Conditional knockout of Sprouty1 and Sprouty2 in the murine lens ectodermal precursors results in failure of lens vesicle detachment, persistent keratolenticular stalks, and premature initiation of lens fiber differentiation. These data indicate that Sprouty1 and Sprouty2, through negative modulation of ERKs, allow lens vesicle separation and are targets of fibroblast growth factor signaling in the lens during initiation of fiber differentiation.
Abstract
Purpose.
The studies reported here were performed to analyze the roles of Sproutys (Sprys), downstream targets and negative feedback regulators of the fibroblast growth factor (FGF) signaling pathway, in lens and corneal differentiation.
Methods.
Spry1 and -2 were conditionally deleted in the lens and corneal epithelial precursors using the Le-Cre transgene and floxed alleles of Spry1 and -2. Alterations in lens and corneal development were assessed by hematoxylin and eosin staining, in situ hybridization, and immunohistochemistry.
Results.
Spry1 and -2 were upregulated in the lens fibers at the onset of fiber differentiation. FGF signaling was both necessary and sufficient for induction of Spry1 and -2 in the lens fiber cells. Spry1 and -2 single- or double-null lenses failed to separate from the overlying ectoderm and showed persistent keratolenticular stalks. Apoptosis of stalk cells, normally seen during lens vesicle detachment from the ectoderm, was inhibited in Spry mutant lenses, with concomitant ERK activation. Prox1 and p57KIP2, normally upregulated at the onset of fiber differentiation were prematurely induced in the Spry mutant lens epithelial cells. However, terminal differentiation markers such as β- or γ-crystallin were not induced. Corneal epithelial precursors in Spry1 and -2 double mutants showed increased proliferation with elevated expression of Erm and DUSP6 and decreased expression of the corneal differentiation marker K12.
Conclusions.
Collectively, the results indicate that Spry1 and -2 (1) through negative modulation of ERKs allow lens vesicle separation, (2) are targets of FGF signaling in the lens during initiation of fiber differentiation and (3) function redundantly in the corneal epithelial cells to suppress proliferation.
The vertebrate lens is composed of proliferating epithelial cells in the anterior portion and postmitotic, terminally differentiated fiber cells in the posterior part. This polarity is maintained throughout life by precise coordination of proliferation, cell cycle exit, and differentiation. An inductive signal from the retina is thought to induce lens epithelial cells at the equatorial region to withdraw from the cell cycle and initiate the fiber differentiation program.1 In vitro and in vivo studies show that FGF stimulation of lens epithelial cells is sufficient to induce premature fiber differentiation.2–5 Expression of secreted dominant negative FGF receptor 3 (FGFR3) or deletion of all six alleles of FGFR1, -2, and -3 in the lens in transgenic or knockout mice, respectively, is sufficient to inhibit initiation of lens fiber differentiation.6,7 These results suggest that FGFR-meditated signaling is necessary for normal fiber differentiation.
Sproutys play an essential role in regulation of Ras-Raf-Erk signaling downstream of FGFR stimulation. Four vertebrate Sprys, Spry1-4, have been identified and have been shown to antagonize Ras-Raf-ERK signaling downstream of FGFR.8–11 During murine embryogenesis, the sites of Spry expression often coincide with centers of FGF signaling.11–15 Spry-knockout mice have revealed interesting roles for these proteins in regulation of organogenesis. Spry1-null mice show kidney and urinary tract defects with ectopic branching of the Wolffian duct and increased ERK activation.16,17 Spry2-null mice show alterations in inner ear development that are rescued by reduction in FGF8 dosage.18 Spry3 is not expressed in the mouse embryo but is expressed in the adult brain and testes.11 Effects of Spry3 deletion in the murine germline have not been reported. Spry4-null mice show growth retardation and polysyndactyly.19 In addition, Spry2 and -4 have been shown to be critical for repression of ERK activation and diastema tooth formation.20 In the brain, Spry1 and -2 regulate cortical patterning, proliferation, and differentiation by repression of FGF-ERK signaling.9 These results establish Sprys as critical modulators of FGF-ERK signaling during organogenesis.
Expression of Spry1 and -2 at different stages of lens maturation has been described previously.21 Nonetheless, the question of whether Sprys are necessary for lens development has not been addressed. By conditional deletion of Spry1 and -2 in ocular tissues, we show that these genes play critical roles in not only lens but also corneal development.
Materials and Methods
Generation of Spry Null Mice
The generation of Spry1 and -2 floxed lines,16,20 Le-Cre,22 FGF8 and -9 transgenic lines2 and FGF receptor mutant lines7 has been described previously. Spry1 and -2 floxed mice were crossed to Le-Cre transgenic mice to delete different combinations of Spry1 and -2 in ocular tissues. Matings were set up such that Cre-positive embryos or pups were hemizygous for the Le-Cre transgene. Primers (designated P1, P2, and P3) used to ascertain recombination and excision of loxP flanked sequences in Spry1 and -2 genes have been described previously.16,18 Animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Histologic Analyses
Pregnant females were killed at appropriate time points, and transgenic or mutant offspring were identified by PCR. Heads of mutant and control embryos were harvested, fixed in 10% formalin, dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
In Situ Hybridization
In situ hybridizations were performed using 35S UTP-labeled riboprobes. The Cre antisense probe was synthesized using BamHI-digested Cre cDNA and T7 RNA polymerase, c-Maf antisense probe using HindIII-digested c-Maf cDNA and T7 RNA polymerase, Sox1 antisense probe using BamHI-digested Sox1 cDNA and T7 RNA polymerase, and DUSP6 antisense probe using BamHI-digested DUSP6 cDNA and T7 RNA polymerase. Spry1, Spry2, Spry4, FoxE3, Prox1, p57KIP2, cyclin D1, cyclin D2, Hes1, Erm, and Pea3 were synthesized as described previously.23 To analyze Spry1 deletion in the conditional knockout mice, sequences between the loxP flanks were subcloned, and a riboprobe was synthesized using NotI-digested Spry1 DNA and T3 RNA polymerase. In situ hybridization was performed using the same hybridization and washing conditions as described previously.24 Bright- and dark-field images were captured separately (Eclipse E600 microscope; Nikon, Tokyo, Japan). Silver grains in the dark-field images were pseudocolored red (Photoshop CS; Adobe Systems, San Jose, CA) and overlaid on corresponding bright-field images.
Immunohistochemistry
Immunohistochemistry (IHC) on paraffin-embedded tissue sections was performed as described previously.23 Sections were mounted using antifade medium containing DAPI (ProLong; Invitrogen, Carlsbad, CA). In figures where IHC data are shown, antigen–antibody complexes are in red, and nuclei are stained blue with DAPI.
Proliferation Assay
The BrdU incorporation assay was performed as described elsewhere.6 Quantification of cell proliferation (BrdU labeling index) was performed by computing the fraction of BrdU-labeled nuclei over the total number of nuclei present in a given section. Sections from a minimum of three different embryos were analyzed per genotype per time point. As the stalk cells did not express either lens or corneal markers, they were excluded from the analysis for quantification of lens and corneal BrdU-labeling indices. Analysis was performed by one-way ANOVA with the post hoc Dunnett test comparing each Spry mutant genotype to Cre− samples at P ≤ 0.05 (Prism 5; GraphPad, La Jolla, CA).
