In advanced retinitis pigmentosa, photopsias tend to be located centrally, over larger regions, and in areas with vision and/or observed more frequently and therefore potentially interfere with patients' functioning or with obtaining vision measures.
Abstract
Purpose.
This study explored whether the location of photopsias (spontaneous phosphenes) in retinitis pigmentosa (RP) is related to the severity of vision loss, as has been suggested.
Methods.
Thirty-two RP subjects self-completed an online survey about photopsias, approximately 1 to 2 months after ETDRS visual acuity (VA), Pelli-Robson contrast sensitivity (CS), and Goldmann visual field (VF) measures were obtained.
Results.
The odds of noting photopsias only or mostly in areas of vision increased as vision was reduced across subjects, by 56% for every 0.1 logMAR VA (95% CI, 1.04–2.33; P = 0.03), 22% for every 0.1 logCS (95% CI, 1.02–1.46; P = 0.03), and 40% for every 1 unit logVF diameter (95% CI, 0.99–1.98; P = 0.06). The odds of noting photopias only in the periphery were reduced by 20% for every 0.1 logCS reduction (95% CI, 0.64–1.02; P = 0.066), and 18% for every 1 unit logVF diameter reduction (95% CI, 0.67–1.001; P = 0.051). For every 0.1 logMAR VA reduction, the odds of indicating that photopsias were located across a larger area over time were 30% greater (95% CI, 1.002–1.70; P = 0.048). The odds of being more aware of photopsias over time were increased as vision was reduced by 48% for every 0.1 logMAR VA (95% CI, 1.04–2.11; P = 0.03) and 18% for every 0.1 logCS (95% CI, 1.01–1.38; P = 0.04). The odds of reporting that photopsias interfere with vision were significantly greater when the photopsias occurred daily, more frequently, or across larger areas over time.
Conclusions.
These cross-sectional data indicate that in later RP stages, photopsias located more centrally, over larger regions, in areas with vision, and/or observed more frequently may obstruct vision at times and are a potential hindrance for patients' functioning or when obtaining vision measures.
Photopsias are simple visual phenomena consisting of unformed, geometric patterns or phosphenes. Patients with retinitis pigmentosa (RP) have described these phenomena as widespread flickering, pulsating, or shimmering lights; snow on a television screen (static noise); fluorescence, quick flashes of light; shapes resembling semicircles; or slow-moving, localized dots (which may move as a consequence of slow eye movements).1–3 Vision loss in RP is typically portrayed as consisting of nightblindness and visual field constriction, whereas the presence of photopsias as an additional visual disturbance is usually not considered by clinicians and researchers. Perhaps this is because, to date, little is known regarding how photopsias may affect RP patients depending on the degree of vision loss and whether photopsias interfere with visual function. These aspects may be important to consider during vision testing or in relation to RP patients' quality of life.
The findings of our previous research using an Internet-based online survey suggested that the location of photopsias in RP was related to disease severity.3 Respondents were significantly more likely to report that their photopsias were located mostly peripherally versus centrally when they reported less difficulty with activities of daily living, such as reading, driving, and mobility. Although the survey was cross-sectional, the results supported the possibility that photopsias may start in the periphery early in RP and then later appear in more central areas, when reported deficits in visual function occur. The conclusions that could be inferred from this preliminary study were further limited by the anonymous nature of the survey, since we could not verify the respondents' RP diagnosis or vision measures through their medical records.
Recently, we studied a new group of individuals with a confirmed RP diagnosis to elucidate the characteristics of photopsias in relation to standard vision tests. In particular, we wanted to quantify the amount of central and peripheral vision loss that was associated with subjects' cross-sectionally reported location of photopsias, changes in photopsia brightness or frequency over time, awareness of photopsias, and whether interference with vision was related to any of the characteristics of photopsias.
Methods
The protocol for the study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine and complied with the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. Data collection occurred from December 2008 through April 2010.
Subjects
Study participants included 32 individuals diagnosed with RP. Most of the subjects (n = 23; 72%) were recruited through the clinical practices of low-vision optometrists and retinal specialists at The Johns Hopkins Wilmer Eye Institute. The remaining subjects self-referred after learning of the study through online listings. Individuals were eligible for the study if they had a confirmed diagnosis of RP, were older than 18 years, had no other ocular diagnoses besides cataracts or macular edema, and had adequate vision to read reverse contrast, large font on a computer to complete the photopsias questionnaire and provide reliable responses to the vision tests used in the study.
