The Th-1 cytokine IFN-γ plays a significant role in pathologic apoptosis in the conjunctiva that develops in dry eye. Immune-based ocular surface inflammation in dry eye promotes apoptosis of the tear-secreting ocular surface epithelia.
Abstract
Purpose.
To investigate the role of interferon (IFN)-γ in dry eye–associated conjunctival apoptosis.
Methods.
Desiccating stress (DS) was created in C57BL/6 (B6) and C57BL/6 IFN-γ-knockout (B6γKO) mice. A separate group of mice of both strains also received subconjunctival injections of exogenous IFN-γ or vehicle control (BSA) at days 0, +2, and +4 after DS. Immunoreactivity to active (Ac)-caspase-3, -8, and -9 and terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) were evaluated in cryosections. Goblet cell apoptosis was assessed by MUC5AC and TUNEL double staining. Levels of caspase-3, -8, -9, Fas, and Fas-associated protein with Death Domain (FADD) mRNA in conjunctiva were measured by real-time PCR. The activity of caspase-3, -8, or -9 was measured using fluorometric assay.
Results.
Increased Ac-caspase-3 and -8 and TUNEL immunoreactivity were noted in conjunctival epithelia in B6 mice compared with B6γKO mice after DS, and exogenous IFN-γ administration further increased these parameters. DS-induced conjunctival apoptosis was greatest in the goblet cell area and was accompanied by a decrease in MUC5AC expression in the B6 and B6-IFN-γ–injected groups compared with the B6γKO and B6-BSA–injected groups. B6γKO mice were resistant to DS-induced apoptosis; however, B6γKO receiving IFN-γ yielded results similar to those for B6 wild-type. Caspase-9 production and activity were not increased with DS in B6 or B6γKO mice; however, the administration of IFN-γ significantly increased caspase-9 production and activity in both strains compared with vehicle-injected mice.
Conclusions.
IFN-γ plays a pivotal role in exacerbating conjunctival apoptosis through dual apoptotic pathways with DS.
Dry eye affects tens of millions of people worldwide, representing one of the most common ocular pathologies.1 The pathogenesis of dry eye has not been clearly established; however, there is increasing evidence to suggest that dry eye is an immune-based disease characterized by increased CD4+ T cell infiltration, T helper (Th)-1 and Th-17 responses, and elevated levels of proapoptotic factors that affect the tears and ocular surface.2–6
A growing body of clinical and experimental studies has shown that pathologic apoptosis has a key role in the pathogenesis of keratoconjunctivitis sicca, and it is a therapeutic target for dry eye.7–10 Apoptosis can be mediated by two predominant pathways. One apoptotic pathway is the extrinsic or “death receptor” pathway mediated by the ligation of cell surface death receptors such as Fas and TNF-related apoptosis-inducing ligand and the subsequent activation of caspase-8. The other is the intrinsic or “mitochondrial” pathway mediated by the recruitment and activation of caspase-9.11 We previously reported increased conjunctival epithelial apoptosis using a Sjögren's syndrome-like animal model.9 However, the precise factors and signal pathways that induce apoptosis in dry eye have not yet been elucidated.
Th-1 cytokine interferon (IFN)-γ is secreted exclusively by T cells (cytotoxic and Th-1) and natural killer cells. This cytokine plays a crucial role in vivo in regulating several immune responses, such as delayed-type hypersensitivity, inflammation, graft rejection, and the pathogenesis of inflammatory diseases (Sjögren's syndrome, mucus membrane pemphigoid, Stevens-Johnson syndrome, and graft-versus-host disease).12–16 In our previous study, we found that desiccating stress (DS) promoted the migration of CD4+ T cells and IFN-γ+ cells into goblet cell zones of the conjunctiva and increased the concentration of IFN-γ in tears under dry eye.17 Increased IFN-γ can lead to conjunctival epithelial squamous metaplasia with progressive goblet cell loss and an increase in cornification marker small proline-rich protein-2a expression in dry eye.17 It has been reported that the Th-1 cytokine IFN-γ, alone or combination with Fas ligand (FasL) and tumor necrosis factor (TNF)-α, has a proapoptotic effect on some mucosal epithelial and tumoral cell lines.11,18–20
The purpose of this study was to investigate the role of IFN-γ in the pathologic apoptosis of conjunctiva that develops in response to DS.