Western Blot Analysis
Total proteins from lenses of wild-type and mutant mice were isolated as described previously.6 Four independent samples (with each sample containing 8 to 10 lenses pooled from four to five different embryos from at least three different litters) were used. pERK/ERK ratios of Spry mutants were normalized to Cre− controls and analyzed by two-tailed Student's t-test at P ≤ 0.05.
Results
Endogenous Expression of Spry1 and -2 in the Lens and Cornea
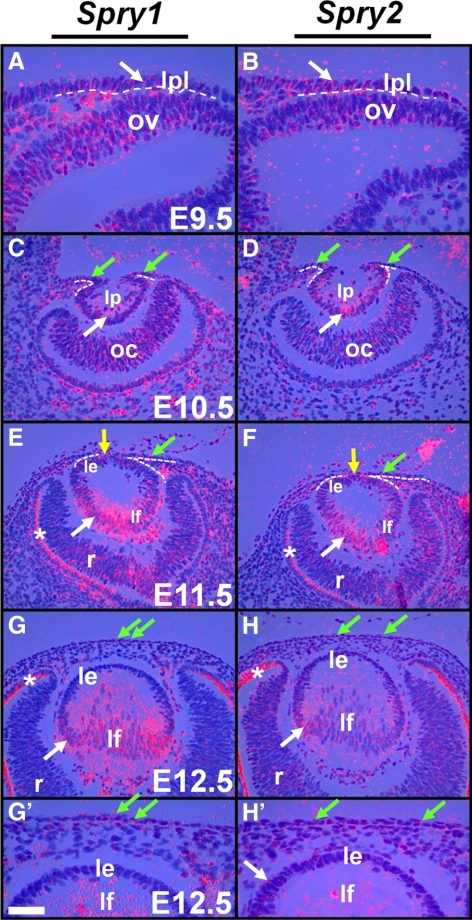
Spry1, -2, and -4 expression during early ocular development was analyzed by in situ hybridization. At E9.5, head ectodermal cells adjacent to the optic vesicle including the lens placodal cells expressed Spry1 and -2 (Figs. 1A, 1B, arrow). At E10.5, the posterior lens pit cells showed strong expression of Spry1 and -2 (Figs. 1C, 1D, white arrow), and the corneal precursor cells adjacent to the lens pit showed weak expression of Spry1 and -2 (Figs. 1C, 1D, green arrows). At E11.5, the cells at the anterior margins of the lens vesicle (at the junction between the lens and the presumptive cornea) that would close to form the lens stalk expressed Spry1 and -2 (Figs. 1E, 1F, yellow arrows). Lens epithelial cells weakly expressed Spry1 and -2 at E11.5 (Figs. 1E, 1F) and Spry2 at E12.5 (Figs. 1G′, 1H′). The lens fiber cells in contrast, showed strong expression of Spry1 and -2 at E11.5 (Figs. 1E, 1F, white arrows) and at E12.5 (Figs. 1G, 1H, white arrows). The corneal epithelial precursors weakly expressed Spry1 and -2 at E11.5 (Figs. 1E, 1F, green arrows) and at E12.5 (Figs. 1G, 1H′, green arrows). Expression of Spry1 and -2 was also seen in a subset of neuroblasts of the optic vesicle (Figs. 1A, 1B), optic cup (Figs. 1C, 1D), and later in the retina (Figs. 1E, 1F, 1H). Spry 4 expression, although not detectable in the lens or corneal epithelial cells between E9.5 and E12.5, was seen in the periocular mesenchymal cells (data not shown).
Figure 1.
Spry expression in the lens. In situ hybridization with 35S-labeled Spry1 and -2 riboprobes was performed on sections of wild-type embryos. Spry1 (A, C, E, G, G′) and Spry2 (B, D, F, H, H′) were expressed initially in the lens placode (A, B, arrows) and later in the lens pit (C, D, arrows) and lens epithelial (E, F) and fiber cells (E, F, G, H, arrows). Spry1 and -2 were weakly expressed in the presumptive cornea (C–F, green arrows), in the cells at the junction between the lens and the presumptive cornea (E, F, yellow arrows) that would close to form the lens stalk, and in retinal neuroblasts (C–H). (A–F, dashed lines) The presumptive lens and corneal epithelium from the periocular mesenchymal cells. (E–H) Staining in the retinal pigmented epithelium (RPE, *) is an artifact of dark-field illumination. le, lens epithelium; lf, lens fibers; lp, lens pit; lpl, lens placode; ov, optic vesicle; oc, optic cup; r, retina. Scale bar in (G): (A, B, G′, H′) 10 μm; (C–H) 20 μm.
FGF Signaling Is Required for Spry1 and -2 Expression in the Lens Fibers
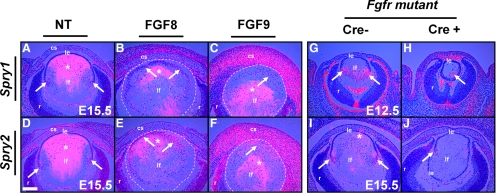
Strong expression of Spry1 and -2 in the lens fiber cells at the onset of fiber differentiation suggested the possibility that they are relevant to the fiber differentiation program. As FGF signaling has been shown to be necessary and sufficient for lens fiber differentiation and as Spry1 and -2 have been shown to be downstream targets of the FGF signaling pathway in several organ rudiments,11,18–20,25 we assessed whether Spry1 and -2 are downstream targets of FGF signaling in the lens during initiation of fiber differentiation by performing in situ hybridization on sections of FGF transgenic (gain of function) and FGF receptor mutant (loss of function) mice. Lens fiber–specific expression of FGF8 or -9 in transgenic mice induces premature fiber differentiation of the overlying epithelial cells.2 In contrast, loss of all six alleles of FGFR1, -2, and -3 (FGFR mutant) leads to inhibition of fiber differentiation.7 At E15.5, lens cells at the transition zone where fiber differentiation is initiated expressed Spry1 and -2 (Figs. 2A, 2D, arrows). In contrast, lens epithelial cells did not express Spry1 and -2 (Figs. 2A, 2D). FGF8 and FGF9 transgenic lens epithelial cells showed weak induction of Spry1 (Figs. 2B, 2C, arrows) but strong upregulation of Spry2 (Figs. 2E, 2F, arrows) suggesting that FGF activation is sufficient for induction of Spry1 and -2 in the differentiating fiber cells. In contrast, FGFR mutant lenses showed reduced expression of Spry1 (Fig. 2H) and -2 (Fig. 2J), suggesting that FGFR-mediated signaling is necessary for expression of Spry1 and -2 in the lens fiber cells. Taken together, these results indicated that FGF signaling is both sufficient and necessary for induction of Spry1 and -2 during lens fiber differentiation. Induction of Spry1 and -2 in the FGF9 corneal stromal cells was also observed (Figs. 2C, 2F).
Figure 2.