Data Collection
All vision tests were administered by a single examiner (AB) and were completed during a single session at our center. Best corrected visual acuity (VA) was measured binocularly with the Early Treatment of Diabetic Retinopathy Study (ETDRS; Lighthouse International, NY, NY) charts at 3 m, or closer if fewer than 10 letters were identified. Best corrected, binocular Pelli-Robson contrast sensitivity (CS; Metropia Ltd., Essex, UK) was assessed at 1 m. The visual field in each eye was measured using the Goldmann visual field (VF) V4e and III4e test targets. We used the results for the eye with the larger isopter diameter and took the log of the V4e diameter for the analyses. We performed ocular coherence tomography (OCT; HRA+OCT; Heidelberg Engineering, Vista, CA) to assess whether macular edema or epiretinal membranes were present.
One to 2 months after enrollment, the subjects were asked to complete an online web-based questionnaire regarding the various characteristics of photopsias. The survey was self-administered on one occasion via computer outside of our center. Subjects were asked if they noticed the following types of photopsias: whiteout glare, slow-moving phosphenes, quick flashes of light, static noise, and fluorescence or background glow. These descriptions of photopsias had been identified with another survey in RP.3 Four subjects in addition to the 32 reported herein were enrolled in the study, but indicated that they did not experience photopsias when they completed the online survey. The characteristics of the four subjects who did not report photopsias are shown in Table 1. Their level of vision or duration of vision loss did not appear to be related to the absence of photopsias. The prevalence of photopsias was 89% in this cohort of RP patients who were also completing computer-based vision tests for a study of factors related to day-to-day vision fluctuations.3
Table 1.
Characteristics of RP Subjects Who Did Not Report Photopsias
| Age (y) | Sex | logMAR VA | logCS | VF Diam. (V4e) | Duration of Vision Loss (y) | Duration of Night Vision Loss (y) |
|---|---|---|---|---|---|---|
| 65 | Male | 0.98 | 0.45 | 146° | 8 | 17 |
| 72 | Female | 0.08 | 1.55 | 15° | 22 | 71 |
| 67 | Female | −0.06 | 1.80 | 140° | 0 | 7 |
| 50 | Male | 0.22 | 0.85 | 140° | Not sure | Not sure |
Data Analysis
Correlation analysis was used to assess vision test results between right and left eyes and between continuous covariates. We used logistic regression analysis, with and without adjustment for age and sex, to determine odds ratios for experiencing photopsias with various characteristics defined as binary outcomes in relation to subjects' binocular VA, CS, and logVF diameter. Mean VA, CS, and logVF diameter were estimated for subjects who reported various characteristics of photopsias and Welch's two-sample t-tests with unequal variances were used to test for significant differences in central visual function by level of each characteristic. We used Pearson's χ2 tests to assess relationships between dichotomized variables for photopsia characteristics (Stata/IC, ver. 10.0; Stata Corp., College Station, TX).
Results
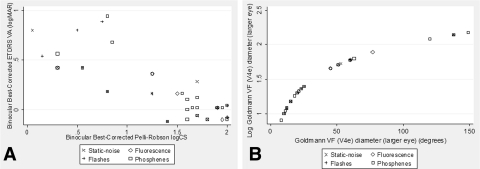
The RP subjects' ages ranged from 20 to 76, with a mean of 48 years, and 53% of the 32 subjects were women. Six percent of the subjects were African-American, 12.5% were Hispanic, and 81% were Caucasian. The subjects had a mean binocular ETDRS VA of 0.23 logMAR (SD 0.33; range, −0.12 to 0.98) and a mean Pelli-Robson CS of 1.3 logCS (SD 0.6; range 0.05–2.0). Figure 1A shows the relationship between VA and CS, as well as the range across subjects and types of photopsias according to these measures of central vision. As would be expected in RP, losses in VA correlated highly with losses in CS (r2 = 0.69), but VA and CS correlated weakly with logVF diameter (r2 < 0.04). The subjects had a mean isopter diameter of 51° (SD 48°; range 8–149°) and 37° (SD 39°; range 5–129°) with the V4e and III4e test targets, respectively. Figure 1B shows the relationship between logVF and VF for the V4e test target in the eye with the larger diameter, as well as the range of VF across subjects and types of photopsias according to VF.