Methods
Mouse Model of Dry Eye
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. DS was created by subjecting female C57/BL6 (B6) mice, 6 to 8 weeks of age, to subcutaneous injection of 0.5 mg/0.2 mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) into alternating hindquarters administered four times a day (8:30 AM, 11 AM, 1 PM, and 4:30 PM) with exposure to an air draft and <40% ambient humidity. Mice were euthanatized after 5 days of treatment (5D). A group of age- and sex-matched mice that did not receive any treatment to induce dry eye served as untreated (UT) control mice.
Exogenous Administration of IFN-γ
To evaluate the role of IFN-γ in conjunctival apoptosis, we subjected B6 and C57BL/6 IFN-γ- knockout (B6γKO; B6.129s7-Ifn-gtm1Ts/J; Jackson Laboratories, Bar Harbor, ME) mice to DS, as described, for 5 days. Mice of each strain were divided into three treatment groups: (1) 5D control mice that received no ocular injections; (2) vehicle control animals that received bilateral subconjunctival injections (20 μL/eye) of 0.1% bovine serum albumin (BSA) in PBS (5D+BSA); (3) 5D+IFN-γ mice that received bilateral subconjunctival injections of recombinant murine IFN-γ (1 × 104 U/eye per injection, dissolved in 20 μL of 0.1% BSA in PBS; Chemicon, Temecula, CA) before and at 2 and 4 days of DS. All mice were euthanatized after 5 days of DS.
Histology
For immunostaining and terminal deoxynucleotidyl transferase-mediated dUTP-nick end labeling (TUNEL), the eyes and adnexa of mice from each group/each strain (n = 3) were excised, embedded in optimal cutting temperature compound (OCT compound; VWR, Suwanee, GA), and flash frozen in liquid nitrogen. Sagittal 8-mm sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and placed on glass slides that were stored at −80°C.
IFN-γRα and Activated Caspase-3, -8, and -9 Immunostaining
Cryosections were evaluated for the expression of activated (AC) caspase-3, -8, and -9. Samples were then blocked with 10% goat serum for 30 minutes at room temperature. After three washes in phosphate-buffered saline (PBS, pH 7.2), tissue samples were incubated with polyclonal rabbit anti–IFN-γ receptor (IFN-γR) (1:100, sc-700; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti–AC-caspase-3 (1:100; BD PharMingen, San Diego, CA), rabbit anti–AC-caspase-8 (1:100; Novus Biologicals, Littleton, CO), or rabbit anti–AC-caspase-9 (1:100; Novus Biologicals) primary antibodies at 4°C overnight. Negative controls were performed at the same time and consisted of sections incubated with PBS in place of primary antibody. The next day, samples were incubated with goat anti–rabbit FITC-conjugated antibody for 45 minutes in the dark at room temperature, followed by three washes in PBS. Nuclei were then counterstained using propidium iodide (PI) (0.7 μg/mL) for 5 minutes. Approximately 30 μL mounting gel (Gel Mount; Fisher Scientific, Pittsburgh, PA) and a 22 × 50-mm coverslip (Fisher Scientific) were then applied.
Digital images (512 × 512 pixels) of representative areas of the tarsal conjunctiva were captured with a laser-scanning confocal microscope with krypton-argon and He-Ne laser (LSM 510; Carl Zeiss Meditec, Ltd., Thornwood, NY). They were acquired with a 40/1.3× oil immersion objective. The intensity of the staining was graded in two conjunctival images, and the results were averaged. Briefly, in each digital image, seven elliptical regions (of 1076 pixels2) were drawn with image analysis software (Metavue6.24r; Molecular Devices, Sunnyvale, CA), and the integrated intensity calculated by the software was recorded on a spreadsheet (Excel; Microsoft, Redmond, WA). The results within each image were summed and are presented as the mean of all images within a group, in fluorescence unit × 105.
