The effect of volume perfused on aqueous outflow resistance during two-level constant pressure perfusion is examined. The effect of age on change in outflow resistance due to perfusion volume is also considered.
Abstract
Purpose.
The effect of total volume perfused on outflow resistance (the reciprocal of outflow facility) and the effect of age on the rate of change in resistance as a function of total volume were determined in rhesus and cynomolgus monkeys.
Methods.
Outflow facility was measured under general anesthesia by two-level constant pressure perfusion in one eye of 22 rhesus and 17 cynomolgus monkeys (ranging in age, respectively, from 4 to 25 and from 3 to 12 years). Total volume perfused was calculated from data obtained during the perfusion.
Results.
Resistance decreased in both cynomolgus and rhesus monkeys as total volume perfused increased (−0.085 ± 0.021 and −0.022 ± 0.011 mm Hg/μL/min/μLtot; P = 0.001 and P = 0.047, respectively). Rate of change in resistance significantly increased in cynomolgus monkeys as total volume perfused increased (0.0018 ± 0.0.0007 mm Hg/μL/min/μLtot, P = 0.033); however, this was not the case in rhesus monkeys. After accounting for total volume perfused, the rate of change in resistance significantly decreased with increasing age in rhesus monkeys (−0.0068 ± 0.0026 [mm Hg/μL/min]/μLtot/y, P = 0.017). There was no significant difference in rate of change in resistance with age, after accounting for total volume, in the cynomolgus monkeys.
Conclusions.
The present study supports previous findings indicating that total washout is largely dependent on perfusion volume. However, in populations with old/elderly animals, such as our rhesus group, we found that age does play a significant role in rate of change in resistance, and may be an even more important factor to consider in the rate of resistance change than volume perfused in aged animals.
In nonhuman primate eyes, aqueous humor outflow resistance progressively decreases with time (outflow facility increases) when outflow facility is measured by two-level constant pressure perfusion, this phenomenon known as the “washout effect.”1–5 Washout during anterior chamber (AC) perfusion with Bárány's solution typically increases by approximately 15% per 30 minutes of drug-free perfusion for the first 1 to 2 hours in the living cynomolgus monkey eye.1 Washout magnitude can vary with perfusion fluid composition.3 Pooled homologous aqueous humor or artificial solutions with amino acid and ascorbate content similar to that of monkey aqueous do not eliminate washout entirely.3 However, the addition of autologous serum to Bárány's perfusand minimized washout in cynomolgus monkeys compared with Bárány's solution alone.5
Although the exact mechanism of washout is unclear, it has been hypothesized that it may be associated with separation of the inner wall endothelium of Schlemm's canal from the juxtacanalicular connective tissue.6–8 Although washout increases with duration of perfusion, it correlates much more strongly with the volume of perfusate that passes through the outflow tissues.8–10 Outflow resistance in the trabecular outflow pathways has been found to increase with age and also in primary open-angle glaucoma in vitro.11–14 Other studies have demonstrated that total outflow resistance increases with age in live rhesus monkeys and humans.10,15–18 Gaining a broader knowledge of the factors that affect resistance and resistance washout during perfusion may facilitate a better understanding of the aging eye and of primary open-angle glaucoma. This knowledge may also be of help when studying the effects of different drugs on outflow resistance, since washout can confound interpretation of pharmacologic effects in that small drug effects may be hard to distinguish from washout induced by perfusion itself.
Previously, we reported that the rate of washout during two-level constant pressure perfusion did not differ with age in either rhesus or cynomolgus monkeys, despite an overall age-related increase in baseline outflow resistance in rhesus monkeys and a tendency for a similar increase in cynomolgus monkeys.10,19,20
In the present study, we investigated whether total volume perfused correlates with outflow resistance and whether the change in the rate of outflow resistance due to total volume perfused is affected by age.