Figure 1.
Scatterplots demonstrating the relationship between (A) binocular best corrected ETDRS VA and CS and (B) logVF diameter and VF diameter in the larger eye with the V4e target. Each point represents a subject and the type of photopsia(s) they noted; overlapping symbols indicate that a subject reported more than one type.
The most common type of photopsia was phosphenes, described by 66% of the subjects as slow-moving, localized dots or shapes, and the second most frequently reported type of photopsia was a group or series of quick flashes of light, noted by 34%. Fluorescence or background glow was experienced by 19%, and static noise (similar to that on a television that has no reception) was seen by 22% of the participants. The subjects were allowed to name more than one type of photopsia. Forty-one percent (n = 13) noted photopsias only or mostly in areas where they had vision, 56% (n = 18) indicated that they were more aware of photopsias over time, 28% (n = 9) noted them across a larger area over time, and 44% (n = 14) experienced them daily. The duration of the photopsias was reported as only a few seconds by 69% (n = 22), a few minutes by 19% (n = 6) and a few hours by 3% (n = 1); 9% (n = 3) reported constant occurrence.
The vision test results correlated moderately to highly between the eyes, with r2 = 0.37 for VA, r2 = 0.78 for CS, and r2 = 0.84 for VF. We chose to use the binocular vision test results for VA and CS in all analyses, since 56% of subjects indicated that photopsias occurred in both eyes equally, and 22% were not sure whether they occurred in one eye or both. We tested for but did not find any statistically significant interactions4 between VA and CS or VF and CS for the characteristics of the photopsia, implying that the effect of VA or VF on the location of the photopsia did not differ according to the level of CS.
The odds of experiencing various characteristics of photopsias in relation to 0.1-unit reductions in logVA and logCS, adjusted for age and sex, are displayed in Table 2. We did not find a statistically significant or qualitative difference after adjustment for age and sex in most cases, except for a larger magnitude in the adjusted odds of photopsias located only or mostly in areas with vision and photopsias that increased in brightness over time, both in relation to VA. Reported results reflect the adjusted values. After including both VA and CS together in adjusted multiple logistic regression analyses, their relationships with photopsias were not statistically significant, as would be anticipated, given the high correlation between VA and CS.
Table 2.
Adjusted Odds of Photopsia Characteristics for Every 0.1 Log Unit Reduction in VA, CS, or VF (V4e) Diameter
| Photopsia Characteristics | VA |
CS |
VF |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Located only/mostly in areas with vision | 1.56 | (1.04–2.33) | 0.03* | 1.22 | (1.02–1.46) | 0.03* | 1.25 | (0.95–1.64) | 0.11 |
| Located only in peripheral areas | 0.80 | (0.58–1.09) | 0.16 | 0.80 | (0.64–1.02) | 0.066† | 0.79 | (0.62–1.005) | 0.055† |
| Located across larger areas over time | 1.30 | (1.002–1.70) | 0.048* | 1.12 | (0.98–1.28) | 0.10 | 1.05 | (0.84–1.32) | 0.66 |
| Increased brightness over time | 1.37 | (0.94–2.01) | 0.10 | 1.20 | (0.99–1.45) | 0.057† | 1.26 | (0.88–1.81) | 0.21 |
| Increased frequency over time | 1.21 | (0.95–1.56) | 0.13 | 1.10 | (0.97–1.25) | 0.14 | 1.07 | (0.87–1.31) | 0.52 |
| Increased awareness over time | 1.48 | (1.04–2.11) | 0.03* | 1.18 | (1.01–1.38) | 0.04* | 1.08 | (0.88–1.33) | 0.45 |
Adjusted for age and sex.
Statistically significant (P < 0.05).
Trended toward statistical significance (P = 0.05–0.07).