TUNEL and MUC5AC Immunostaining
TUNEL assay was performed using a commercially available kit (ApopTag; Intergen Co., Purchase, NY).9 Cryosections were fixed in 1% paraformaldehyde and permeabilized with 2:1 ethanol/acetic acid solution. The samples were incubated with TdT enzyme and 11-digoxigenin dUTP at 37°C for 4 hours. After quenching the reaction, samples were blocked with blocking solution and incubated with anti–digoxigenin FITC-conjugated antibody for 60 minutes at room temperature.
After completion of the initial TUNEL procedure, the cryosections were stained for expression of MUC5AC (rabbit anti–MUC5AC primary antibody; 1:100, sc-20118; Santa Cruz Biotechnology), as described. Digital images (512 × 512 pixels) of representative areas of the tarsal conjunctiva were captured, and the TUNEL-positive cells in the conjunctival epithelia in 200-μm length in the sagittal sections were counted.
Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
Total RNA collected from the conjunctiva was extracted using a purification kit (RNeasy Micro Kit; Qiagen, Valencia, CA) according to the manufacturer's instructions, quantified by a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Wilmington, DE), and stored at −80°C. Three samples per group/stain were used, and one sample consisted of pooled samples from five mice. Samples were treated with DNase to prevent genomic DNA contamination, according to the manufacturer's instructions (Qiagen). First-strand cDNA was synthesized with random hexamers by M-MuLV reverse transcription (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Inc., Arlington Heights, NJ), as previously described.21
Real-time PCR was performed with specific MGB probes (TaqMan; Applied Biosystems, Inc. [ABI], Foster City, CA) and PCR master mix (TaqMan Gene Expression Master Mix; ABI) in a commercial thermocycling system (Mx3005P QPCR System; Stratagene, La Jolla, CA), according to the manufacturers' recommendations. Murine MGB probes were GAPDH (Mm99999915), IFN-γRα (Mm00599890), Fas (Mm00433237), FADD (Mm00438861), caspase-3 (Mm00438045), caspase-8 (Mm00802247), and caspaspe-9 (Mm01348848). The GAPDH gene was used as an endogenous reference for each reaction. Results of the relative-quantitative real-time PCR were analyzed by the comparative threshold cycle (CT) method22 and normalized by GAPDH as an internal control.
Caspase-3, -8, and -9 Activation Fluorometric Assays
The activation of caspase-3, -8, and -9 was measured according to the protocol of the respective fluorometric kit (K105-25, K112-25, and K189-100, respectively; BioVision, Inc., Mountain View, CA). Conjunctivas were surgically excited and placed in the lysis buffer provided with the kits. Protein concentration was measured using a micro bovine serum albumin (BSA) protein assay kit (Thermo Fisher Scientific, Waltham, MA), and 50 μg conjunctival lysates was used for each assay. Lysis buffer without any protein was used as blank control. Three samples per group/strain were used, and one sample consisted of pooled conjunctiva samples from four mice. Fluorescence was read using a fluorescence and absorbance reader (SpectraFluor; Tecan, Männedorf, Switzerland) with 400-nm excitation filter and 505-nm emission filter.
Statistical Analysis
The unpaired t-test was used to compare the effect of DS (UT vs. 5D) and the effect of IFN-γ subconjunctival injection (5D+BSA vs. 5D+IFN-γ) in each strain (B6 and B6γKO) as well as the effect of B6γKO with DS (B6 5D vs. B6γKO 5D) on AC-caspase-3, -8 and -9 fluorometric intensity, TUNEL-positive cell ratio, caspase-3, -8 and -9 activities, and the results of relative quantitative real-time PCR.