Methods
Animals and Anesthesia
Single eyes of 17 cynomolgus (Macaca fascicularis) and 22 rhesus (Macaca mulatta) monkeys from previous perfusion protocols were studied. On the basis of lifespan data of captive monkeys, we used a ratio of 1:3 when comparing ages of rhesus and cynomolgus monkeys to humans.21–24 Cynomolgus monkeys ranged in age from 3 to 12 years (human equivalent of approximately 9–36 years) and rhesus monkeys from 4 to 25 years (human equivalent of approximately 12–75 years). Each animal contributed a single data set from only one eye and had been previously used in no more than four outflow facility determinations. For each animal, data from the most recent outflow facility were used when possible. We have found that cannulation of the AC, with at least a 4- to 6-week recovery period between cannulations, is well tolerated in cynomolgus monkeys. Most animals can have at least 8 to 10 needle cannulations on separate occasions, with no ill effects aside from some minimal corneal scarring, as evidenced by a return to normal intraocular pressure (IOP) and reproducible baseline outflow facility (Kaufman PL, unpublished data, 1988–1989, 1995–1997). After recovery from any outflow facility procedure the monkeys were examined to ensure they were in good health and that the eyes were clinically normal by slit-lamp biomicroscopy. This method enabled us to gather retrospective data from enough animals of each species to make comparisons of volume versus change in outflow resistance and also to use baseline outflow facility values that were within normal range (0.13–0.69 μL/min/mm Hg).
Total volume perfused was taken as the amount of perfusate entering the eye, based on the change in weight of the perfusion reservoir. The monkeys examined in the present study were from the same cohort looked at in our previous age versus washout study. Given that this is a study using retrospective data, data on total volume perfused were not available for all monkeys; consequently, the present study examines fewer animals than the previous age versus washout study since only those with complete sets of available data were used.
Anesthesia for outflow facility measurements was induced with intramuscularly (IM) administered ketamine (5 to 10 mg/kg) and maintained with intravenously (IV) administered pentobarbital sodium (15 mg/kg initial; 10 mg/kg supplemental doses as needed) in cynomolgus monkeys. In rhesus monkeys anesthesia was induced with IM ketamine as before and maintained with IV pentobarbital sodium or IM pentobarbital sodium (15 mg/kg IV or 35 mg/kg IM initial dose; 10 mg/kg supplemental as needed). Slight differences in the anesthetic regimen (IV pentobarbital sodium versus IM pentobarbital sodium) occur because this study uses baseline data of monkeys from several different protocols. This was attributed to differences in the actual experimental protocols as well as to changes in Institutional Animal Care and Use Committee requirements for anesthesia. All experiments were conducted in compliance with the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research.
Outflow Facility
In each protocol, total outflow facility (Ctot) was measured by two-level constant pressure perfusion of the AC for 35 to 40 minutes.4 Total outflow facility is comprised of facility of outflow via the trabecular pathway (Ctrab), facility of outflow via the uveoscleral pathway (Cu), and the facility of inflow (Cin) such that20
Because trabecular outflow facility comprises the majority of total outflow facility25 and outflow resistance mainly occurs in tissues that comprise the trabecular pathway,26 uveoscleral outflow facility and facility of inflow were assumed to be constant.
Depending on experimental protocol, the ACs of both eyes were cannulated with either one (rhesus) or two (cynomolgus) 26-gauge needles as described in the following text. The perfusion pressures were 2.5 and 11.9 mm Hg above spontaneous IOP and outflow facility values were corrected for the internal resistance of the perfusion apparatus.27 Measurements were taken with the same perfusion apparatus in all experiments.
In cynomolgus monkeys the ACs of both eyes were cannulated with one branched (superiorly) and one nonbranched (inferiorly) 26-gauge needle. One end of the branched needle was attached to an elevated reservoir containing Bárány's perfusand4 and the other to a physiological pressure transducer (P23 Db Series; Gould–Statum Inc., Oxnard, CA) via polyethylene tubing. Flow and pressure measurements were obtained via the branched needle. The reservoir was suspended from a strain gauge that generated voltage readings.3 Pressure and reservoir weight were recorded approximately every 5 seconds over a period of 4 minutes, so that there were approximately 48 measurements taken in each 4-minute period. Pressure and flow measurements were calculated for each 4-minute interval and these were used to calculate outflow facility values by successive weighted averaging for three periods, the middle period being counted twice, minimizing “sawtoothing.”4,28 Bárány found that individual facility and pressure values from single estimates fluctuated and formed a curve that deviated from the assumed “true ” course for facility. He hypothesized that this was most likely caused by noise in the system. In a live animal, factors such as heart rate, respiration, and slight eye movement can produce unsteadiness in the rate of flow. He found that successive weighted averaging of the individual facility values compensated for these errors and smoothed the curve to more closely represent true facility.28 Voltage measurements corresponding to flow from the reservoir and to pressure were also sent to a chart recorder. Total volume perfused was determined from the chart recorder tracing by taking the sum of each flow measurement calculated during each 4-minute segment for each eye of each monkey. Outflow resistance was calculated as the reciprocal of outflow facility as described by Bárány.4 Generally, five to seven outflow facility values generated over a 35- to 40-minute period were averaged, giving a mean value for total outflow facility. The nonbranched needle was attached to clamped polyethylene tubing and was not used while obtaining baseline outflow facility readings used in this study. Baseline outflow facility was measured for 30 to 40 minutes.
