Lacritin is a novel human tear glycoprotein that when added topically promotes tearing in normal New Zealand White rabbits. After a two-week treatment, lacritin stimulated basal tearing, and cyclosporine triggered mild to moderate corneal irritation and a temporary elevation in tearing.
Abstract
Purpose.
Lacritin is a novel human tear glycoprotein that promotes basal tear peroxidase secretion by rat lacrimal acinar cells in vitro. This study investigates whether lacritin is prosecretory when added topically to the ocular surface of normal living rabbits, and if so, what is its efficacy and tolerability versus cyclosporine and artificial tears.
Methods.
Purified recombinant human lacritin (1, 10, 50, or 100 μg/mL), inactive lacritin truncation mutant C-25 (10 μg/mL), cyclosporine (0.05%), or artificial tears were topically administered to eyes of normal New Zealand White rabbits either as a single dose or three times daily for 14 days with monitoring of basal tear production. Basal tearing under proparacaine anesthesia was repeatedly assessed throughout and 1 week after chronic treatment ceased. Eyes were examined weekly by slit-lamp biomicroscopy.
Results.
Lacritin acutely increased basal tearing to 30% over vehicle at 240 minutes. Three times daily treatment with 10–100 μg/mL lacritin was well tolerated. Basal tearing became progressively elevated 4, 7, and 14 days later and was 50% over baseline (50 μg/mL lacritin) 1 week after treatment had ceased. Cyclosporine elevated tearing to a similar level on days 4 and 7 but had little or no effect on day 14 and had returned to baseline 1 week after ending treatment. C-25 and artificial tears had no effect.
Conclusions.
Lacritin acutely stimulates basal tear flow that is sustained for at least 240 minutes. Two weeks of lacritin treatment three times daily was well tolerated and progressively elevated the basal tear flow. One week after treatment ended, basal tearing was still 50% over baseline. In contrast, cyclosporine triggered mild to moderate corneal irritation and a temporary elevation in tearing.
Dry eye is the most common eye disease, affecting at least 5% of the world's population, with higher prevalence in postmenopausal women (6%–9.8%)1 and the elderly (as high as 34%).2 Symptoms of this multifactorial disease include ocular surface discomfort and damage, tear film instability, problems with visual acuity, increased tear osmolarity and inflammation, and elevated susceptibility to infection. The International Dry Eye Workshop Report3 distinguishes aqueous deficient (ADDE) and evaporative (EDE) dry eye and subcategories. ADDE is subdivided into Sjögren's (primary/secondary) and non-Sjögren's syndrome. Primary Sjögren's syndrome is ADDE associated with autoantibodies, an inflammatory focal score in minor salivary glands and dry mouth with reduced salivation. Secondary Sjögren's syndrome has added evidence of connective tissue autoimmune disease. The more common non-Sjögren's syndrome ADDE is described as an aging-associated and autoimmune disease–independent lacrimal gland deficiency. EDE is excessive ocular surface water loss from evaporation, most commonly from blepharitis or meibomian gland dysfunction. Other causes include vitamin A deficiency (insufficient development of conjunctival goblet cells and lacrimal acinar cells), contact lens wear, topical drugs, and allergy.3
Treatment of dry eye is still at an early stage. Commonly used “artificial tears” temporarily alleviate symptoms of dry eye without addressing the cause. An ophthalmic formulation of the anti-inflammatory agent cyclosporine has been widely promoted for treatment of moderate to severe dry eye.4 Although two independent FDA phase 3 clinical trials involving 877 patients revealed a basal tearing benefit (Schirmer with anesthesia, 10 mm or more) in only 15% of dry eye patients versus 5% with placebo,4 subsequent studies have been more promising.5,6 Other proposed treatment approaches include topical vitamin A6, androgen,7 UTP analog INS365,8 and muscarinic agonist pilocarpine.9,10