Results
Expression of IFN-γR in Conjunctiva
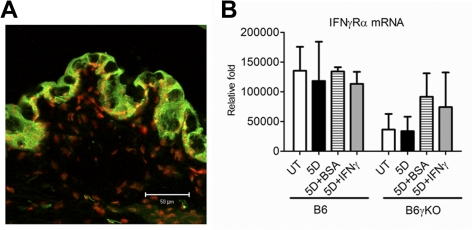
The IFN-γR is composed of two main chains, IFN-γRα and IFN-γRβ. IFN-γRα and IFN-γRβ are concomitantly expressed, representing the ligand binding component and the signal transducing component of IFN-γR, respectively. In this study, we used immunostaining of IFN-γRα to evaluate the expression of IFN-γR in conjunctiva. Cryosections from the untreated B6 wild-type mice were assayed (n = 3). We found that IFN-γRα was expressed in all cell layers of the conjunctival epithelia (Fig. 1A). DS and exogenous IFN-γ administration had no effect on the IFN-γRα mRNA transcripts after 5 days of DS treatment in both strains of mice (Fig. 1B).
Figure 1.
Merged images of IFN-γR (green) immunofluorescence staining for conjunctiva with PI counterstaining (red) in conjunctiva (A). IFN-γRα mRNA transcripts in the conjunctiva after DS and exogenous IFN-γ treatment (B).
DS Increased Apoptosis in Conjunctiva through Activation of the Extrinsic Apoptotic Pathway in B6 Mice
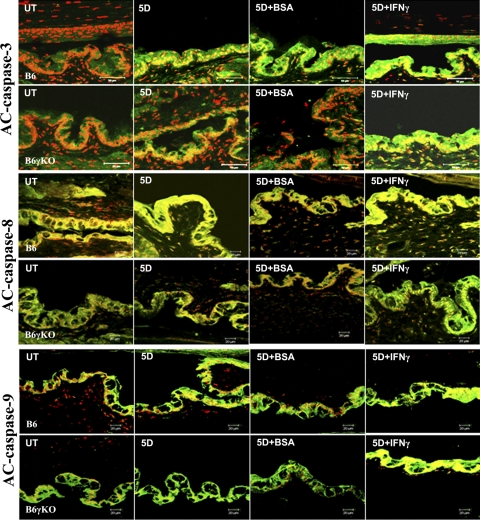
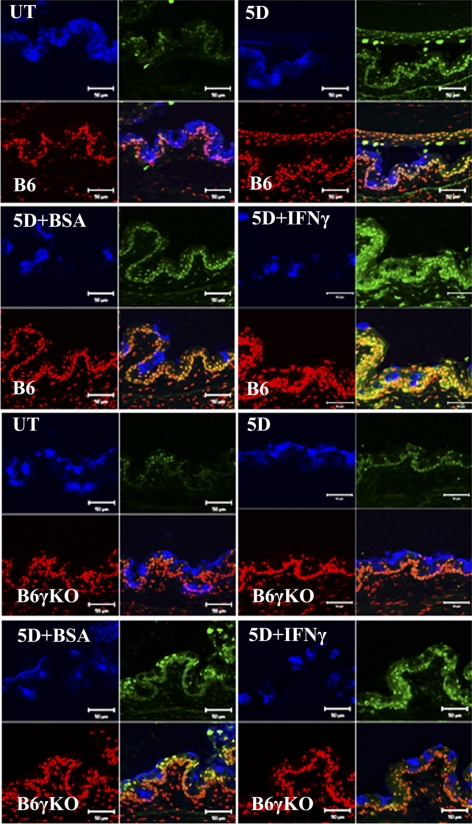
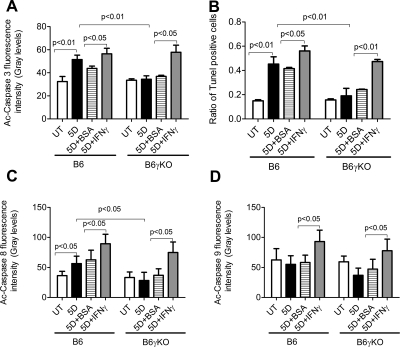
Caspase-3 is activated by upstream caspase-8 and caspase-9, and, because it serves as a convergence point for different signaling pathways, it is well suited as a readout in an apoptosis assay. TUNEL is a method to assay apoptosis at a late stage by detecting DNA fragmentation by labeling the terminal end of nucleic acids. In this study, DS significantly increased Ac-caspase-3 and TUNEL immunoreactivity in conjunctival epithelia in B6 mice (Figs. 2–4, P < 0.05). Significantly increased conjunctival casapse-3 mRNA levels and activity were noted in B6 mice after 5 days of DS treatment (Fig. 5, P < 0.05). In addition, the apoptosis of conjunctival epithelial cells was greatest in the goblet cell area and was accompanied by a decrease in MUC5AC expression after 5 days of DS treatment in B6 mice (Fig. 3).