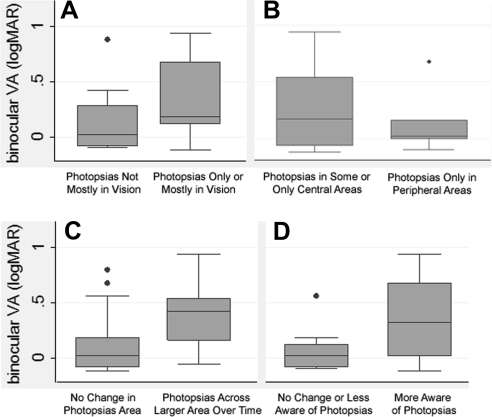
As seen in Table 2, the odds of noting photopsias only or mostly in areas of vision were significantly greater (by 56%) for every 0.1 logMAR VA reduction across subjects, significantly greater (by 22%) for every 0.1-unit logCS reduction across subjects, and 2.64 times greater for every 1-unit logVF V4e diameter reduction across subjects (P = 0.11). Figures 2A, 3A, and 4A provide the data for VA, CS, and logVF, respectively. Figure 2A indicates higher median and interquartile ranges for VA (i.e., worse VA) in subjects reporting photopsias only or mostly in areas with vision. Figures 3A and 4A show a lower median and interquartile range for CS and logVF (i.e., worse CS and smaller VF) among those reporting photopsias only or mostly in areas with vision. After including both VA and logVF together in the adjusted logistic model, loss of VA was the better predictor of the location of photopsias in areas with vision, independent of VF loss (OR, 1.55; 95% CI, 1.005–2.38; P = 0.047).
Figure 2.
Box plots of binocular VA by characteristics of photopsias: (A) location in vision; (B) location in central versus peripheral areas; (C) located across larger area over time; and (D) awareness of photopsias over time. The bottom and top of the box are the 25th and 75th percentiles (the lower and upper quartiles, respectively), and the band near the middle of the box is the 50th percentile (the median). The ends of the whiskers represent the lowest datum within 1.5 times the interquartile range of the lower quartile, and the highest datum still within 1.5 times the interquartile range of the upper quartile. Dot: datum not included between the whiskers and regarded as an outlier.
Figure 3.
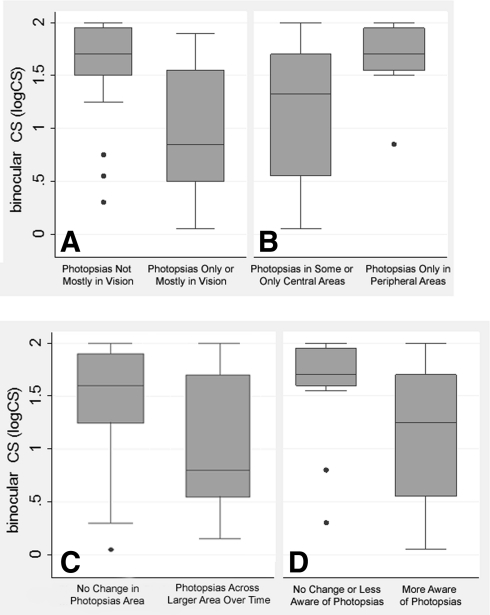
Box plots of binocular CS by characteristics of photopsias: (A) location in vision, (B) location in central versus peripheral areas, (C) located across larger area over time, and (D) awareness of photopsias over time.
Figure 4.
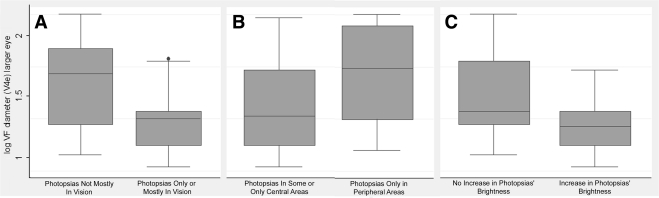
Box plots of logVF diameter in the larger eye by characteristics of photopsias: (A) location in vision, (B) location in central versus peripheral areas, and (C) changes in brightness over time.
Subjects were 20% less likely to note photopsias only in the periphery for every 0.1 reduction in logMAR VA or logCS and were borderline significantly (64%) less likely to note them only in the periphery with every 1-unit log(V4e) diameter reduction across subjects, as shown in Table 2. Figures 2B, 3B, and 4B provide a graphic depiction of the data, indicating a higher median and interquartile range for CS and logVF (i.e., better CS and larger VF) among those reporting photopsias only in peripheral areas. After including both CS and logVF together in an adjusted logistic model, neither was a borderline statistically significant predictor of the location of photopsias in the peripheral versus central areas (P > 0.10).
As indicated in Table 2, for every 0.1-unit logMAR VA reduction, the odds of indicating that photopsias were located across a larger area over time were significantly (30%) greater. Figure 2C provides a graphic depiction of the data, indicating a higher median and interquartile range for VA (i.e., worse VA) among those reporting that photopsias were located across a larger area over time during the one-time survey.
