functional or absent magA to cause ocular disease in mice. In the present study, the intraocular growth of NTUHK2044 was similar to that of the HMV strain in the previous study. Each strain grew to greater than 8 log10 CFU/eye by 18 hours. In contrast, the magA mutant reported here was not as fit for intraocular growth because it reached only 5 log10 CFU/eye compared with greater than 7 log10 CFU/eye for the previously reported non-HMV strain. Despite the differences in intraocular growth, the retinal function retained in eyes infected by HMV+/magA+ or non-HMV/ΔmagA were similar. At 18 hours, NTUHK2044 and the HMV+ strain in the previous study caused approximately 65% loss in A-wave function and > 80% loss in B-wave function. Similarly, the ΔmagA and non-HMV strains in the previous study caused 20% to 30% loss in A-wave amplitude and approximately 50% loss in B-wave amplitude. The most surprising difference between the results of the two studies is the degree of inflammation observed at 18 hours. Strains with the NTUHK2044 background elicited between 300 and 550 ng MPO per eye, whereas strains in the previous study elicited 3.8- to 6.6-fold less MPO per eye. As in the previous study, infection with wild-type K. pneumoniae resulted in phthisis by 24 hours, whereas eyes infected with the ΔmagA remained intact. These results suggest that other factors in the NTUHK2044 background may cause this particular K. pneumoniae clone to be more inflammatory, more virulent, or both.
The virulence of many ocular pathogens can be attributed to the production of toxins. Staphylococcus aureus strains deficient in α-toxin are significantly attenuated in their ability to cause endophthalmitis.23 S. aureus and B. cereus, which do not produce toxins in a density-dependent (i.e., deficient in agr and plcR, respectively) manner, eventually caused damage similar to wild-type bacteria, but intraocular infections were highly attenuated.24,25 In contrast, K. pneumoniae are not known to produce membrane-damaging toxins such as hemolysins, cytolysins, or phospholipases. To confirm this, we cultured K. pneumoniae on casein and blood agar, and no zones of clearing were observed with any of the strains used in this study. In experimental B. cereus endophthalmitis, it has been previously shown that PMNs are the major cell population infiltrating the posterior segment and that the increase of CD18+/Gr-1+ cells correlated with an increase in MPO detected over time.16 Much of the intraocular virulence of B. cereus is attributed to its potent toxins and to its inflammogenic cell wall. Despite the lack of these classical virulence factors, NTUHK2044 elicits an amount of inflammation during experimental endophthalmitis similar to or greater than that elicited by B. cereus. It is likely that other bacterial products, such as endotoxin (LPS) on the surface of K. pneumoniae, may account for the robust inflammatory response in the absence of classical toxin production.
It has been reported that magA is required to mask LPS recognition by TLR-4 in vitro.26 In that report, cells deficient in TLR-4 were unable to secrete TNF-α when stimulated with UV-inactivated magA+ bacteria. However, a cell line competent for TLR-4 secreted more TNF-α when treated with heat-killed bacteria than when treated with UV-inactivated bacteria, suggesting that the integrity of the capsule is essential for evading TLR-4 recognition.26 Here we report that an equivalent amount of MPO was elicited by all strains at both 12 and 18 hours. Interestingly, at these time points, there were 1.5- to 3-fold less bacteria in the ΔmagA and magA complement groups. In the case of the ΔmagA strain, this suggests that the lack of magA renders the bacterium more inflammogenic, in accordance with previous findings. Although the magA complement strain did produce capsule that could be visualized and quantified, it is possible that the trans-complementation of magA did not restore the proper localization of the polysaccharide capsule. We speculate that the capsule, while present, may not be completely intact so as to function as a barrier to the innate immune response. This may account for the fact that the ΔmagA and magA complement groups elicited amounts of inflammation similar to those of the wild-type strain but at much lower bacterial loads. Toll-like receptor 4 is the pathogen-associated molecular pattern receptor for bacterial LPS and is expressed in the human eye and is localized to resident perivascular antigen-presenting cells and the retinal pigment epithelium. Expression and localization of TLR-4 is not well understood in the mouse eye. It is unclear whether TLR-4 is important in the clearance of Gram-negative bacteria during endophthalmitis. It is possible that TLR-4 dependent recruitment of immune cells into the eye after infection with Gram-negative bacteria could exacerbate visual outcomes by causing bystander damage to nearby retinal neurons. We have undertaken studies to determine the role of TLR-4 in experimental endophthalmitis, and preliminary results suggest that in TLR-4−/− mice, the infiltration of immune cells is delayed several hours compared with wild-type mice when infected with K. pneumoniae.
Prompt diagnosis and treatment of endophthalmitis are important to preserve visual function. Visual acuity in patients with endogenous K. pneumoniae endophthalmitis is generally poor, and practitioners should be aware of this complication in patients treated for pyogenic abscesses caused by K. pneumoniae. Although aggressive treatment, including vitrectomy and lensectomy for treatment of K. pneumoniae endophthalmitis, was shown to be effective when performed 8 hours from onset of ocular symptoms,27 a retrospective study on endogenous endophthalmitis found that 74% of eyes infected with Klebsiella retain, at best, hand motion perception.14 In recent years, multiply drug-resistant K. pneumoniae have gained significant attention. Strains have been described that are resistant to most clinically relevant antibiotics. Considering the increasing frequency of community-acquired K. pneumoniae infections and their secondary consequences, it will be important to find new therapeutic targets to aid in the control of this organism.