Spry1 and -2 expression in FGF transgenic and FGFR mutant mice. In situ hybridization with 35S-labeled Spry1 and -2 riboprobes was performed on sections of nontransgenic (NT) (A, D), FGF8 (B, E), and FGF9 (C, F) transgenic and FGFR mutant (G–J) embryos. Spry1 and -2 were upregulated at the transition zone in the nontransgenic lenses (A, D, arrows). Lens fiber–specific expression of FGF8 (B, E) or FGF9 (C, F), weakly induced Spry1 (B, C, arrows), and strongly induced Spry2 (E, F, arrows) in the lens epithelial cells. (B–F, dashed lines) The lens. Spry1 (H, arrow) and Spry2 (J, arrow) expression was reduced in FGFR mutant lenses in contrast to Cre− controls (G, I). Staining within the lens core (*) in (A–F) and in the RPE (G) are artifacts of dark-field illumination. True hybridization signals appeared as dots (arrows), and diffraction artifacts had a hazy, less-defined appearance that was visible even in the complete absence of a signal. cs, corneal stroma; le, lens epithelium; lf, lens fibers; r, retina. Scale bar, 40 μm.
Spry1 and -2 Deletion in the Lens and Cornea
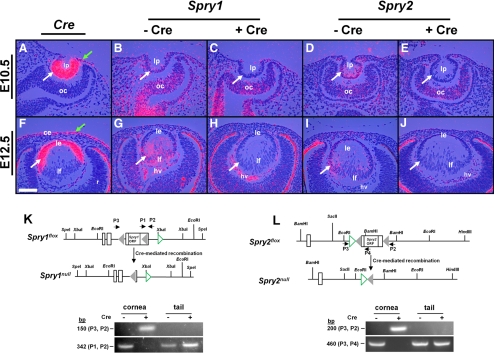
To assess whether Spry1 and -2 are necessary for lens and corneal differentiation, we used the Cre-loxP system to conditionally delete these genes in the head ectodermal cells that form lens and corneal epithelial precursors. Mice carrying floxed alleles of Spry1 and -2 were mated to the Le-Cre transgenic line that expressed Cre recombinase driven by the Pax6 P0 promoter.22 This promoter is active initially in the preplacodal ectodermal precursors and later in the lens (Figs. 3A, 3F, white arrows), corneal (Figs. 3A, 3F, green arrows), and conjunctival epithelial cells (data not shown).22 Deletion of Spry1 and -2 in the lens precursors was analyzed by in situ hybridization (Figs. 3B–E, 3G–J). Cre+ embryos containing floxed Spry1 and -2 alleles did not show expression of Spry1 (Figs. 3C, 3H, arrows) and -2 (Figs. 3E, 3J, arrows) in the lens pit or epithelial or fiber cells in contrast to Cre− controls (Figs. 3B, 3D, 3G, 3I). Spry1 and -2 expression however, remained unaltered in other ocular tissues, such as the optic cup (Figs. 3B–E) and hyaloid vascular cells (Figs. 3G–J) where Cre recombinase was not expressed. Loss of Spry1 and -2 in the corneal epithelial precursors could not be confirmed by in situ hybridization because of low levels of expression of these two genes. Nonetheless, PCR analysis performed on genomic DNA isolated from new born Spry1 and -2 mutant corneal epithelial cells showed excision of loxP-flanked sequences in Spry1 and -2 genes in Cre+ but not in Cre− samples (Figs. 3K, 3L). These results, taken together, correlated Spry1 and -2 deletion in the lens and corneal epithelial cells to Cre recombinase expression.
Figure 3.
Conditional deletion of Spry1 and -2 in the lens. In situ hybridization (A–J) was performed with 35S-labeled Cre and Spry1 and -2 riboprobes on sections of Cre transgenic (A, F) and Spry1 (B–H) and Spry2 (D–J) mutant embryos. Cre recombinase was expressed in the lens pit (A, white arrow), lens epithelium (F), and presumptive corneal epithelial cells (A, F, green arrows) in the Le-Cre mice, as reported previously. Cre+ embryos showed loss of Spry1 (C, H) and Spry2 (E, J) expression in the lens but not in the optic cup (oc) (B–E) or the hyaloid vasculature (hv) (G–J) where Cre recombinase was not expressed. (K, L) Spry1 and -2 floxed alleles (adapted and modified with permission from Basson MA, Akbulut S, Watson-Johnson J, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 2005;8:229–239. © Elsevier, and Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell 2005;8:553–564. © Elsevier). Cre-mediated recombination in the cornea was determined by PCR using primers (P1, P2, and P3) flanking the loxP sequences (gray triangles) of Spry1 and -2 genes. Tail genomic DNA was used as negative controls. Open rectangles: exons. Green open triangles: frt sequences. After recombination, P2 and P3 amplicons were seen in Cre+ corneas but not in Cre− corneas, as they are too large to be amplified. ce, corneal epithelium; le, lens epithelium; lf, lens fibers; lp, lens pit; ORF, open reading frame; r, retina. Scale bar, 20 μm.
Failure of Lens Detachment in Spry1- and -2-Null Mice
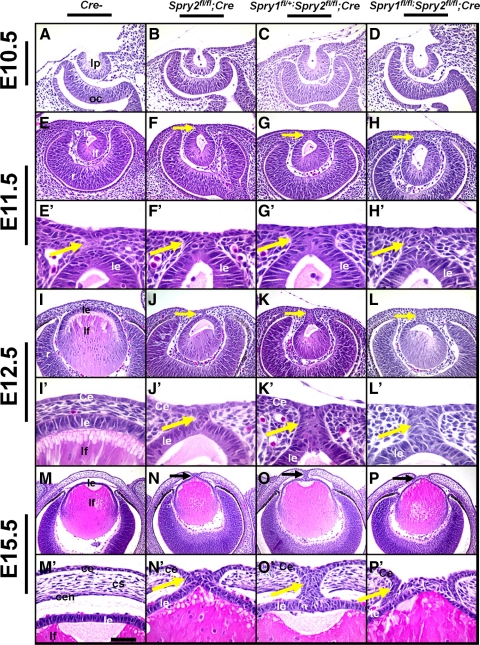
To assess alterations in lens and corneal development in Spry-null mice, sections of embryos that had lost two, three, or all four alleles of Spry1 and -2 in the lens, corneal, and conjunctival epithelial cells were analyzed by histology (Fig. 4). Embryos that lacked Cre recombinase (Cre−) were used as controls. At E10.5, embryos lacking two alleles of Spry2 (Fig. 4B), one allele of Spry1 and two alleles of Spry2 (Fig. 4C), and all four alleles of Spry1 and -2 (Fig. 4D) were indistinguishable from control embryos (Fig. 4 A). In these mutants, the lens pit formed normally. At E11.5, a remnant of a lens stalk, a transient structure connecting the lens vesicle to the presumptive cornea, was seen in control embryos (Figs. 4E, 4E′, arrow). Spry mutants, in contrast, displayed a thicker stalk with more cells (Figs. 4F–H, 4F′–4H′, arrows). At E12.5, the lens vesicle in the control embryo had separated from the presumptive corneal epithelium, and periocular mesenchymal cells were present in the region between the lens and the presumptive cornea (Figs. 4I, 4I′). In contrast, Spry mutant embryos still contained the lens stalk and failed to separate from the overlying corneal epithelium (Figs. 4J–L, 4J′–L′, arrows). The stalks in the Spry mutant embryos were persistent at later ages including E15.5 (Figs. 4N–P, 4N′–P′). The posterior part of the stalk was continuous with the lens and the anterior part with the corneal epithelium (Figs. 4N′–P′). Occasionally, cells within the stalk piled up (Figs. 4P, 4P′) and lenses were ruptured in the Spry mutant embryos (Figs. 6L, 8F, 9E). Fiber cells in the posterior portion of the lens in Spry mutants (Figs. 4J–L, 4N–P) appeared similar to those in control embryos (Figs. 4I, 4M). The mean lens diameter was not significantly altered in Spry mutants compared with Cre− controls at E12.5 (0.208 ± 0.022 mm [Spry1;Spry2 double mutants] vs. 0.191 ± 0.014 mm [Cre−]) or at E15.5 (0.490 ± 0.019 mm [Spry1;Spry2 double mutants] vs. 0.480 ± 0.011 mm [Cre−]). The corneal stroma and endothelium in Spry mutants (in the posterior part of the cornea) were discontinuous, presumably due to the presence of the lens stalk (Figs. 4N–P, 4N′–P′). Embryos that lacked two alleles of Spry1 (Supplementary Figs. S1A–B′, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental) or one allele each of Spry1 and -2 (Supplementary Figs. S1A–B′, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental) also displayed lens stalks and were identical with other Spry mutants. As loss of two, three, or four alleles of Spry1 and/or -2 leads to similar alterations in early lens differentiation, detailed analysis was performed on embryos that lacked all four alleles of Spry1 and -2 (unless specified otherwise). In this article, we report changes in lens and corneal differentiation. Alterations in ocular gland development and eyelid closure were also seen but are not reported here.