In rhesus monkeys the ACs of both eyes were cannulated with a single branched 26-gauge needle connected to an elevated reservoir and pressure transducer and baseline outflow facility was measured for 35 to 40 minutes as before.
Data Analysis
Statistical analysis used linear regression for the mean outflow resistance for the entire 30- to 40-minute period versus total volume (Vtot) perfused, and multiple regression analysis for change in resistance per minute of perfusion versus total volume perfused (Δ resistance/Vtot), change in resistance per minute of perfusion versus age (Δ resistance/age), and change in resistance after accounting for total volume perfused versus age. There were no interspecies comparisons. Data are defined as follows:
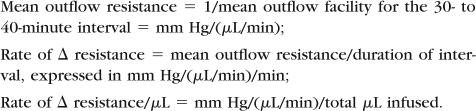 |
Data values are slope ± SE for linear and multiple regressions.
Results
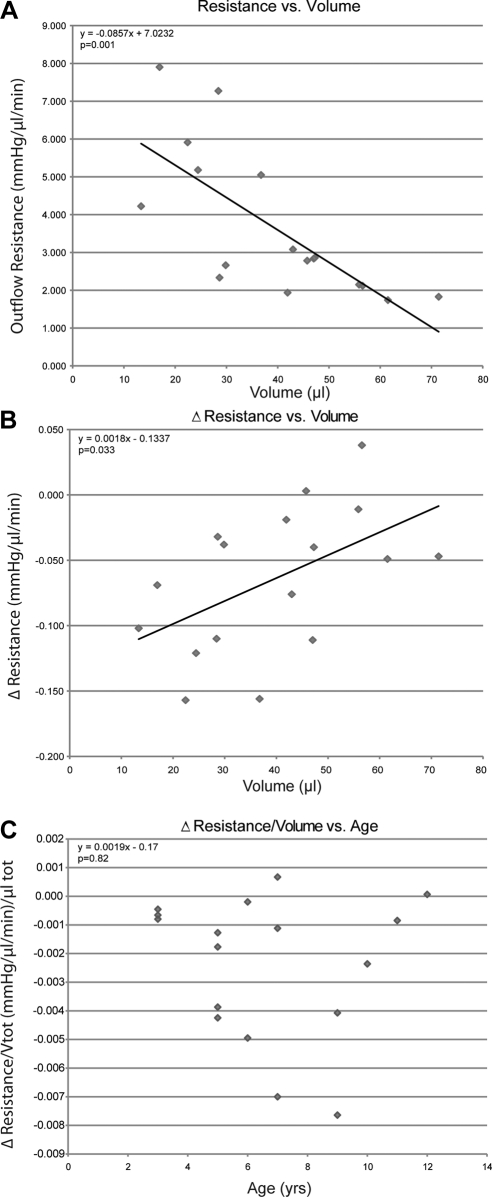
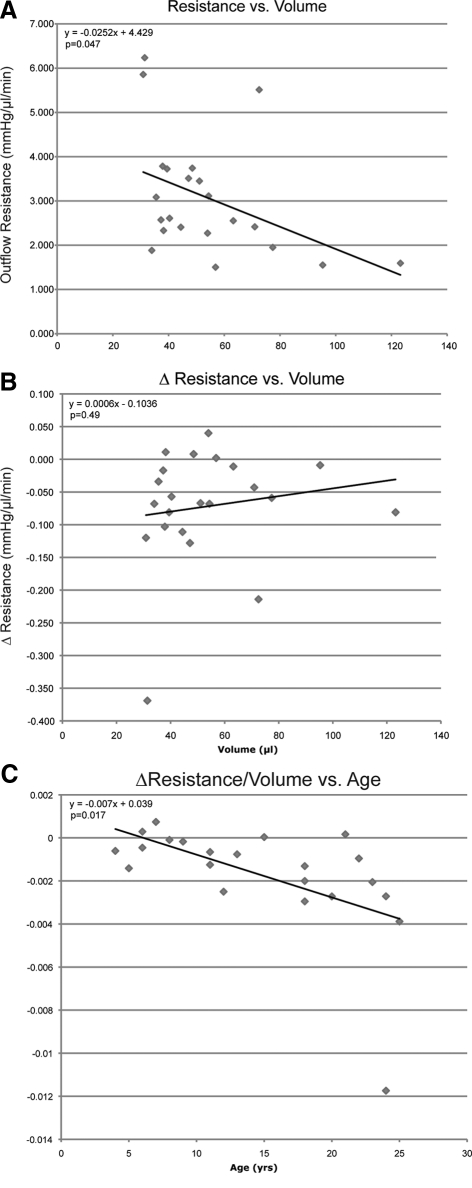
Total outflow resistance decreased as the total volume perfused increased in both cynomolgus (Fig. 1A) and rhesus monkey (Fig. 2A) eyes (−0.085 ± 0.021 and −0.022 ± 0.011 mm Hg/(μL/min)/μLtot, P = 0.001 and P = 0.047, respectively). In cynomolgus monkey eyes (Fig. 1B), the rate of change in resistance increased significantly as total volume perfused increased (0.002 ± 0.0007 [mm Hg/(μL/min)]/μLtot, P = 0.033); however, this was not the case in rhesus monkey eyes (Fig. 2B). Total volume perfused tended to decrease with age in cynomolgus and rhesus monkey eyes, approaching significance for each species. After accounting for total volume perfused, the rate of change in resistance decreased significantly with age in rhesus monkey eyes (Fig. 2C; −0.0068 ± 0.0026 [mm Hg/(μL/min)]/μLtot/y, P = 0.017); however, there was no age-related change in resistance in cynomolgus monkey eyes (Fig. 1C).
Figure 1.
Total volume perfused versus resistance (A) and rate of change in resistance (B) at baseline in 17 cynomolgus monkeys each contributing one eye. Solid line represents least-squares linear regression of change in resistance on volume. Age (years) versus rate of change in resistance corrected for total volume [(mm Hg/μL/min)/μLtot] (C) at baseline in 17 cynomolgus monkeys each contributing one eye. Equation represents multiple regression analysis of change in resistance due to volume after accounting for age.