The tear film is a rich source of growth factors, proteases, protease inhibitors, antioxidants,11 mucins, and lipids that has been only partially characterized. Future-capture ELISAs of specific tear proteins and tear proteomics together offer potentially useful indicators of the health of the ocular surface.12 Mechanisms underlying dysfunction might also be gleaned; for example, the lipophile lipocalin-113 and phospholipase A2 are increased in tears of patients intolerant to wearing contact lenses.14 Comparison of tears from Sjögren's syndrome dry eye versus normal subjects by mass spectrometry revealed seven proteins peaks downregulated and three upregulated.15 Three human dry eye-related conditions have been scrutinized to date by unbiased screens coupled to proteomics and sequencing. Although HGF, IGF, NGF, and EGF can be detected in normal tears,16 “lacritin” was the only growth-like factor (and only one of nine tear proteins) downregulated of hundreds of proteins identified in tears from patients suffering from blepharitis.17 Blepharitis is a common inflammation of the eyelid, associated as noted above with EDE. The other eight downregulated proteins were albumin, Ig k chain-VIII, pyruvate kinase, α1-antitrypsin, prolactin-inducible protein, cystatin SA-III, and lysozyme.17 Lactoferrin, lipocalin-1, lysozyme, and prolactin-inducible protein were reported to be downregulated in a screen of tears from patients with non-Sjögren's syndrome dry eye.18 Recently Nichols and Green reported that lacritin is selectively downregulated more than any other tear protein in contact lens–related dry eye.19 Lacritin stimulates MUC16 production by human corneal epithelial cells at levels matching or exceeding that of serum (Laurie GE, et al. IOVS 2006;47:ARVO E-Abstract 1606). Autologous serum is a reportedly successful method of treating dry eye.20 Lacritin also promotes basal tear secretion by cultured rat21 and monkey22 lacrimal acinar cells and stimulates human corneal epithelial cell growth.23
Lacritin is a 12.3 kDa secreted glycoprotein that is apically released from human lacrimal acinar cells during reflex tearing24–26 and can be detected in mixed reflex and basal human tears by ELISA21 and Western blotting.27 Lacritin is also produced by corneal, conjunctival,27 meibomian,28 and salivary epithelia as one of the most eye-restricted genes. Few cell types appear capable of being targeted by lacritin. Targeted cells include lacrimal acinar, salivary ductal/HeLa, human corneal, and embryonic kidney cells, but no others among 17 different cell lines tested.23 Its co-receptor syndecan-1 is widely expressed on ocular surface epithelia.29 Thus, lacritin appears to be a multifunctional eye-specific factor with a potential role in tear secretion and corneal epithelial renewal. Here we ask whether lacritin promotes tearing when administered topically to eyes of normal rabbits. We observe that a single dose of lacritin or lacritin chronically administered for 2 weeks elevates basal tearing. The latter is remarkably sustained for 1 week after treatment ended.
Materials and Methods
Animals
A total of 18 age- and weight-matched New Zealand White adult female rabbits (3–5 kg) with normal eyes and no ocular pathology were used in this study. Experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Eastern Virginia Medical School Institutional Animal Care and Use Committee. Animals were acclimated for 1 week before experimentation.
Recombinant Lacritin and C-25
Human recombinant lacritin and a lacritin truncation mutant lacking 25 amino acids from its C terminus (C-25) were cloned into bacteria expression plasmids as previously described.23 For protein expression, cultures of E. coli strain ER2566 harboring the plasmid of interest were grown to midlog (37°C), induced with 0.5 mM isopropyl-β-D-thiogalactopyranoside for 4 hours (23°C), and harvested by centrifugation. Cell pellets frozen at −70°C were thawed at room temperature, lysed by sonication in 50 mM Tris (pH 8), 0.5 M NaCl, 0.45% Triton X-100, and centrifuged. Supernatant was loaded onto chitin columns (IMPACT-CN System; New England Biolabs, Ipswich, MA) equilibrated with 10 column volumes of 50 mM Tris (pH 8), 0.5 M NaCl. Columns were washed with 20 column volumes of the same buffer. On-column cleavage of lacritin or C-25 from C-terminal intein was accomplished by incubation for 16 hours at room temperature with 0.39% (V/V) 2-mercaptoethanol in the same buffer. Eluates were concentrated by ultrafiltration and dialyzed extensively against PBS (4°C). The concentrated and dialyzed chitin fraction was loaded onto a fast-flow column equilibrated with PBS (DEAE Sepharose Fast Flow; GE Healthcare, Uppsala, Sweden). The unbound flow through was collected and assayed for total protein concentration by the BCA assay and purity by SDS PAGE (Fig. 1). Aliquots were frozen, lyophilized, and stored at −70°C. To prepare topical eye drops, lacritin was dissolved under aseptic conditions in sterile water.
Figure 1.
SDS PAGE of purified lacritin. Lane 1: molecular weight markers; lane 2: purified lacritin. Coomassie blue staining.
Cyclosporine and Artificial Tears
Cyclosporine (0.05%, Restasis; Allergan, Irvine, CA) and artificial tears (Refresh Tears; Allergan) were purchased from local pharmacies.