Figure 4.
Failure of lens detachment in Spry mutants. Sections of E10.5 (A–D), E11.5 (E–H′), E12.5 (I–L′), and E15.5 (M–P′), control (Cre−, A, E, E′, I, I′, M, M′), Spry2fl/fl; Cre (B, F, F′, J, J′, N, N′), Spry1fl/+;Spry2 fl/fl;Cre (C, G, G′, K, K′, O, O′), and Spry1fl/fl;Spry2fl/fl;Cre (D, H, H′, L, L′, P, P′) embryos were analyzed by hematoxylin and eosin staining. (E′–P′) Higher magnifications of (E–P). Lens placode invagination (B–D) was unaffected in Spry1 and -2 mutants. At E11.5, Spry mutants showed prominent stalks (F–H′) in contrast to control embryos (E′). At E12.5 and at E15.5, Spry mutant lenses failed to separate and displayed persistent lens stalks (J–L′, N–P′, arrows) in contrast to controls (I, I′, M, M′). Corneal stroma (cs) and endothelium (cen) were discontinuous in Spry mutants at E15.5 (N–P′). ce, corneal epithelium; cs, corneal stroma; cen, corneal endothelium; le, lens epithelium; lf, lens fibers; lp, lens pit; oc, optic cup; r, retina. Scale bar in (M): (A–H) 30 μm; (I–L,M–P) 60 μm; (E′–H′, I′–L′) 10 μm; (M′–P′) 15 μm.
Figure 6.
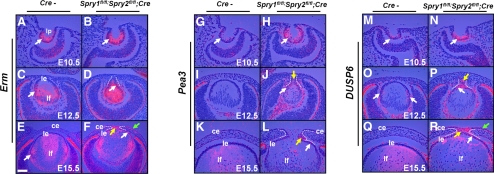
Induction of FGF targets in the Spry mutants. In situ hybridizations were performed on sections of control (Cre−) and Spry mutant embryos using 35S-labeled Erm, Pea3, and DUSP6 riboprobes. Erm and DUSP6 were induced in the Spry mutant lens epithelial (D, F, R, white arrows), stalk (F, P, R, yellow arrow), and corneal epithelial cells (F, R, green arrows). Pea3 expression was seen in the Spry mutant lenses (H, J, L, white arrows) and stalks (J, L, yellow arrows). The stalks were, in some cases, more ventrally placed and in these cases, peripheral sections were chosen (D, J) to include the stalks. The lenses in these sections therefore, appear smaller. ce, corneal epithelium; le, lens epithelium; lf, lens fibers. Scale bar in (E): (A–E, G–R) 30 μm; (E, F) 60 μm.
Figure 8.
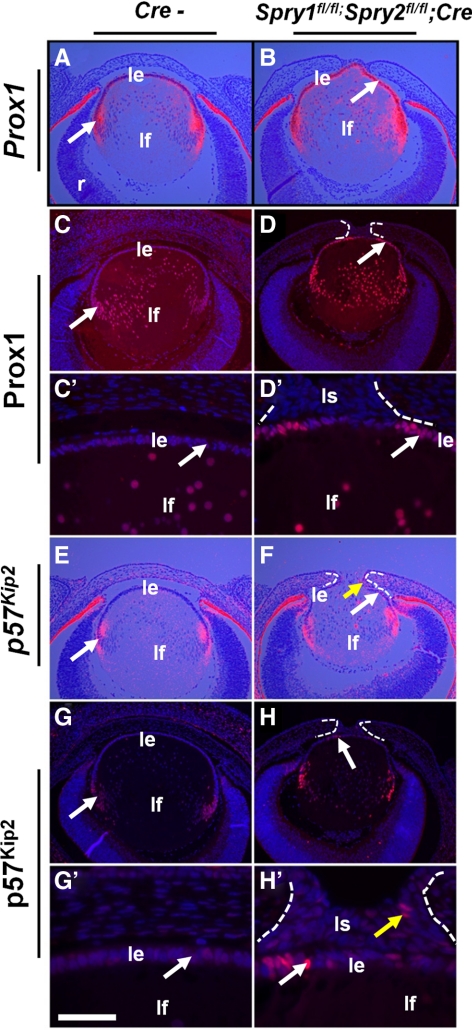
Lens fiber differentiation in Spry mutant embryos. In situ hybridizations (A, B, E, F) and immunohistochemistry (C–D′, G–H′) were performed on E15.5 control (Cre−) and Spry mutant embryos to detect expression of Prox1 (A–D′) and p57KIP2 (E–H′). In situ hybridizations were performed using 35S-labeled riboprobes (A, B, E, F). Prox1 (B, D, D′, arrows), and p57KIP2 (F, H, H′, arrows) were upregulated in Spry mutant lens epithelial cells. Some of the stalk cells also expressed p57KIP2 (H′, yellow arrow). ce, corneal epithelium; le, lens epithelium; lf, lens fibers; r, retina. Scale bar in (G′): (A–D, E–H) 60 μm; (C′, D′, G′, H′) 10 μm.
Figure 9.