For every 0.1-log-unit reduction in vision, the odds of reporting that photopsias increased in brightness over time during the survey were 37% greater for VA and 20% greater for CS. The odds of subjects responding that they were more aware of photopsias over time were significantly greater (48%) for every 0.1-logMAR VA reduction, and 18% greater for every 0.1-logCS reduction. These results are displayed in Table 2. Figures 2D and 3D provide a graphic depiction of the data for VA and CS, respectively, indicating a higher median and interquartile range for VA (i.e., worse VA) and lower median and interquartile range for CS (i.e., worse CS) among those reporting that they were more aware of photopsias over time.
Forty-four percent (n = 14) of the subjects indicated that photopsias interfered with their vision. After adjustment for age and sex, the odds of reporting that photopsias interfered with vision were significantly (9 times) greater for the subjects who experienced photopsias daily (95% CI, 1.67–50.15; P = 0.01), nearly 15 times greater when photopsias increased in frequency over time (95% CI, 1.97–111.1; P = 0.009), and 10 times greater when photopsias were located across a larger area over time (95% CI, 1.45–72.6; P = 0.02), based on subjects' responses to the one-time survey. All seven subjects who described their photopsias as static noise indicated that photopsias interfered with their vision (χ2 = 10.98; P = 0.001). After adjustment for age and sex, the data suggest that the subjects who indicated that their photopsias appeared as static noise were 21 times more likely to report that their photopsias increased in frequency over time (95% CI, 1.56, 292.9; P = 0.02); however, this estimate may be unreliable, given that it was based on a small sample of seven subjects and resulted in a wide confidence interval.
Our data also indicated that patients who noted seeing phosphenes, rather than other types of photopsias, tended to report less awareness and interference with vision. Only 3 (14%) of the 21 subjects who described their photopsias as phosphenes indicated that they thought they occurred across a larger area over time, compared with 55% of those who had other types of photopsias (P = 0.016). Most (82%) of the respondents who did not describe their photopsias as phosphenes indicated that they thought they were more aware of their photopsias over time, but only 9 (43%) of the 21 subjects who reported phosphenes noted increased awareness (P = 0.035). Only a third of the subjects who saw phosphenes responded that they interfere with vision, whereas nearly two thirds (64%) of those who saw other types of photopsias noted interference (P = 0.10).
We found some interesting trends for flashes of light and other characteristics of photopsias over time. Five (46%) of the 11 subjects who described their photopsias as flashes indicated that the phenomena appeared to occur across a larger area over time, compared with only 19% of those who did not describe their photopsias as flashes (P = 0.12). Seven (64%) of the 11 subjects who described their photopsias as flashes indicated that they occurred daily, compared with only one third of those who did not describe their photopsias as flashes (P = 0.10). We explored whether any of the types of photopsias varied according to central or peripheral retinal location, but did not find any significant trends or relationships to location in our relatively small sample.
Table 3 shows the difference in mean VA, CS, and logVF according to the presence or absence of certain characteristics of photopsias. For all the characteristics of photopsias that we examined in relation to mean VA and CS in Table 3, there were either statistically significant relationships or trends toward statistical significance (all P ≤ 0.12). For the results in Table 3 in relation to larger logVF diameter in the better eye, only or mostly located in areas with or without vision, peripheral versus central location, and increased brightness over time had P ≤ 0.12.
Table 3.