Tear Fluid Analysis
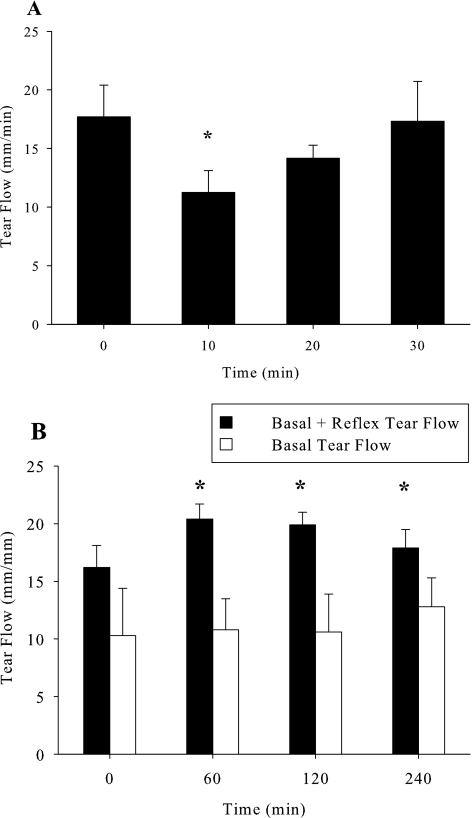
Before experimentation, baseline tear flow, intraocular pressure, and tear composition were assessed. Eyes were first anesthetized for 10 minutes with 0.5% proparacaine to minimize reflex tearing (Fig. 2) and then polyester wicks (Filtrona R15643; Company, Colonial Heights, VA) or calibrated Schirmer strips (TearFlo; Rose Stone Enterprises, Alta Loma, CA) were placed in the medial canthus for 1 minute. We refer to tears collected with anesthetic as “basal tears” and their production as “basal tear secretion.” Rabbits were randomly assigned to receive 50 μL in each eye of lacritin (1, 10, and 50 μg/mL), C-25 (10 μg/mL), 0.05% cyclosporine, or artificial tears. In acute studies, basal tear flow was bilaterally measured 60, 120, and 240 minutes after agonist addition. In chronic studies, eyes were treated three times daily for 14 days, and then basal tear flow was measured with Schirmer strips after 4, 7, and 14 days of treatment, and 7 days after treatment had stopped. Basal tears were collected 60, 120, and 240 minutes after the morning dose of lacritin, C-25, cyclosporine, or artificial tears. Data were then averaged as the “daily tear flow.” Tears were eluted by soaking wicks for 20 minutes in 30 μL of deionized water (conductivity 18 μΩ), followed by centrifugation at 13,000 rpm for 10 minutes. Tear pH, sodium, and potassium were respectively monitored with microelectrodes MI-410, MI-420, and MI-442 (Microelectrodes, Inc., Bedford, NH). Protein concentration was determined using a BCA protein assay (Thermo Scientific/Pierce, Rockford, IL), which was linear from 0.025 to 2.0 μg/μL at 570 nm (PowerWaveX microplate spectrophotometer; Bio-Tek Instruments Inc., Winooski, VT).
Figure 2.
Optimization of anesthesia for basal tear collection. (A) Proparacaine anesthesia before Schirmer strip application is optimal at 10 minutes. Tear flow into Schirmer strips was measured at baseline, and then 10, 20, or 30 minutes after proparacaine administration. *Tear flow compared to baseline (P = 0.05, n = 6). (B) Constant basal (white bars; with proparacaine) versus variable basal plus reflex (black bars; no proparacaine) tear flow at consecutive 60-minute intervals over 240 minutes. *Significantly greater than basal tear flow (P < 0.001, n = 6).
Ocular Surface Analysis
Before and after treatment, rabbits were lightly sedated with acepromazine (2.5 mg/kg) and ketamine (25 mg/kg), and then all eyes were examined by slit-lamp biomicroscopy (HAAG-STREIT, Bern, Switzerland) using a semiquantitative modified McDonald-Shadduck scale for ocular irritation and inflammation.30 Evaluations were performed by an independent knowledgeable observer. Parameters included conjunctival congestion, swelling, and discharge, aqueous flare/anterior chamber reaction, loss of light reflex, iris hyperemia, corneal opacity, and vascularization. Each parameter was rated on a four-point scale, where zero represents normal.
Intraocular Pressure Analysis
Intraocular pressure was monitored using pneumotonometry (Mentor, Norwel, MA) in rabbits lightly sedated with acepromazine (2.5 mg/kg) and ketamine (25 mg/kg). Two consecutive measurements made at the same time of day by the same observer were averaged.
Statistical Analysis
Results are reported as the mean ± SE. Data were analyzed by paired t-test or ANOVA as appropriate. Differences were considered significant at P < 0.05.