Figure 2.
Merged images of Ac-caspase-3, -8, and -9 (green) immunofluorescence staining for conjunctiva with PI counterstaining (red).
Figure 3.
Merged images of TUNEL (green) immunofluorescence staining for conjunctiva with MUC5AC (blue) and PI (red) counterstaining.
Figure 4.
Ac-caspase-3 (A), -8 (C), and (D) immunofluorescence intensity and the ratio of TUNEL-positive cells (B) in conjunctival epithelia. Data were shown in mean ± SEM.
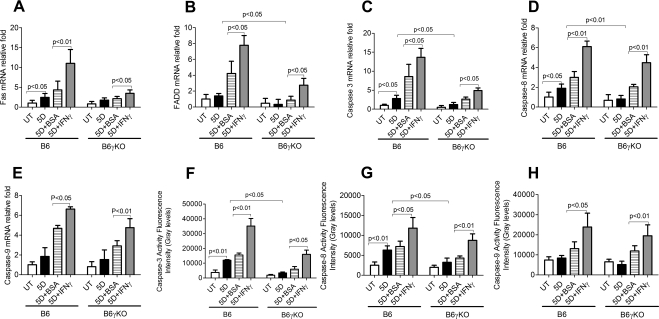
Figure 5.
Relative levels of Fas (A), FADD (B), caspase-3 (C), caspase-8 (D), and caspase-9 (E) mRNA transcripts evaluated by real-time PCR as well as caspase-3 (F), caspase-8 (G), and caspase-9 (H) activities. Data were shown in mean ± SEM.
Because both Ac-caspase-3 and TUNEL are not specific for the apoptotic pathway, we investigated the extrinsic and intrinsic apoptotic pathway mediators using real-time PCR for Fas, FADD, caspase-8 and caspase-9 mRNA levels, immunostaining for AC-caspase-8 and -9 expression, and fluorometric assays for caspase-8 and -9 activity. We found DS significantly increased the levels and activation of the extrinsic apoptotic pathway mediators, as evidenced by increased Fas and caspase-8 mRNA levels (Figs. 5A, 5D), AC-caspase-8 expression (Figs. 2, 5C), and caspase-8 activity (Fig. 5G) in B6 mice (P < 0.05). DS had no effect on caspase-9 mRNA level (Fig. 5E), caspase-9 activity (Fig. 5H), or AC-casapse-9 expression (Fig. 2) in B6 mice (P > 0.05). Taken together, these results indicate that DS increases conjunctival apoptosis through caspase-8–mediated extrinsic apoptotic pathways but not through caspase-9–mediated intrinsic apoptotic pathways in B6 mice.
B6γKO Mice Showed Resistance to DS-Induced Apoptosis in Conjunctiva
To evaluate the role of IFN-γ in apoptosis, we used B6γKO mice to evaluate whether IFN-γ deficiency would modulate DS-induced apoptosis in the conjunctiva. We found that DS has no effect on Ac-caspase-3, -8, and -9, TUNEL immunoreactivity (Figs. 2–4), Fas, FADD, caspase-3, -8, and -9 mRNA levels (Figs. 5A–E), or caspase-3, -8, and -9 activities (Figs. 5F–H) in conjunctiva in B6γKO mice (P > 0.05). B6γKO mice also showed resistance to DS-induced MUC5AC expression loss in conjunctiva (Fig. 3).