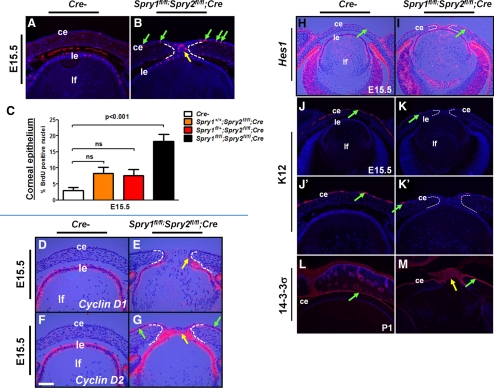
Cell proliferation and differentiation in Spry mutant corneal epithelial cells. (A–C) BrdU incorporation assay. Immunohistochemistry was performed on E15.5 Spry mutant (B) and control (A) embryos. BrdU proliferation index in the corneal epithelial cells (C) was quantified. Each genotype was compared to Cre− controls. Error bars, SEM. BrdU incorporation was significantly increased in the Spry mutant corneal epithelial cells (B, green arrows, C). (D–G) Cell cycle targets in the lens and cornea. In situ hybridizations were performed on E15.5 control (Cre−) and Spry mutant embryos to detect expression of cyclin D1 (D, E) and cyclin D2 (F, G). Cyclin D2 (G, green arrows) but not cyclin D1 (E) was upregulated in the Spry mutant corneas. Both cyclin D1 (E, arrow) and D2 (G, yellow arrow) were expressed in the stalk cells of Spry mutants. (H–M) Corneal epithelial differentiation. In situ hybridization (H, I) and immunohistochemistry (J–M) were performed on sections of control (Cre−) and Spry mutant embryos. In situ hybridization was performed with a 35S-labeled Hes1 riboprobe. (J′, K′) Higher magnifications of (J) and (K). Increased Hes1 expression in the Spry mutant corneal epithelial cells (I, green arrows) suggests an expansion of progenitor cells. Expression of K12, but not 14-3-3σ, a corneal epithelial differentiation marker, was reduced in Spry mutant corneas (K, K′, M). ce, corneal epithelium; le, lens epithelium; ir, iris; lf, lens fibers; r, retina. Scale bar in (F): (A, B, D–G, J′, K′) 20 μm; (H–M) 40 μm.
Increased Erk Activation in Spry Mutants
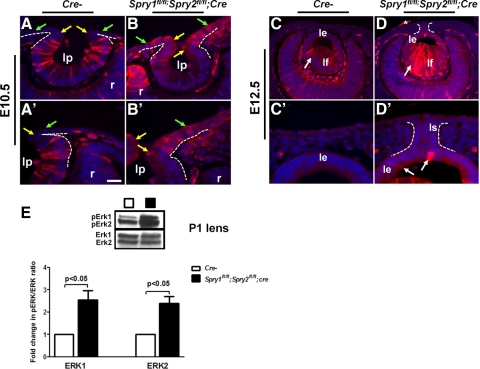
As Spry1 and -2 are negative-feedback regulators of the FGF-Ras-Erk pathway in several mammalian tissues, alterations in ERK phosphorylation in Spry1 and -2 double-null embryos (Figs. 5A–D′) were assessed by immunohistochemistry and Western blot analysis (Fig. 5E). Spry1;Spry2 double-null embryos showed elevated pERK1/2 at the junction between the lens pit and the surface ectoderm (Figs. 5B, B′, yellow arrows) and in the prospective corneal epithelial cells (Figs. 5B, B′, green arrows). Spry1 and -2 single-null embryos also showed a similar increase (data not shown). In the lens pit, pERK1/2 levels were reduced compared with controls (Figs. 5B, B′). At E12.5, pERK1/2 levels were increased in the lens epithelial and fiber cells (Figs. 5D, D′, arrows). Increase in pERK1/2 levels in the newborn Spry1;Spry2 double-null lenses was quantified by Western blot analysis (Figs. 5E). Quantification of pERK/ERK ratios suggested that pERK1 and -2 levels were increased approximately 2.3- to 2.5-fold in the Spry1;Spry2 double-null lenses (Fig. 5G). Spry mutant corneal epithelial cells did not show detectable levels of pERK1/2 at E12.5 (Figs. 5D, 5D′) or at E15.5 (data not shown).
Figure 5.
ERK activation in Spry mutants. Immunohistochemistry was performed on sections of E10.5 (A–B′) and E12.5 (C–D′) control and Spry1fl/fl;Spry2fl/fl;Cre embryos using an anti-pERK1/2 antibody. (A′–D′) Higher magnifications of (A–D). Elevated pErk1/2 levels were detected at the anterior margins of the lens pit (compare B to A, B′ to A′, yellow arrows) and corneal epithelial precursors (B, B′, green arrows) in the Spry mutant eyes. Similarly, pERK1/2 levels were elevated in the Spry mutant lens epithelial and fiber cells at E12.5 (D, D′, arrows). (D, *) pERK1/2 staining in the incompletely dissected amnion. (E) Western blot analysis of lens lysates from postnatal day 1 (P1) control (Cre−) and Spry mutants. The blots were probed either with the anti-ERK1/2 (bottom) or the anti-pERK1/2 (top) antibody. pERK/ERK ratios were quantified and normalized to Cre− controls. Error bars, SEM. pERK1 and -2 levels were significantly increased in Spry1fl/fl;Spry2fl/fl;Cre mutant lenses. ce, corneal epithelium; le, lens epithelium; lf, lens fibers; ls, lens stalk; r, retina. Scale bar in (A′): (A, B) 15 μm; (C, D) 30 μm; (E, F) 60 μm; (A′–D′) 10 μm.
Induction of Downstream Targets of FGF Signaling
The ETS domain transcription factors, Erm and Pea3, and the MAP kinase phosphatase DUSP6 (MKP3) have been shown to be targets of FGF signaling.26–28 At E10.5, the lens pit cells expressed Erm (Fig. 6A), Pea3 (Fig. 6G), and DUSP6 (Fig. 6M). At E12.5, Erm (Fig. 6C, arrow), and DUSP6 (Fig. 6O, arrows) were strongly expressed at the transition zone where fiber differentiation is initiated. Spry mutants showed a strong induction of Erm, Pea3, and DUSP6 in their lens pits (Figs. 6B, 6H, 6N, arrows) and lens epithelial cells (Figs. 6D, 6F, 6J, 6L, 6P, 6R, white arrows). Erm, Pea3, and DUSP6 were also induced in the stalk cells (Figs. 6F, 6J, 6L, 6P, 6R, yellow arrows). Interestingly, at E15.5, the Spry mutant corneal epithelial cells also showed strong upregulation of Erm and DUSP6 (Figs. 6F, 6R, green arrows). These results suggest that the FGF signaling axis is active in the Spry mutant stalk, lens, and corneal epithelial cells.