Results
Basal Tearing after Treatment with Lacritin, C-25, Artificial Tears, or Cyclosporine
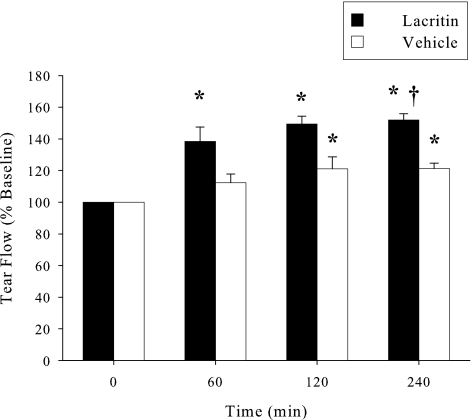
We initially optimized 0.5% proparacaine anesthesia to thoroughly block irritation-induced reflex tearing associated with wick or Schirmer strip insertion.31 Without anesthesia, Schirmer strips collected 17.7 ± 2.1 mm (n = 6) of rabbit tears in 1 minute, a value representing mixed basal and reflex tears. However, 10, 20, and 30 minutes after proparacaine, strips collected 11.3 ± 3.4, 14.2 ± 2.7, and 17.3 ± 8.3 mm (n = 6) of rabbit tears, respectively. These data indicated that proparacaine was most effective at 10 minutes (P = 0.05) or less. Thereafter reflex tearing gradually resumed (Fig. 2A) in keeping with the brevity of proparacaine anesthesia. We wished to assess basal tearing at multiple time points and wondered whether an interval as short as 60 minutes was appropriate. To address this question (Fig. 2A), proparacaine was administered 10 minutes before tear collection at each of 0, 60, 120, and 240 minutes later. Basal tear flow was 10.3 ± 4.1 mm at 0 min (n = 6) and remained essentially unchanged at each subsequent time point. At 240 minutes, for example, it was 10.4 ± 2.6 mm (n = 6). However, in the absence of proparacaine, tear flow was 16.2 ± 1.9 mm (n = 6) at 0 min. Flow increased after Schirmer strips were inserted at 60 and 120 minutes, but not after 240 minutes (Fig. 2B). Taken together, basal tear collection 10 minutes after initiating proparacaine anesthesia and at 60-minute intervals are appropriate for analysis of basal tearing. To address whether lacritin was a basal tear agonist, lacritin (50 μg/mL) was topically administered to the right eyes. Left eyes received PBS (vehicle) alone. Tear flow was subsequently measured at 60, 120, and 240 minutes with anesthesia. Lacritin significantly increased basal tear flow over baseline at each time point (n = 6; P < 0.01) and exceeded the vehicle by 30% at 240 minutes (Fig. 3; n = 6; P < 0.001).
Figure 3.
A single topical dose of lacritin (50 μg/mL) increases tear flow for at least 240 minutes. Contralateral eyes received vehicle (PBS) alone. Augmented tear production versus vehicle-treated eyes was significant after 240 minutes (P < 0.05, n = 6). *Significantly greater than baseline (P < 0.001). †Significantly greater than vehicle (P < 0.05).
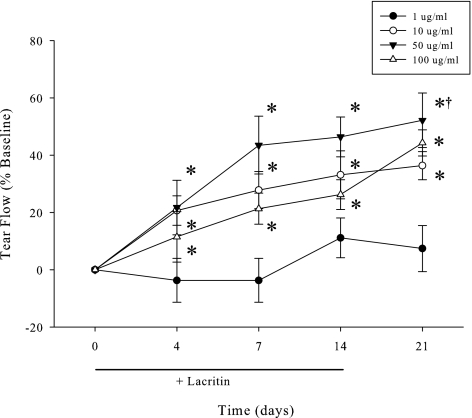
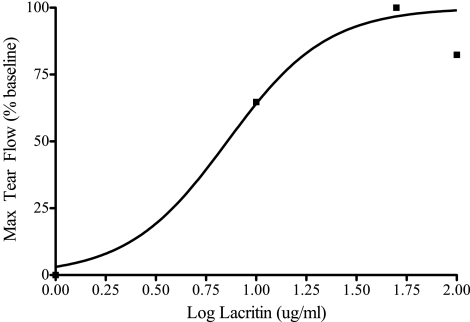
Although basal tearing is elevated for at least 240 minutes after a single lacritin dose, we wished to know whether the response might diminish with repeated treatment and whether there was a concentration effect. To address these questions, eyes were topically treated with 1, 10, 50, or 100 μg/mL of lacritin three times daily for 14 days. Basal tears were collected during the course of treatment at 0, 4, 7, and 14 days. To explore possible long-term effects, tears were also collected 7 days after lacritin treatment had ended (Fig. 4). Instead of a loss of effect, basal tearing became progressively elevated and displayed a concentration dependant response with a lacritin dose optimum of 50 μg/mL. More unexpectedly, basal tearing was 50% over baseline (P < 0.001, n = 6) 1 week after lacritin treatment had ceased. This latter effect (possibly trophic) displayed an approximate EC50 of 10 μg/mL (Fig. 5) when maximum basal tear flow was plotted versus log dose. Thus single doses of lacritin given repeatedly have a benefit that is sustained after washout.