Difference in Mean logMAR VA, LogCS, and LogVF Diameter According to Photopsias' Characteristics
| Photopsia Characteristics | VA | 95% CI | P | CS | 95% CI | P | log VF | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|
| Located only/mostly in areas with vision (n = 13) | 0.38 | (0.17 to 0.59) | 0.99 | (0.61 to 1.36) | 1.28 | (1.12 to 1.44) | |||
| Located only/mostly in vision loss (n = 19) | 0.11 | (−0.02 to 0.23) | 1.56 | (1.32 to 1.81) | 1.60 | (1.40 to 1.80) | |||
| Difference in means | −0.27 | (−0.04 to 0.51) | 0.03* | 0.57 | (0.15 to 1.00) | 0.01* | 0.32 | (0.07 to 0.56) | 0.01* |
| Located only in peripheral areas (n = 10) | 0.09 | (−0.06 to 0.25) | 1.67 | (1.42 to 1.91) | 1.66 | (1.32 to 2.00) | |||
| Located in some or only central areas (n = 22) | 0.28 | (0.12 to 0.43) | 1.18 | (0.89 to 1.47) | 1.39 | (1.24 to 1.54) | |||
| Difference in means | 0.18 | (−0.03 to 0.39) | 0.09 | −0.49 | (−0.13 to −0.85) | 0.01* | 0.27 | (−0.08 to 0.63) | 0.12 |
| Located across larger areas over time (n = 9) | 0.40 | (0.14 to 0.67) | 1.02 | (0.51 to 1.53) | 1.40 | (1.10 to 1.70) | |||
| Stable over time (n = 23) | 0.15 | (0.02 to 0.27) | 1.45 | (1.20 to 1.69) | 1.50 | (1.33 to 1.66) | |||
| Difference in means | −0.26 | (0.02 to 0.54) | 0.07 | 0.43 | (−0.11 to 0.97) | 0.11 | 0.10 | (−0.24 to 0.43) | 0.54 |
| Increased brightness over time (n = 6) | 0.38 | (0.16 to 0.60) | 0.87 | (0.29 to 1.44) | 1.26 | (0.97 to 1.54) | |||
| Stable brightness over time (n = 26) | 0.18 | (0.05 to 0.32) | 1.44 | (1.20 to 1.68) | 1.52 | (1.36 to 1.68) | |||
| Difference in means | −0.20 | (0.03 to −0.43) | 0.09 | 0.57 | (0.003 to 1.14) | 0.049* | 0.26 | (−0.04 to 0.56) | 0.09 |
| Increased frequency over time (n = 15) | 0.32 | (0.14 to 0.51) | 1.14 | (0.76 to 1.53) | 1.43 | (1.21 to 1.64) | |||
| No change in frequency over time (n = 17) | 0.13 | (−0.02 to 0.28) | 1.49 | (1.23 to 1.75) | 1.50 | (1.30 to 1.70) | |||
| Difference in means | −0.19 | (0.03 to −0.42) | 0.09 | 0.35 | (−0.09 to 0.80) | 0.12 | 0.07 | (−0.21 to 0.36) | 0.60 |
| Increased awareness over time (n = 18) | 0.35 | (0.17 to 0.53) | 1.11 | (0.80 to 1.42) | 1.42 | (1.24 to 1.60) | |||
| No change in awareness over time (n = 14) | 0.05 | (−0.05 to 0.16) | 1.61 | (1.33 to 1.90) | 1.54 | (1.29 to 1.78) | |||
| Difference in means | −0.29 | (−0.10 to −0.49) | 0.005* | 0.51 | (0.10 to 0.91) | 0.02* | 0.12 | (−0.17 to 0.41) | 0.41 |
Differences in means were analyzed with two-sample t-tests.
Statistically significant (P < 0.05).
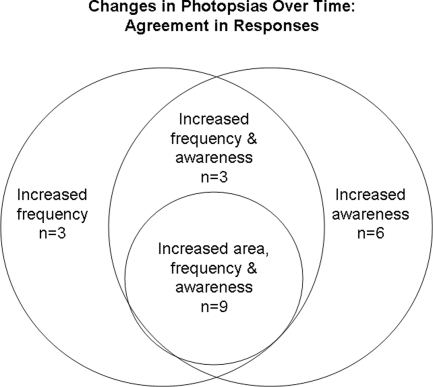
We tested whether the subjects tended to report the same characteristics of photopsias and found that this was sometimes but not necessarily always the case. There was a general agreement for the following responses, displayed in Table 4. All the 14 subjects who did not note a change in their awareness of photopsias over time also noted them across a stable area over time (χ2 = 9.7; P = 0.002). All 17 of the subjects who indicated no change in photopsia frequency over time also tended to indicate that photopsias were located across a stable area over time during the cross-sectional survey (χ2 = 14.2; P < 0.001). Twelve of the 15 subjects who indicated in the survey that photopsias increased in frequency over time reported that they were more aware of the photopsias (χ2 = 6.47; P = 0.01). Figure 5 illustrates the agreement in responses for changes over time in awareness, frequency, and area. Eleven of the 13 subjects who noted photopsias only or mostly in areas with vision, indicated that they were not mostly in the periphery (χ2 = 2.56; P = 0.11).