Acknowledgments
The authors thank Nanette Wheatley (Dean McGee Eye Institute) and Mark Dittmar (Dean A. McGee Eye Institute Animal Research Facility) for their invaluable technical assistance, Paula Pierce (Excalibur Pathology, Moore, OK) for histology expertise, Joe Wilkerson (Oklahoma Medical Research Foundation Imaging Core Facility) for electron microscopy expertise, and Bo Novosad and Phil Coburn (Oklahoma University Health Sciences Center) for their helpful comments.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2010, and at the International Society for Eye Research Biennial Meeting, Montreal, Quebec, July 2010.
Supported by Research to Prevent Blindness Lew R. Wasserman Award (MCC); National Institutes of Health (NIH) Grant R01EY012985 (MCC); NIH CORE Grant P30EY12191 (Robert E. Anderson, OUHSC); National Center for Research Resources COBRE Grant P20RR017703 (Robert E. Anderson, OUHSC); and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: J.J. Hunt, None; J.-T. Wang, None; M.C. Callegan, None
References
- 1. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916 [PubMed] [Google Scholar]
- 2. Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100:322–331 [DOI] [PubMed] [Google Scholar]
- 3. Casella F, Finazzi L, Repetti V, et al. Liver abscess caused by Klebsiella pneumoniae: two case reports. Cases J. 2009;2:6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frazee BW, Hansen S, Lambert L. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann Emerg Med. 2009;53:639–642 [DOI] [PubMed] [Google Scholar]
- 5. Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis. 2008;6:228–233 [DOI] [PubMed] [Google Scholar]
- 6. Sobirk SK, Struve C, Jacobsson SG. Primary Klebsiella pneumoniae liver abscess with metastatic spread to lung and eye: a North-European case report of an emerging syndrome. Open Microbiol J. 2010;4:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turton JF, Englender H, Gabriel SN, Turton SE, Kaufmann ME, Pitt TL. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J Med Microbiol. 2007;56:593–597 [DOI] [PubMed] [Google Scholar]
- 8. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293 [DOI] [PubMed] [Google Scholar]
- 10. Chiu CT, Lin DY, Liaw YF. Metastatic septic endophthalmitis in pyogenic liver abscess. J Clin Gastroenterol. 1988;10:524–527 [DOI] [PubMed] [Google Scholar]
- 11. Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess: their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151:1557–1559 [PubMed] [Google Scholar]
- 12. Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423 [DOI] [PubMed] [Google Scholar]
- 13. Ang LP, Lee HM, Au Eong KG, Yap EY, Lim AT. Endogenous Klebsiella endophthalmitis. Eye. 2000;14:855–860 [DOI] [PubMed] [Google Scholar]
- 14. Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107:1483–1491 [DOI] [PubMed] [Google Scholar]
- 15. Chee SP, Ang CL. Endogenous Klebsiella endophthalmitis—a case series. Ann Acad Med Singapore. 1995;24:473–478 [PubMed] [Google Scholar]
- 16. Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31:955–965 [DOI] [PubMed] [Google Scholar]
- 17. Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiskur BJ, Hunt JJ, Callegan MC. Hypermucoviscosity as a virulence factor in experimental Klebsiella pneumoniae endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49:4931–4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol. 2010;192:3144–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434–1438 [DOI] [PubMed] [Google Scholar]
- 21. Hui JY, Yang MK, Cho DH, et al. Pyogenic liver abscesses caused by Klebsiella pneumoniae: US appearance and aspiration findings. Radiology. 2007;242:769–776 [DOI] [PubMed] [Google Scholar]
- 22. Han SH. Review of hepatic abscess from Klebsiella pneumoniae: an association with diabetes mellitus and septic endophthalmitis. West J Med. 1995;162:220–224 [PMC free article] [PubMed] [Google Scholar]
- 23. Callegan MC, Engelbert M, Parke DW, 2nd, Jett BD, Gilmore MS. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002;15:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Booth MC, Atkuri RV, Nanda SK, Iandolo JJ, Gilmore MS. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Invest Ophthalmol Vis Sci. 1995;36:1828–1836 [PubMed] [Google Scholar]
- 25. Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun. 2003;71:3116–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu MF, Yang CY, Lin TL, et al. Humoral immunity against capsule polysaccharide protects the host from magA+ Klebsiella pneumoniae-induced lethal disease by evading Toll-like receptor 4 signaling. Infect Immun. 2009;77:615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishii K, Hiraoka T, Kaji Y, Sakata N, Motoyama Y, Oshika T. Successful treatment of endogenous Klebsiella pneumoniae endophthalmitis: a case report. Int Ophthalmol. 2011;31:29–31 [DOI] [PubMed] [Google Scholar]