Figure 4.
Comparison of lacritin doses suggests that 50 μg/mL lacritin optimally stimulates basal tearing. Elevated tearing is sustained for at least one week after treatment ceased (“7-Day Washout”; n = 6/dose). Rabbits were treated three times daily for 14 days. Tear flow was measured at 60, 120, and 240 minutes after the morning dose of lacritin. Shown is the 240 minutes timepoint. *Significantly greater tear flow than baseline (P < 0.01, n = 6). †Significantly greater tear flow than 1 or 10 μg/mL lacritin (P < 0.001, n = 6).
Figure 5.
Elevated posttreatment basal tearing versus lacritin treatment dose. Tear flow dose response 7 days after cessation of three times per day treatment with 1, 10, 50, or 100 μg/mL lacritin (n = 4/group). Apparent EC50 during treatment phase is 10 μg/mL.
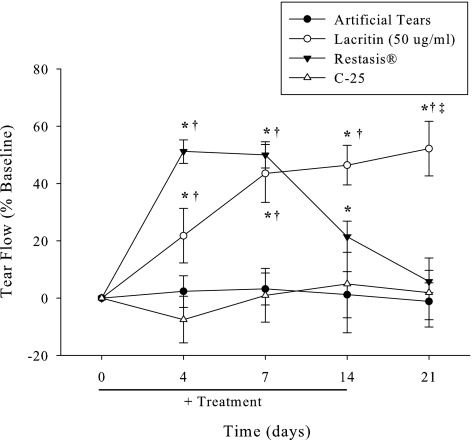
Although our rabbits were not suffering from autoimmune dry eye, a question left unanswered by Toshida32 was whether 0.05% cyclosporine might alter basal tearing, and if so how might tearing compare to eyes treated with artificial tears, lacritin, or inactive lacritin C-25. C-25 lacks the syndecan-1 binding domain necessary for lacritin cell targeting.33 Eyes received topical 0.05% cyclosporine, artificial tears, lacritin, or C-25 lacritin three times daily over 2 weeks with parallel basal tear collection at 0, 4, 7, and 14 days, and then 7 days after treatment ended (Fig. 6). Despite proparacaine anesthesia, cyclosporine stimulated a transient but rapid rise in basal tearing that formed a plateau at days 4 and 7 and then subsequently fell off to baseline by 7 days posttreatment. Artificial tears and C-25 had no effect. This contrasted with lacritin that again promoted a steady rise in basal tearing. Remarkably, lacritin-dependent tearing was sustained for 7 days posttreatment.
Figure 6.
Only lacritin promotes sustained tearing posttreatment. Lacritin (50 μg/mL), C-25 (10 μg/mL), artificial tears, or cyclosporine (0.05%) were administered three times daily for 14 days (n = 6/group). Tear production was measured at 0, 4, 7, and 14 days, and 7 days after treatment cessation (wash out). Inactive lacritin, C-25, served as the control group. *Significantly greater tear flow than baseline (P < 0.01, n = 6). †Significantly greater tear flow than artificial tears or C-25 (P < 0.01, n = 6). ‡Significantly greater tear flow than artificial tears, cyclosporine or C-25 (P < 0.01, n = 6).
Lacritin Tolerability, Tear Composition, and Intraocular Pressure (IOP) Assessment
To assess relative lacritin tolerability, eyes were treated three times daily for 14 days with lacritin, cyclosporine, or artificial tears and examined with a slit-lamp. Ocular irritation was then graded using the semiquantitative modified McDonald-Shadduck scale. Lacritin is well tolerated after 14 days of treatment three times daily (Table 1). Mild conjunctival congestion was observed in all groups after 14 days of treatment. However, neither lacritin nor C-25 treatment stimulated conjunctival swelling or discharge, aqueous flare/anterior chamber reaction, loss of light reflex, iris hyperemia, or corneal opacity or vascularization. Lacritin did not cause corneal vascularization, which suggests lack of cross-talk with VEGF signaling. Also tear composition after chronic lacritin treatment was consistent with normal tears as tear sodium, potassium, pH, and protein concentration levels were unaffected. Tear composition was assessed by measuring the sodium, potassium, and total protein content of tears before and after lacritin (100 μg/mL) single dose treatment. These measurements remained within normal limits and were not different from baseline IOP, and were maintained at an average value of 17.0 ± 0.9 mm Hg throughout the study (Table 2). In contrast, significant conjunctival discharge and iris hyperemia were noted after cyclosporine treatment. IOP in lacritin-treated eyes was also unchanged.