Figure 2.
Total volume perfused versus resistance (A) and rate of change in resistance (B) at baseline in 22 rhesus monkeys each contributing one eye. Solid line represents least-squares linear regression of resistance or change in resistance on volume. Age (years) versus rate of change in resistance corrected for total volume perfused [(mm Hg/μL/min)/μLtot] (C) at baseline in 22 rhesus monkeys each contributing one eye. Solid line represents least-squares linear regression of resistance washout, corrected for total volume, on age. Equation represents multiple regression analysis of change in resistance, accounting for volume, on age.
Discussion
In the present study, we demonstrated that resistance decreased as volume perfused through the AC increased during outflow facility determination by two-level constant pressure perfusion in both rhesus and cynomolgus monkeys. We also found an increase in the rate of change in resistance as a function of volume perfused that was significant for cynomolgus monkeys, but not for rhesus monkeys. As in previous studies10,17 we found an overall decrease in baseline outflow resistance (increase in outflow facility) with volume perfused in both rhesus and cynomolgus monkeys that was not correlated with age in either species. However, after correcting for total volume perfused, we did find a significant age-related decrease in the rate of change in resistance in rhesus monkeys that was not present in cynomolgus monkeys. This indicates that age may play a role in the rate of resistance change during AC perfusion. That there was not a significant difference in the rate of change in resistance with age in cynomolgus monkey eyes may be explained by the fact that we were able to obtain data only from young and middle-aged animals, unlike the rhesus monkey group that included more middle-aged and elderly animals. We also found a nearly significant decrease in total volume perfused with age in rhesus monkeys and a similar, although not significant, trend in cynomolgus monkeys.