Table 4.
Relationships between Photopsia Characteristics
| Located Only in Peripheral Areas | Located in Some or Only Central Areas | P | Located across Larger Areas Over Time | Stable Over Time | P | Increased Frequency Over Time | No Change in Frequency Over Time | P | Increased Awareness Over Time | No Change in Awareness Over Time | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Located across larger areas over time | 2 | 7 | ||||||||||
| Stable over time | 8 | 15 | 0.49 | |||||||||
| Increased frequency over time | 5 | 10 | 9 | 6 | ||||||||
| No change in frequency over time | 5 | 12 | 0.81 | 0 | 17 | <0.001* | ||||||
| Increased awareness over time | 5 | 13 | 9 | 9 | 12 | 6 | ||||||
| No change in awareness over time | 5 | 9 | 0.63 | 0 | 14 | 0.002* | 3 | 11 | 0.01* | |||
| Located only/mostly in areas with vision | 2 | 11 | 3 | 10 | 7 | 6 | 8 | 5 | ||||
| Located only/mostly in vision loss | 8 | 11 | 0.11 | 6 | 13 | 0.60 | 8 | 11 | 0.51 | 10 | 9 | 0.62 |
Pearson χ2 tests. Data are the number of subjects reporting similar characteristics.
Statistically significant (P < 0.05).
Figure 5.
Diagram demonstrating the numbers of subjects who reported the same responses for increases in photopsias' frequency, awareness, and/or area over time.
Reduction in the logVF (V4e) diameter was significantly associated with reports that photopsias were located only or mostly in areas with vision, as displayed in the right column of Table 3. Reduced logVF (V4e) diameter trended toward being significantly associated with reports that photopsias increased in brightness over time versus no change in brightness (P = 0.09). Figure 4C provides a graphic depiction of the data, indicating a lower median and interquartile range for VF (i.e., worse VF) among those reporting that photopsias increased in brightness over time. There was a trend toward a relationship between reduced logVF (V4e) diameter and noting photopsias in at least some central areas versus only peripherally (P = 0.12), as shown in Figure 4B.
Most (70%; n = 7) of the subjects who indicated that photopsias lasted longer than a few seconds indicated that they interfered with vision (χ2 = 4.07; P = 0.04). A duration of photopsias longer than a few seconds, noted by 31% of subjects, was not significantly related to VA (P = 0.74), CS (P = 0.65), or logVF (P = 0.29). All six of the subjects who reported that their photopsias appeared as fluorescence indicated that it lasted only a few seconds (χ2 = 3.5; P = 0.06). However, the duration of photopsias lasting longer than a few seconds was not significantly related to the other types of photopsias: static noise (P = 0.50), flashes (P = 0.15), or phosphenes (P = 0.53).
Thirty-four percent (n = 11) of the subjects had macular edema in at least one eye. We tested whether any of the characteristics or types of photopsias were related to the presence of macular edema and found no statistically significant associations.
Discussion
In the present study, we demonstrated a relationship between reduced-vision measures (VA, CS, and/or logVF) and RP subjects' reports that photopsias occur in areas where they have vision, more centrally, and across larger areas over time. Our data indicate that RP subjects with reduced measures of central vision (VA and/or CS) were more likely to report increased awareness of photopsias over time and that photopsia interfere with their vision. The data also suggest a possible association between a reduction in central vision measures and increased brightness and/or frequency of photopsias over time. From these findings, it appears that photopsias do not tend to occur only in end-stage RP. Alternatively, we hypothesize that photopsias can occur at all stages of RP, but patients become more aware of them or report interference with vision when changes in the location and severity of photopsias occur as vision is progressively lost.
The present study in a new group of RP patients confirmed previous survey results,3 which suggested that photopsias tended to be located in at least some central areas when RP subjects had more advanced disease, indicated by their reports of increased difficulty with daily activities requiring vision. The current results quantified the amount of central and peripheral vision loss associated with certain characteristics of photopsias that may cause increased awareness or obstruction with vision. This information is helpful to eye care providers and vision researchers who are trying to understand the amount of vision loss that is typically associated with the possible occurrence of photopsias that may impede vision during testing. With this increased knowledge, they can offer reassurance to RP patients, many of whom do not know that photopsias are related to RP. The present study suggests the need for additional research in RP patients who report that their photopsias interfere with their vision (e.g., those with the static noise type of photopsias) to determine the magnitude of reductions in vision measures at times when photopsias occur and whether there are any modifiable factors (e.g., changes in lighting or perceived stress).