Table 1.
Slit-Lamp Analysis and McDonald-Shadduck Scale for Ocular Irritation
| Criteria | Baseline | After 14-Day Treatment (3 times daily) |
|||
|---|---|---|---|---|---|
| Lacritin (100 μg/mL) | Cyclosporine (0.05%) | Artificial Tears* | C-25 (10 μg/mL) | ||
| Conjunctival congestion | 0 | 1 ± 0.2 | 1 ± 0.1 | 1.3 ± 0.4 | 1 ± 0.1 |
| Conjunctival swelling | 0 | 0 | 0 | 0 | 0 |
| Conjunctival discharge | 0 | 0 | 2 ± 0.3 | 0 | 1 ± 0.2 |
| Aqueous flare | 0 | 0 | 0 | 0 | 0 |
| Light reflex | 0 | 0 | 0 | 0 | 0 |
| Iris hyperemia | 0 | 0 | 1 ± 0.3 | 0 | 0 |
| % Corneal opacity | 0 | 0 | 0 | 0 | 0 |
| % Corneal vascularization | 0 | 0 | 0 | 0 | 0 |
Individual parameters were graded on a scale of 0 to 4, where 0 = normal. All values are mean ± SE, n = 6.
Proprietary non-prescription formula (Refresh Tears; Allergan).
Table 2.
Effect of 14-Day Lacritin (100 μg/mL) Administration on Tear Quality, and Intraocular Pressure (IOP) as Compared with Baseline
| Baseline | Lacritin (100 μg/mL) | |
|---|---|---|
| pH | 8.1 ± 1.2 | 7.6 ± 0.8 |
| Sodium | 116 ± 18 mM | 87 ± 10 mM |
| Potassium | 23 ± 4 mM | 18 ± 3 mM |
| Protein | 39 ± 3 μg/μL | 32 ± 8 μg/μL |
| IOP | 17.0 ± 0.9 mm Hg | 17.0 ± 1.4 mm Hg |
n = 6.
Discussion
We document that administration of lacritin to the eye stimulates basal tear flow in rabbits. Flow is elevated for at least 240 minutes after a single dose. Eyes treated three times daily for 2 weeks display a steady rise in tearing that is remarkably sustained for at least 1 week after the last treatment and is well tolerated. Cyclosporine transiently increases basal tearing, apparently coincident with elevated ocular surface irritation.
As a normal component of human tears, lacritin flows from the lacrimal gland onto the ocular surface with contributions from the meibomian gland,28 conjunctiva, and cornea.27 In cell culture, lacritin stimulates calcium signaling within seconds and subsequently promotes proliferation of subconfluent human corneal epithelial cells.21 It also induces NFAT and mTOR mitogenic signaling in HSG/HeLa23 cells via heparanase-dependent binding of the cell surface proteoglycan syndecan-1 and activation of a G-protein coupled receptor. Both heparanase34 and syndecan-129 are expressed throughout the normal corneal epithelia, and heparanase has been detected in normal tears (Ma P and Laurie GW, unpublished, 2006).