Early Lens Differentiation Is Altered in Spry Null Embryos
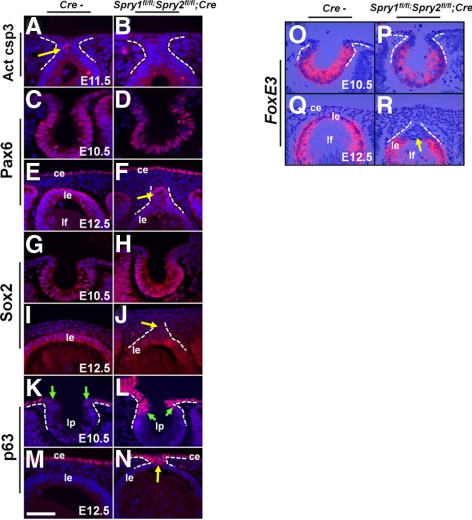
Cells in the lens stalk undergo apoptosis as the lens vesicle separates from the overlying ectoderm.29 To assess whether the Spry mutant stalk cells persist because of decreased apoptosis, we performed immunohistochemistry, using an active caspase 3 antibody (Figs. 7A, 7B). At E11.5, activated caspase 3–positive cells were seen in the stalks of the control (Fig. 7A, arrow) but not the Spry mutants (Fig. 7B), suggesting an inhibition of the apoptotic program. Next, we examined expression of genes that are critical for lens placode specification and vesicle separation by immunohistochemistry and in situ hybridization (Figs. 7C–R). Pax6, Sox2, and FoxE3 are transcription factors that are critical for lens specification (Pax6), placode thickening and invagination (Pax6, Sox2), and vesicle separation (Pax6, FoxE3).22,30–32 All three proteins were expressed in the correct spatial pattern in the Spry mutant embryos (Figs. 7C–J, 7O–R) suggesting that the persistent stalk phenotype is not due to altered expression of these proteins. Of note, Spry mutant stalk cells did not express Sox2 or FoxE3 in contrast to lens epithelial cells (Figs. 7J, 7R, arrow). At E10.5, p63, a gene essential for epidermal differentiation,33 was restricted to the cells of the surface ectoderm and was excluded from the lens pit (Fig. 7K, arrows). In Spry mutant embryos, at E10.5, the expression domain of p63 had expanded into the anterior margins of the lens pit (Fig. 7L, arrows). At E12.5, corneal but not lens epithelial precursors expressed p63 (Fig. 7M). At E12.5, p63 expression was unaltered in the corneal epithelial cells (Fig. 7N). All the stalk cells, however, expressed p63 (Fig. 7N, arrow). Expression of E- and N-cadherin, two proteins shown to regulate lens epithelial adhesion, survival, and vesicle separation34 was examined (Supplementary Figs. S3A–H′, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental). The Spry mutant stalks expressed E-cadherin (Supplementary Figs. S3A, S3B, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental) but not N-cadherin (Supplementary Figs. S3F–H′, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental) or K12, a corneal epithelial marker (Figs. 9K, 9K′). These results, taken together, suggest that Spry mutant stalk cells do not differentiate as lens or corneal epithelial cells, but instead, remain as undifferentiated ectodermal precursors.
Figure 7.
Early lens differentiation in Spry mutant embryos. Immunohistochemistry (A–N) and in situ hybridizations (O–R) were performed on control (Cre−) and Spry mutant embryos, to detect expression of activated caspase 3 (A, B), Pax6 (C–F), Sox2 (G–J), p63 (K–N), and FoxE3 (O–R). In situ hybridizations were performed using 35S-labeled riboprobes (O–R). Activated caspase 3 was seen in the stalks of Cre− controls (A, arrow) but not in Spry mutants. Pax6 (D, F) and Sox2 (H, J) were expressed in their normal spatial pattern in Spry mutants. Spry mutant lens stalks, however, showed reduced Sox2 expression compared to lens epithelial cells (J, arrow). p63, normally excluded from the lens placodal cells (K, green arrows), was expressed at the anterior margins of the lens pit (L, arrows) and in the stalks (N, arrow) of Spry mutants. FoxE3 expression in the invaginating lens placodal cells at E10.5 (O) and epithelial cells (Q) was similar to controls (O). Spry mutant stalk cells did not express FoxE3 (R, arrow). ce, corneal epithelium; le, lens epithelium; lf, lens fibers; lp, lens pit. Scale bar in (M): (A–D, M, N) 10 μm; (E, F, I–L, O–R) 15 μm.
Premature Initiation of Fiber Differentiation in Spry Mutant Lenses
The onset of lens fiber differentiation near the equator is marked by an upregulation of the homeobox transcription factor, Prox1, and the cyclin-dependent kinase (CDK) inhibitor, p57KIP2 (Figs. 8A, 8C, 8E, 8G, arrows).35–37 Prox1 mRNA (Fig. 8B) and protein (Figs. 8D, 8D′) and p57KIP2 mRNA (Fig. 8F) and protein (Figs. 8H, 8H′) were upregulated in most of the Spry mutant lens epithelial cells, indicating a premature induction of markers of fiber differentiation. Expression of transcription factors critical for fiber differentiation such as c-Maf and Sox1 and the terminal fiber differentiation markers β- and γ-crystallin in Spry mutant fiber cells were similar to controls (Supplementary Figs. S3I–S3R, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental), suggesting that fiber cell maturation is not affected in Spry mutant lenses. In a significant finding, β- and γ-crystallins were not induced in the Spry mutant lens epithelial cells (Supplementary Figs. S3P, S3R, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental).
Cell Proliferation in Spry Null Embryos
To determine whether the persistent stalks seen in Spry mutants were due to increased proliferation, we performed a BrdU incorporation assay on E10.5, E12.5, and E15.5 Spry mutant embryos (Figs. 9A–C; Supplementary Figs. S4A–E, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental). Quantification of the BrdU labeling index of E10.5 Spry mutant embryos did not reveal a significant change in the BrdU incorporation rates, either in cells lining the lens pit or at the junction between the lens and the presumptive cornea (data not shown), suggesting that the persistence of lens stalks is unlikely to be due to an increase in proliferation. At E12.5 and E15.5, BrdU incorporation rates of Spry1 and -2 single- or double-null lens epithelial cells were indistinguishable from those of control embryos (Supplementary Figs. S4C–E, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental), suggesting that Spry1 and -2 were dispensable for lens epithelial proliferation. These results were surprising, given the increase in p57Kip2 expression in Spry mutant lens epithelial cells (Figs. 8A, 8C, 8E, 8G). That cell cycle exit is not induced despite elevated p57 expression could be due to either qualitative (p57 protein expression in seen in most but not all Spry mutant lens epithelial cells; Figs. 8H, 8H′) or quantitative (p57 expression is presumably not at high enough levels to block activities of all cdk2 and cdk4 molecules in lens epithelial cells) differences. In addition, expression of p27Kip1, another cdk inhibitor shown to have overlapping role with p57 in initiating lens epithelial cell cycle exit,37 was unaltered in Spry mutants (data not shown). At E12.5 (Supplementary Fig. S4D, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental) and E15.5 (Fig. 9B, yellow arrow), however, the stalk cells incorporated BrdU. Interestingly, the corneal epithelial cells of Spry1;Spry2 double-null (Fig. 9B, green arrows, 9C) but not Spry1 (Supplementary Fig. S1E, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental) or Spry2 single-null embryos (Fig. 9C) showed increased BrdU incorporation. Also, cyclin D2 but not cyclin D1 was induced in Spry1;Spry2 double-null (Figs. 9E, 9G, green arrows) and Spry1 single-null corneas (Supplementary Figs. S1F–I, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-7531/-/DCSupplemental). These results suggest that Spry1 and -2 function redundantly to suppress corneal epithelial proliferation.