The exact mechanism(s) for washout are not entirely clear. Changes in resistance could be the result of mechanical disruption or removal of extracellular material from the outflow pathways.5,29,30 Outflow resistance in the trabecular meshwork (TM) outflow pathways has been shown to increase with age in humans and rhesus monkeys.11,12,14 Buildup of extracellular material within the interstices of the TM has been implicated in the age-related increase in outflow resistance seen in humans.31,32 Similarly, increases in sheath-derived plaques and fibrillar material in the juxtacanalicular tissue region and a decrease in overall cellularity of the TM have been found with increasing age in rhesus monkeys.23 Loss of trabecular cells with age could result in a reduction in matrix metalloproteinase (MMP) activity in the TM. This may result in a reduced capacity of the TM to break down extracellular material.23 In fact, passage-number–related reduction in MMP activity has been found in porcine trabecular cell cultures exposed to 15–50 mm Hg of pressure for 72 hours (Ehrich D, et al. IOVS 2001;42:ARVO Abstract 748) and in human TM cells in vitro (Williams GC, et al. IOVS 2001;42:ARVO Abstract 764). This increase in extracellular material and loss of cellularity seen in older animals might affect the rate of change in outflow resistance during AC perfusion. Removal of extracellular material from the TM during AC perfusion may need to reach a “critical mass” in these older monkeys (a certain amount of time may need to elapse and/or a sufficient amount of fluid may need to pass through the outflow tissues) before the rate of change in resistance is similar to that seen in young and middle-aged monkeys. In the present study, we measured outflow resistance for only 30 to 45 minutes, whereas outflow resistance washout typically occurs for the first 1 to 2 hours during AC perfusion.1 Therefore, it may be useful to determine whether the rate of change in resistance is similar in groups of young, middle-aged, and older monkeys when perfused for a longer period.
Previous studies have indicated that decreased resistance may be due to a disruption in the connectivity between the inner wall endothelium of Schlemm's canal and the juxtacanalicular tissue6,8,33,34) that may lead to an increase in the effective filtration area of aqueous outflow.35 Scott et al.8 found that a greater separation of the inner wall and juxtacanalicular tissue was significantly correlated with a larger absolute value of outflow facility in enucleated bovine eyes. In fact, studies have shown that enucleated human eyes do not exhibit the washout effect36; moreover, human eyes, evaluated by light and electron microscopy after undergoing long-duration perfusion (∼3 hours), were found to have no apparent separation between the inner wall and juxtacanalicular tissue,8 supporting the theory that decreased resistance may be caused by this separation of outflow tissues rather than actual “washout” of extracellular material.
Our findings support previous reports indicating that outflow resistance decrease during perfusion is largely, and often primarily, dependent on volume rather than on age.9,11,37) However, in populations with old/elderly animals, such as our rhesus group, we found that age does play a significant role in the rate of change of resistance, and may be an even more important factor than total perfusion volume when examining the rate of resistance washout. In our study, eyes were perfused for the same length of time, so that any change in outflow resistance over the course of the perfusion would have been due to some factor other than time, such as volume perfused or age. The number of previous experimental procedures an animal has undergone could theoretically influence the rate of change in resistance, thus contributing to the change we observed in the older rhesus monkeys. However, this seems highly unlikely since all animals in the rhesus monkey group had only one perfusion and no monkey in either group had more than four previous perfusions. Since the older rhesus did not undergo any more ocular experimental manipulations than did the younger rhesus, the number of procedures cannot explain the decrease in rate of change in resistance seen with age. Many of the cynomolgus monkeys underwent more than one perfusion, but no difference in rate of change in resistance was observed among monkeys in this group, irrespective of whether they underwent one perfusion or more than one perfusion.
The present study suggests that the factors that lead to changes in outflow resistance, such as removal of extracellular material and/or the separation of the inner wall and juxtacanalicular tissue, may occur at a slower rate with increasing age. This may be partly explained by the fact that the age-related increase in overall outflow resistance allows less volume to pass through the outflow pathways, which leads to a slower rate of resistance washout.
Acknowledgments
The authors thank Ting-Li Lin for assistance with data analysis and the Wisconsin National Primate Research Center.