Although a tear protein itself, how might lacritin increase ocular surface wetting by promoting basal tearing? In unpublished studies, lacritin induces protein expression of the hydrophilic membrane mucin MUC16 (Laurie GE, et al. IOVS 2006;47:ARVO E-Abstract 1606), which is necessary for ocular surface wetting.35 For basal tearing, an immediate consideration is whether lacritin may directly stimulate corneal sensory neurons, possibly via a G-protein coupled receptor as a negative or positive modulator of a transient receptor potential (TRP) cationic channel. As an example, alpha 2A adrenoreceptor activation inhibits the TRPM8 innocuous cold receptor in rat dorsal root ganglion sensory neurons.36 Alternatively, sensory neurons could be indirectly activated by lacritin. Sensory nerve endings in human37 and chick cornea are enveloped in specialized epithelial membrane invaginations, referred to as synapse-like structures,38 through which mediators might be released after lacritin-stimulated corneal epithelial cell signaling. Sensory neurons penetrate the corneal epithelium37 to form the afferent arm of the lacrimal functional unit.39 Although incompletely understood at the molecular level, corneal sensory neurons detect and convey delicate or dramatic changes in ocular surface temperature, hyperosmolarity,40 chemical irritation, and mechanical pressure39 to the brain stem's lacrimal nucleus. Preganglionic parasympathetic axons originating from the lacrimal nucleus then promote lacrimation commensurate with the level of the efferent signal, with low levels sufficient for basal tearing.39 It is also possible that lacritin might target corneal water or ion transport that are each subject to regulation by different agonists, for example, by altering the cellular distribution of aquaporin-2.41
Notably, basal tearing was elevated 1 week after chronic lacritin treatment ended. Perhaps lacritin is trophic for sensory neurons, particularly in the context of a germative corneal epithelium that is being continually renewed. Neuronal sensitivity, differentiation, and axonal stabilization are in part G-protein coupled receptor dependent,42 and targeting of the alpha 2 adrenoreceptor promotes signaling that transactivates nerve growth factor receptor NTRK1.43 The sustained effect of lacritin contrasts with other ocular surface therapeutics. Artificial tears had no effect on basal tearing, and cyclosporine elevated basal tearing only temporarily and was irritating. It should be noted that a longer-term treatment (>4 weeks) is recommended for dry eye patients and may produce more relief. Although the rationale for cyclosporine is to suppress inflammatory dry eye, a condition not experienced by our rabbits, others have noted that cyclosporine secondarily provokes a transient rise in tear flow collected without anesthesia in rabbits.32,44,45 Cyclosporine has no effect when added to cultured lacrimal gland fragments.32 Since flow in rabbits is abrogated by lacrimal gland parasympathectomy, Toshida et al.32 proposed an effect on reflex tearing from irritation. With the strength of the parasympathetic signal controlling the level of lacrimation, an alternative interpretation is that the effect was at the level of basal tearing. Unfortunately, tears were collected in the absence of ocular surface anesthesia.
In summary, lacritin is a natural human protein with unique properties. Addition of recombinant lacritin to the ocular surface alone or in combination with other agonists may help restore a natural tear film, particularly under dry eye conditions where lacritin or elements of its cell targeting mechanism may be deficient.
Acknowledgments
The authors thank Patricia Loose-Thurman, Natasha Gandia, Danea Campbell, Kristen Pelosky, and Scott Eubank for their technical assistance; and Cassandra Sherry for critical review of this manuscript.
Footnotes
Supported by Grants R42 EY015376-03S109 (JDS) and R01 EYO18222 (GWL).
Disclosure: S.S. Samudre, None; F.A. Lattanzio Jr, None; V. Lossen, None; A. Hosseini, None; J.D. Sheppard Jr, None; R.L. McKown, None; G.W. Laurie, P; P.B. Williams, None
References
- 1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–1326 [DOI] [PubMed] [Google Scholar]
- 2. Lin PY, Cheng CY, Hsu WM, et al. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2005;46:1593–1598 [DOI] [PubMed] [Google Scholar]
- 3. DEWS Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:179–193 [DOI] [PubMed] [Google Scholar]
- 4. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–342 [DOI] [PubMed] [Google Scholar]
- 5. Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005;112:1790–1794 [DOI] [PubMed] [Google Scholar]
- 6. Kim EC, Choi JS, Joo CK. A comparison of vitamin A and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009;147:206–213 [DOI] [PubMed] [Google Scholar]
- 7. Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009;15:1553–1572 [PMC free article] [PubMed] [Google Scholar]
- 8. Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001;42:96–100 [PubMed] [Google Scholar]
- 9. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren's syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169–177 [DOI] [PubMed] [Google Scholar]