Impaired Corneal Differentiation in Spry Mutants
Alterations in corneal differentiation were assessed by in situ hybridization and immunohistochemistry. Hes1, a transcription factor and downstream target of the Notch pathway, is expressed in the corneal epithelial cells (Fig. 9H, arrow) and has been shown to be necessary for maintenance of corneal epithelial progenitor/stem population.38 Spry mutant corneal epithelial cells (and stalk cells) showed a stronger expression of Hes1 than Cre− controls (Figs. 9H, 9I, arrow). Expression of keratin 12 (K12), an early marker of corneal epithelial differentiation, was detectable in the corneal epithelial cells by E15 (Figs. 9J, 9J′, arrow). Spry mutant corneas, however, showed a significant reduction in expression of K12 (Figs. 9K, 9K′, arrow) suggesting an inhibition of terminal differentiation of Spry mutant corneal epithelial cells. In contrast, expression of 14-3-3σ, a protein expressed in, but not restricted to, the corneal epithelium, remained unaltered in the Spry mutant corneas (Figs. 9L, 9M).
Discussion
Conditional deletion of Spry1 and -2 in ocular tissues revealed interesting roles for these genes during early lens and corneal development. The significance of our results as it relates to FGF signaling and past studies are discussed in the following sections.
Our Spry expression results differ from a previously published report of Spry expression in the lens. Boros et al.21 reported a higher expression of Spry1 in the lens epithelium than fibers and ubiquitous expression of Spry2 within the lens before E13.5. The difference in expression pattern between our study and that of Boros et al. may be attributable to the following. The riboprobes that they used were derived from rat Spry cDNAs and were designed to hybridize to specific regions within Spry1 and -2 coding sequences. The riboprobes in our study were against full-length mouse Spry1 and -2 that included 3′ untranslated sequences. These probes have been used to describe Spry expression pattern during early embryogenesis and limb development.11 In addition, we used 35S-labeled riboprobes for our study. In contrast, Boros et al. used a nonradioactive method to detect Spry1 and -2 expression in situ. Nonetheless, both studies agree on the absence of Spry4 expression in the lens or corneal epithelial cells before E12.5.
Lens placode specification, invagination, and vesicle formation are apparently normal in Spry mutants, suggesting that Spry function is dispensable for modulation of signaling pathways that regulate these developmental events. However, lens vesicle separation from the overlying ectoderm was not successfully completed in the Spry mutants. Inhibition of apoptosis may be one reason for failure of vesicle detachment. Whether Sprys play a direct or an indirect role in inhibition of apoptosis is not known. Before vesicle closure, cells at the anterior margins of the lens pit that would close to form the stalk showed elevated ERK activity in the Spry mutants. Although these results suggest the possibility that increased ERK signaling may play a role in promoting survival of stalk cells, it is unlikely to be FGF-dependent, as concomitant induction of FGF targets in these cells were not seen. After vesicle closure, the stalk cells that escape programmed cell death showed induction of FGF signaling targets such as Erm, Pea3, and DUSP6. Continued survival of stalk cells therefore could at least in part, be attributable to a local increase in FGF signaling. This interpretation is consistent with a recent report that shows that decreased FGF signaling during early placode development by conditional deletion of FGFR1 and -2 in the preplacodal cells results in increased apoptosis.39 Also of relevance are the results of our previous studies that show that Ras activation in the placodal cells leads to failure of lens detachment.23 Considered together, these results support the notion that the process of lens vesicle separation is sensitive to changes in FGF-Ras-ERK activity and that Spry-mediated suppression of ERK is critical for proper separation of the lens vesicle. However, we cannot rule out the possibility that elevated ERK activity in Spry mutant lenses is an indirect consequence of altered expression of transcription factors or other intercellular signaling pathways. In addition, it is possible that the persistent lens stalk phenotype is due to Spry1 and -2 deficiency in the corneal epithelial precursors. Future experiments to specifically delete Spry1 and -2 in the corneal (but not lens) epithelial precursors should allow us to test this hypothesis.
Our results are in contrast to those in a previous study that showed persistent lens stalks in transgenic mice that expressed kinase deficient FGFR1 in the lens placodal cells.40 This result was interpreted to indicate that decreased FGF signaling caused failure of lens vesicle separation. However, in these studies, the persistent lens stalk phenotype was seen in only one of two transgenic lines. In addition, induction of FGF targets or elevation in pERK activity was not reported making it difficult to assess whether FGF signaling was indeed reduced in the lens placodes of these mice. A recent report suggests the possibility that these mice may present a gain-of-function rather than a loss-of-function phenotype, as they do not match the Fgfr1;Fgfr2 double-null phenotype.39
Our pERK immunohistochemistry results show an initial reduction at E10.5 and a later increase E12.5 in pERK levels in the posterior lens cells of the Spry mutants. Although the reasons for this change are unknown, it appears to be of little functional consequence, as lens fiber differentiation remained unaltered.
Our results suggest that Spry1 and -2 are dispensable for lens epithelial proliferation. These genes, however, are downstream targets of FGF signaling and negative regulators of ERK activity during initiation of lens fiber differentiation. Spry1 and -2 are upregulated in the primary fiber cells at E12.5 and at the transition zone where secondary fiber differentiation is initiated at E15.5. FGF stimulation is both necessary and sufficient for induction of Spry1 and -2 in the differentiating fiber cells. (However, it is unclear whether induction of Spry expression in FGF transgenic mice is a direct or an indirect effect of FGF stimulation.) Loss of Spry1 and -2 does not fully phenocopy the effects of FGF stimulation of lens epithelial cells (i.e., induce terminal fiber differentiation, as assayed by β- or γ-crystallin expression). Instead, loss of Spry1 and -2 in the lens epithelial cells leads to premature induction of early fiber differentiation markers Prox1, P57Kip2 with concomitant increase in ERK activity and induction of FGF signaling targets. We interpret these results to mean that Sprys function more as fine tuners than as major regulators of FGF signaling during fiber differentiation. Whether Spry overexpression or activation is sufficient to reduce FGF signaling in the lens has not been tested. Nonetheless, conditional deletion of Shp2, a phosphatase that can dephosphorylate and inactivate the function of Spry2, in the lens leads to reduced ERK activity and delayed initiation of lens fiber differentiation.41 These results complement our Spry loss-of-function studies that show increased ERK activity and premature induction of fiber differentiation markers.