Footnotes
Supported in part by National Eye Institute Grant EY002698; Research to Prevent Blindness (New York, NY), unrestricted departmental and physician–scientist awards; Ocular Physiology Research and Education Foundation; and the Walter Helmerich Chair from the Retina Research Foundation.
Disclosure: J.A. Kiland, None; B.T. Gabelt, None; P.L. Kaufman, None
References
- 1. Kaufman PL, True-Gabelt B, Erickson-Lamy KA. Time-dependence of perfusion outflow facility in the cynomolgus monkey. Curr Eye Res. 1988; 7: 721– 726 [DOI] [PubMed] [Google Scholar]
- 2. Kiland JA, Peterson JA, Gabelt BT, Kaufman PL. Effect of DMSO and exchange volume on outflow resistance washout and response to pilocarpine during anterior chamber perfusion in monkeys. Curr Eye Res. 1997; 16: 1215– 1220 [DOI] [PubMed] [Google Scholar]
- 3. Erickson KA, Kaufman PL. Comparative effects of three ocular perfusates on outflow facility in the cynomolgus monkey. Curr Eye Res. 1981; 1: 211– 216 [DOI] [PubMed] [Google Scholar]
- 4. Bárány EH. Simultaneous measurements of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol. 1964; 2: 135– 143 [PubMed] [Google Scholar]
- 5. Kee C, Gabelt BT, Gange SJ, Kaufman PL. Serum effects on aqueous outflow during anterior chamber perfusion in monkeys. Invest Ophthalmol Vis Sci. 1996; 37: 1840– 1848 [PubMed] [Google Scholar]
- 6. Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002; 43: 3455– 3464 [PubMed] [Google Scholar]
- 7. Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004; 78: 137– 150 [DOI] [PubMed] [Google Scholar]
- 8. Scott PA, Overby DR, Freddo TF, Gong H. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007; 84: 435– 443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan DB, Trope GE, Ethier CR, Menon IA, Wakeham A. Effects of hydrogen peroxide–induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthalmol Vis Sci. 1991; 32: 2515– 2520 [PubMed] [Google Scholar]
- 10. Kiland JA, Croft MA, Gabelt BT, Kaufman PL. Atropine reduces but does not eliminate the age-related decline in perfusion outflow facility in monkeys. Exp Eye Res. 1997; 64: 831– 835 [DOI] [PubMed] [Google Scholar]
- 11. Sit AJ, Gong H, Ritter N, Freddo TF, Kamm R, Johnson M. The role of soluble proteins in generating aqueous humor outflow resistance in the bovine and human eye. Exp Eye Res. 1997; 64: 813– 821 [DOI] [PubMed] [Google Scholar]
- 12. Ethier CR, Coloma FM, Sit AJ, Johnson M. Two pore types in the inner-wall endothelium of Schlemm's canal. Invest Ophthalmol Vis Sci. 1998; 39: 2041– 2048 [PubMed] [Google Scholar]
- 13. Johnson M, Chan D, Read AT, Christensen C, Sit A, Ethier CR. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002; 43: 2950– 2955 [PubMed] [Google Scholar]
- 14. Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retina Eye Res. 2005; 24: 612– 637 [DOI] [PubMed] [Google Scholar]
- 15. Becker B. The decline in aqueous secretion and outflow facility with age. Am J Ophthalmol. 1958; 4: 731– 736 [DOI] [PubMed] [Google Scholar]
- 16. Gaasterland D, Kupfer C, Milton R, Ross K, McCain L, MacLellan H. Studies of aqueous humour dynamics in man VI. Effect of age upon parameters of intraocular pressure in normal human eyes. Exp Eye Res. 1978; 26: 651– 656 [DOI] [PubMed] [Google Scholar]
- 17. Gabelt BT, Crawford K, Kaufman PL. Outflow facility and its response to pilocarpine decline in aging rhesus monkeys. Arch Ophthalmol. 1991; 109: 879– 882 [DOI] [PubMed] [Google Scholar]
- 18. Croft MA, Oyen MJ, Gange SJ, Fisher MR, Kaufman PL. Aging effects on accommodation and outflow facility responses to pilocarpine in humans. Arch Ophthalmol. 1996; 114: 586– 592 [DOI] [PubMed] [Google Scholar]