It has been hypothesized that photopsias in RP may be manifestations of spontaneous discharges from degenerating retinal cells due to remodeling in the inner retina5 and/or release phenomena resulting from a lack of afferent neuronal impulses.6 It is less likely that the quick flashes of light noted by RP patients over periods of several years are related to more transient vitreous traction on the retina or other mechanical factors. Some patients described the photopsias as shapes, and therefore, cortical involvement cannot be ruled out, although phosphenes resulting from electrical stimulation of the retina have also been documented as shapes.7 It would be interesting for future longitudinal studies to explore whether the increased occurrence of photopsias is a predictor of impending or current vision loss in RP. A limitation of the present study is the cross-sectional design, which may be susceptible to recall bias, as subjects were asked to indicate changes in photopsias over time. Many RP patients (81% in the present study) stated that they try to filter out or ignore their photopsias, and in doing so, they may underestimate their impact or not realize when subtle changes are occurring. Therefore, we recommend prospective research designs for future studies of photopsias.
In the present study, 44% of participants indicated that photopsias interfered with their vision, whereas more than two-thirds of previously surveyed RP patients indicated interference.3 We previously acknowledged that the prior survey results may have been affected by some selection bias, since those who were most affected by photopsias may have been more interested in completing the survey. The current participants did not primarily join the study to complete the photopsias survey, but were interested in contributing to research to determine whether various factors (e.g., perceived stress, sleepiness, and lighting) were related to day-to-day fluctuations in vision. The proportion of respondents indicating that photopsias interfere with their vision may be affected by the severity of vision loss, which may vary depending on the source of the study population. Patients with advanced RP with an implanted retinal prosthesis may also be adversely affected by naturally occurring photopsias, and it may be difficult to distinguish electrically stimulated percepts from photopsias, especially when they are initially learning to interpret the prosthetic vision. Therefore, strategies to reduce photopsias would also be valuable for retinal prosthesis patients, and future studies should evaluate whether controlled lighting or behavioral intervention programs to reduce perceived stress may help to decrease photopsias.
Conclusions
Subjects in the earlier stages of RP with larger VF diameters tended to note photopsias only in the periphery, whereas those with more advanced RP and a loss of VA tended to report that photopsias were located in areas where they still had vision and across larger areas over time. Although photopsias can be noted early in RP before significant loss in central visual function, RP patients with decreased VA and/or CS reported that over time, changes in the location and frequency of photopsias impeded vision at times. Therefore, the impact of photopsias in advanced RP should be considered, as it may affect the variability of vision testing, as well as patients' visual functioning and quality of life.
Acknowledgments
The authors thank Liancheng Yang for the programming related to the Internet-based photopsia questionnaire used in the study.
Footnotes
Supported by National Institutes of Health Grant K23EY018356 (AKB).
Disclosure: A.K. Bittner, None; M. Diener-West, None; G. Dagnelie, None
References
- 1. Murtha T, Stasheff SF. Visual dysfunction in retinal and optic nerve disease. Neurol Clin. 2003;21(2):445–481 [DOI] [PubMed] [Google Scholar]
- 2. Heckenlively JR, Yoser SL, Friedman LH, Oversier JJ. Clinical findings and common symptoms in retinitis pigmentosa. Am J Ophthalmol. 1988;105:504–511 [DOI] [PubMed] [Google Scholar]
- 3. Bittner AK, Diener-West M, Dagnelie G. A survey of photopsias in self-reported retinitis pigmentosa: location of photopsias is related to disease severity. Retina. 2009;29(10):1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norton EC, Wang H, Ai C. Computing interaction effects in logit and probit models. Stata J. 2004;4(2):154–167 [Google Scholar]
- 5. Marc RE, Jones BW, Anderson JR, et al. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48(7):3364–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kölmel HW. Visual illusions and hallucinations. Baillieres Clin Neurol. 1993;2(2):243–264 [PubMed] [Google Scholar]
- 7. Nanduri D, Humayun MS, Greenberg RJ, McMahon MJ, Weiland JD. Retinal prosthesis phosphene shape analysis. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1785–1788 [DOI] [PubMed] [Google Scholar]