Spry mutant corneal epithelial precursors show increased proliferation with a concomitant increase in Erm and DUSP6 and a transient increase in pERK levels. Reduced expression of corneal differentiation markers such as K12 and increased expression of the progenitor marker Hes1 and the cell cycle regulatory gene cyclin D2 are consistent with the notion that increased ERK signaling in the cornea promotes proliferation rather than differentiation. This is consistent with our previous results that suggested FGF stimulation and Ras activation also increased corneal epithelial proliferation.23,24 Therefore, the lens and cornea, although of common embryonic origin, show distinctive biological responses to loss of Sprys: the cornea shows increased proliferation and the lens, premature initiation of differentiation. However, both corneal and lens epithelial precursors in Spry mutants show elevated ERK activation and induction of ERK targets such as Erm and DUSP6. How does activation of the same signaling pathway lead to distinctive biological responses in these two tissues? Our results provide correlative evidence that the distinctive responses could be due to induction of different targets. For instance, Prox1 and p57KIP2 (a cell cycle inhibitor) were elevated in the Spry mutant lens but not in the cornea. Nonetheless, more experimental evidence is needed to rigorously test this hypothesis.
Loss of any two alleles of Spry1 and -2 is sufficient to cause alterations in lens development suggesting that Spry1 and -2 perform unique roles in regulation of lens differentiation. In contrast, loss of all four Spry alleles is needed to alter corneal differentiation suggesting that Spry1 and -2 function redundantly in the cornea. The reason why the cornea relies on both Spry1 and -2 and the lens, on either Spry1 or -2 to modulate ERK activity is not clear. A future challenge will be to unravel the complexities of negative feedback regulation of Ras-ERK signaling in these two tissues. The studies described here provide a framework for these investigations.
Supplementary Material
Acknowledgments
The authors thank Paul Overbeek and Frank Lovicu (FGF8, FGF9 mice), Gail Martin (Spry2 flox mice, Spry1, Spry2, and Spry4 riboprobes), Ruth Ashery Padan (Le-Cre mice), Andy Groves (Cre), Milan Jamrich (FoxE3), Brigid Hogan (Erm, Pea3), Guillermo Oliver (Prox1), Stephen Elledge (p57KIP2), and Samuel Zigler (α- and γ-crystallin antibodies).
Footnotes
Supported by National Cancer Center Grant CA59998 (JDL), National Eye Institute Grant EY017610 (VG), and National Center for Research Resources Grant G20RR024001 (Creighton University).
Disclosure: M.R. Kuracha, None; D. Burgess, None; E. Siefker, None; J.T. Cooper, None; J.D. Licht, None; M.L. Robinson, None; V. Govindarajan, None
References
- 1. Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science. 1963;142:1489–1490 [DOI] [PubMed] [Google Scholar]
- 2. Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377 [DOI] [PubMed] [Google Scholar]
- 3. McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228 [DOI] [PubMed] [Google Scholar]
- 4. Robinson ML, Ohtaka-Maruyama C, Chan CC, et al. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol. 1998;198:13–31 [DOI] [PubMed] [Google Scholar]
- 5. Robinson ML, Overbeek PA, Verran DJ, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514 [DOI] [PubMed] [Google Scholar]
- 6. Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–1627 [DOI] [PubMed] [Google Scholar]
- 7. Zhao H, Yang T, Madakashira BP, et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chambers D, Medhurst AD, Walsh FS, Price J, Mason I. Differential display of genes expressed at the midbrain-hindbrain junction identifies sprouty2: an FGF8-inducible member of a family of intracellular FGF antagonists. Mol Cell Neurosci. 2000;15:22–35 [DOI] [PubMed] [Google Scholar]
- 9. Faedo A, Borello U, Rubenstein JL. Repression of Fgf signaling by sprouty1–2 regulates cortical patterning in two distinct regions and times. J Neurosci. 30:4015–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–858 [DOI] [PubMed] [Google Scholar]
- 11. Minowada G, Jarvis LA, Chi CL, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475 [DOI] [PubMed] [Google Scholar]
- 12. Chambers D, Mason I. Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech Dev. 2000;91:361–364 [DOI] [PubMed] [Google Scholar]
- 13. de Maximy AA, Nakatake Y, Moncada S, Itoh N, Thiery JP, Bellusci S. Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. Mech Dev. 1999;81:213–216 [DOI] [PubMed] [Google Scholar]
- 14. Tefft JD, Lee M, Smith S, et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–222 [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Lin Y, Itaranta P, Yagi A, Vainio S. Expression of Sprouty genes 1, 2 and 4 during mouse organogenesis. Mech Dev. 2001;109:367–370 [DOI] [PubMed] [Google Scholar]
- 16. Basson MA, Akbulut S, Watson-Johnson J, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239 [DOI] [PubMed] [Google Scholar]
- 17. Basson MA, Watson-Johnson J, Shakya R, et al. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477 [DOI] [PubMed] [Google Scholar]
- 18. Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564 [DOI] [PubMed] [Google Scholar]
- 19. Taniguchi K, Ayada T, Ichiyama K, et al. Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902 [DOI] [PubMed] [Google Scholar]
- 20. Klein OD, Minowada G, Peterkova R, et al. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boros J, Newitt P, Wang Q, McAvoy JW, Lovicu FJ. Sef and Sprouty expression in the developing ocular lens: implications for regulating lens cell proliferation and differentiation. Semin Cell Dev Biol. 2006;17:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess D, Zhang Y, Siefker E, et al. Activated Ras alters lens and corneal development through induction of distinct downstream targets. BMC Dev Biol. 2010;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200 [DOI] [PubMed] [Google Scholar]
- 25. Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54 [DOI] [PubMed] [Google Scholar]
- 26. Kawakami Y, Rodriguez-Leon J, Koth CM, et al. MKP3 mediates the cellular response to FGF8 signalling in the vertebrate limb. Nat Cell Biol. 2003;5:513–519 [DOI] [PubMed] [Google Scholar]
- 27. Li C, Scott DA, Hatch E, Tian X, Mansour SL. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development. 2007;134:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharrocks AD. The ET. S-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837 [DOI] [PubMed] [Google Scholar]
- 29. Mohamed YH, Amemiya T. Apoptosis and lens vesicle development. Eur J Ophthalmol. 2003;13:1–10 [DOI] [PubMed] [Google Scholar]
- 30. Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254 [PMC free article] [PubMed] [Google Scholar]
- 31. Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93 [DOI] [PubMed] [Google Scholar]
- 32. Smith AN, Miller LA, Radice G, Ashery-Padan R, Lang RA. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development. 2009;136:2977–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713 [DOI] [PubMed] [Google Scholar]
- 34. Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lovicu FJ, McAvoy JW. Spatial and temporal expression of p57(KIP2) during murine lens development. Mech Dev. 1999;86:165–169 [DOI] [PubMed] [Google Scholar]
- 36. Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322 [DOI] [PubMed] [Google Scholar]
- 37. Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura T, Ohtsuka T, Sekiyama E, et al. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells. 2008;26:1265–1274 [DOI] [PubMed] [Google Scholar]
- 39. Garcia CM, Huang J, Madakashira BP, et al. The function of FGF signaling in the lens placode. Dev Biol. 2011;351:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438 [DOI] [PubMed] [Google Scholar]
- 41. Pan Y, Carbe C, Powers A, Feng GS, Zhang X. Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development. 2010;137:1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.