- 19. Kiland JA, Gabelt BT, Kaufman PL. Effect of age on outflow resistance washout during anterior chamber perfusion in rhesus and cynomolgus monkeys. Exp Eye Res. 2005; 81: 724– 730 [DOI] [PubMed] [Google Scholar]
- 20. Gabelt BT, Kaufman PL. Aqueous humor hydrodynamics. In: Kaufman P, Alm A.eds. Adler's Physiology of the Eye, Clinical Applications. 10th ed. St. Louis: Mosby; 2002:237–289 [Google Scholar]
- 21. Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001; 281: E757– E765 [DOI] [PubMed] [Google Scholar]
- 22. Fernandes A, Bradley DV, Tigges M, Tigges J, Herndon JG. Ocular measurements throughout the adult life span of rhesus monkeys. Invest Ophthalmol Vis Sci. 2003; 44: 2373– 2380 [DOI] [PubMed] [Google Scholar]
- 23. Gabelt BT, Gottanka J, Lütjen-Drecoll E, Kaufman PL. Aqueous humor dynamics and trabecular meshwork and anterior ciliary muscle morphologic changes with age in rhesus monkeys. Invest Ophthalmol Vis Sci. 2003; 44: 2118– 2125 [DOI] [PubMed] [Google Scholar]
- 24. Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004; 45: 89– 115 [DOI] [PubMed] [Google Scholar]
- 25. Bill A. Conventional and uveo-scleral drainage of aqueous humor in the cynomolgus monkey (Macaca irus) at normal and high intraocular pressures. Exp Eye Res. 1966; 5: 45– 54 [DOI] [PubMed] [Google Scholar]
- 26. Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009; 88: 648– 655 [DOI] [PubMed] [Google Scholar]
- 27. Bárány EH, Rohen JW. Localized contraction and relaxation within the ciliary muscle of the vervet monkey (Cercopithecus ethiops). In: Rohen JW.ed. The Structure of the Eye, Second Symposium. Stuttgart, Germany: FK Schattauer Verlag; 1965: 287–311 [Google Scholar]
- 28. Bárány EH. The mode of action of miotics on outflow resistance. A study of pilocarpine in the vervet monkey Cercoithecus ethiops. Trans Ophthalmol Soc UK. 1966; 86: 539– 578 [PubMed] [Google Scholar]
- 29. Gual A, Llobet A, Gilabert R, et al. Effects of time of storage albumin, and osmolality changes on outflow facility of bovine anterior segment in vitro. Invest Ophthalmol Vis Sci. 1997; 38: 2165– 2171 [PubMed] [Google Scholar]
- 30. Johnson M, Gong H, Freddo TF, Ritter N, Kamm R. Serum proteins and aqueous outflow facility resistance in bovine eyes. Invest Ophthalmol Vis Sci. 1993; 34: 3549– 3557 [PubMed] [Google Scholar]
- 31. Lütjen-Drecoll E, Shimuzu R, Rohrbach M, Rohen JW. Quantitative analysis of “plaque material ” in the inner and outer wall of Schlemm's canal in normal and glaucomatous eyes. Exp Eye Res. 1986; 42: 443– 457 [DOI] [PubMed] [Google Scholar]
- 32. Rohen JW, Lütjen-Drecoll E. Age change in the morphology of anterior segment of the eye. In: De Vincentis M.ed. The Fundamental Aging Processes of the Eye. Florence, Italy: Baccini & Chiappi; 1987:47–57 [Google Scholar]
- 33. Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused bovine aqueous outflow pathways. Exp Eye Res. 1988; 7: 799– 807 [DOI] [PubMed] [Google Scholar]
- 34. Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992; 33: 1670– 1675 [PubMed] [Google Scholar]
- 35. Scott PA, Lu Z, Liu Y, Gong H. Relationships between increased aqueous outflow facility during washout with the changes in hydrodynamic pattern and morphology in bovine aqueous outflow pathways. Exp Eye Res. 2009; 89: 942– 949 [DOI] [PubMed] [Google Scholar]
- 36. Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest Ophthalmol Vis Sci. 1990; 31: 2384– 2388 [PubMed] [Google Scholar]
- 37. Johnson M, Chen A, Epstein DL, Kamm RD. The pressure and volume dependence of the rate of wash-out in the bovine eye. Curr Eye Res. 1991; 10: 373– 385 [DOI] [PubMed] [Google Scholar]