- 10. Tsifetaki N, Kitsos G, Paschides CA, et al. Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren's syndrome: a randomised 12 week controlled study. Ann Rheum Dis. 2003;62:1204–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laurie GW, Olsakovsky LA, Conway BP, McKown RL, Kitagawa K, Nichols JJ. Dry eye and designer ophthalmics. Optom Vis Sci. 2008;85:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46:3589–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glasson M, Stapleton F, Willcox M. Lipid, lipase and lipocalin differences between tolerant and intolerant contact lens wearers. Curr Eye Res. 2002;25:227–235 [DOI] [PubMed] [Google Scholar]
- 15. Tomosugi N, Kitagawa K, Takahashi N, Sugai S, Ishikawa I. Diagnostic potential of tear proteomic patterns in Sjögren's syndrome. J Proteome Res. 2005;4:820–825 [DOI] [PubMed] [Google Scholar]
- 16. Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res. 2004;23:449–474 [DOI] [PubMed] [Google Scholar]
- 17. Koo BS, Lee DY, Ha HS, Kim JC, Kim CW. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Proteome Res. 2005;4:719–724 [DOI] [PubMed] [Google Scholar]
- 18. Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8:4889–4905 [DOI] [PubMed] [Google Scholar]
- 19. Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009;28:1109–1117 [DOI] [PubMed] [Google Scholar]
- 20. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol. 1999;83:390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanghi S, Kumar R, Lumsden A, et al. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol. 2001;310:127–139 [DOI] [PubMed] [Google Scholar]
- 22. Morimoto-Tochigi A, Nakajima T, Fujii A, Shearer TR, Azuma M. Functions of primate lacritin: enhanced secretion of tear proteins from lacrimal acinar cells and promotion of corneal epithelial cell adhesion. Invest Ophthalmol Vis Sci. 2010;51:4395–440620375347 [Google Scholar]
- 23. Wang J, Wang N, Xie J, et al. Restricted epithelial proliferation by lacritin via PKCalpha-dependent NFAT and mTOR pathways. J Cell Biol. 2006;174:689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma P, Wang N, McKown RL, Raab RW, Laurie GW. Focus on molecules: lacritin. Exp Eye Res. 2008;86:457–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKown RL, Wang N, Raab RW, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88:848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morimoto-Tochigi A, Walkup RD, Nakajima E, Shearer TR, Azuma M. Mechanism for carbachol-induced secretion of lacritin in cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2010;51:4395–4406 [DOI] [PubMed] [Google Scholar]
- 27. Nakajima T, Walkup RD, Tochigi A, Shearer TR, Azuma M. Establishment of an appropriate animal model for lacritin studies: cloning and characterization of lacritin in monkey eyes. Exp Eye Res. 2007;85:651–658 [DOI] [PubMed] [Google Scholar]
- 28. Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stepp MA, Gibson HE, Gala PH, et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531 [DOI] [PubMed] [Google Scholar]
- 30. Samudre SS, Lattanzio FA, Jr, Williams PB, Sheppard JD., Jr Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther. 2004;20:533–547 [DOI] [PubMed] [Google Scholar]
- 31. Lamberts DW, Foster CS, Perry HD. Schirmer test after topical anesthesia and the tear meniscus height in normal eyes. Arch Ophthalmol. 1979;97:1082–1085 [DOI] [PubMed] [Google Scholar]
- 32. Toshida H, Nguyen DH, Beuerman RW, Murakami A. Neurologic evaluation of acute lacrimomimetic effect of cyclosporine in an experimental rabbit dry eye model. Invest Ophthalmol Vis Sci. 2009;50:2736–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma P, Beck SL, Raab RW, et al. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J Cell Biol. 2006;174:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berk RS, Dong Z, Alousi S, Kosir MA, Wang Y, Vlodavsky I. Murine ocular heparanase expression before and during infection with Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 2004;45:1182–1187 [DOI] [PubMed] [Google Scholar]
- 35. Gipson IK, Hori Y, Argüeso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–148 [DOI] [PubMed] [Google Scholar]
- 36. Bavencoffe A, Gkika D, Kondratskyi A, et al. The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway. J Biol Chem. 2010;285:9410–9419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542 [DOI] [PubMed] [Google Scholar]
- 38. Kubilus JK, Linsenmayer TF. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010;51:3969–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Junaid A, Cui L, Penner SB, Smyth DD. Regulation of aquaporin-2 expression by the alpha(2)-adrenoceptor agonist clonidine in the rat. J Pharmacol Exp Ther. 1999;291:920–923 [PubMed] [Google Scholar]
- 42. Rowe SJ, Messenger NJ, Warner AE. The role of noradrenaline in the differentiation of amphibian embryonic neurons. Development. 1993;119:1343–1357 [DOI] [PubMed] [Google Scholar]
- 43. Karkoulias G, Flordellis C. Delayed transactivation of the receptor for nerve growth factor is required for sustained signaling and differentiation by alpha2-adrenergic receptors in transfected PC12 cells. Cell Signal. 2007;19:945–957 [DOI] [PubMed] [Google Scholar]
- 44. Toshida H, Nakayasu K, Kanai A. Effect of cyclosporin A eyedrops on tear secretion in rabbit. Jpn J Ophthalmol. 1998;42:168–173 [DOI] [PubMed] [Google Scholar]
- 45. Yoshida A, Fujihara T, Nakata K. Cyclosporin A increases tear fluid secretion via release of sensory neurotransmitters and muscarinic pathway in mice. Exp Eye Res. 1999;68:541–546 [DOI] [PubMed] [Google Scholar]