Exogenous Administration of IFN-γ Increased DS-Induced Apoptosis in Conjunctiva in Both B6 and B6γKO Mice through Dual Apoptotic Pathways
To further evaluate the role of IFN-γ in conjunctival apoptosis, we performed subconjunctival injection of IFN-γ in both strains of mice. Administration of IFN-γ in B6 mice was used to evaluate whether exogenous IFN-γ would aggravate disease. In B6γKO mice, it was used to evaluate whether DS-induced apoptosis could be induced by IFN-γ. Significantly increased Ac-caspase-3, -8, and -9 and TUNEL immunoreactivity (Figs. 2–4), Fas, FADD, caspase-3, -8, and -9 mRNA levels (Fig. 5), and caspase-3, -8 and -9 activities (Fig. 5) in conjunctiva were noted in the IFN-γ–injected group compared with the BSA-injected group in both strains of mice after 5 days of DS treatment (P < 0.05 for each comparison). IFN-γ–injected mice showed decreased MUC5AC expression compared with BSA-injected mice in both strains after 5 days of DS treatment. These results indicate that IFN-γ plays a pivotal role in exacerbating conjunctival apoptosis through dual apoptotic pathways.
Discussion
This study evaluated the role of the Th-1 cytokine IFN-γ in the pathologic apoptosis that occurs in the conjunctiva in response to DS. In this study, we found DS induces apoptosis in the conjunctival epithelia that expresses the IFN-γR through a caspase-8–mediated extrinsic apoptotic pathway in B6 wild-type mice. By contrast, B6γKO mice showed resistance to DS-induced apoptosis in conjunctiva. Exogenous administration of IFN-γ increased DS-induced apoptosis in the conjunctiva in both B6 and B6γKO mice through dual apoptotic pathways under DS. Taken together, these indicate that IFN-γ plays a pivotal role in exacerbating conjunctival apoptosis in dry eye.
Goblet cell density is a critical parameter that reflects the overall health of the ocular surface.23 These cells synthesize, store, and secrete large gel-forming mucins that lubricate and protect the ocular surface. Previous studies have demonstrated that decreased numbers of mucin-containing goblet cells is characteristic of dry eye.7,24 Although the mechanism leading to goblet cell loss in dry eye is still unknown, evidence suggests that cytokines liberated by the infiltrating T cells may contribute to the disappearance of conjunctival goblet cells in DS. There is an inverse correlation between the number of CD4+ T cells and the number of goblet cells in the conjunctiva.17 Furthermore, an increase in conjunctival goblet cell density has been observed in dry eye patients treated topically with the T-cell immunomodulatory agent cyclosporine A.10,25 In our previous study, we showed that DS had no effect on conjunctival goblet cell density in B6γKO mice; however, exogenous administration of IFN-γ significantly decreased goblet cell density after 5 days of DS.17 In this study, we found that apoptosis was greatest in the goblet cell area and was accompanied by decreased MUC5AC expression in B6 and B6-IFN-γ–injected mice compared with B6γKO and BSA-injected mice. Reconstitution of IFN-γ in B6γKO mice during DS produced changes in MUC5AC expression similar to those of B6 wild-type mice. This evidence suggests that the Th-1 cytokine IFN-γ can promote apoptosis and lead to a loss of goblet cells under DS.
The initiation of apoptosis depends on the type of apoptotic stimuli that can activate one or both of the predominant apoptotic pathways.11 One predominant apoptotic pathway is the extrinsic or “death receptor” pathway of apoptosis that is triggered by the presence of proinflammatory cytokines such as Fas or TNF-related apoptosis-inducing ligand.11 This leads to the recruitment and activation of an adaptor protein, FADD, and to subsequent activation of caspase-8/Flice.11 Fas has been implicated in the induction of apoptosis of several cell types, and its effects are exacerbated by IFN-γ.11,26 In this study, we found that exogenous administration of IFN-γ significantly increased mRNA levels of Fas and FADD in both strains of mice, indicating that IFN-γ may exacerbate conjunctival epithelial apoptosis through the Fas-FADD–mediated extrinsic pathway under DS.
The activation of pro-caspase-8 to active caspase-8 leads to the cleavage of its targets. Caspase-8 cleavage of c-FlipL triggers the processing of c-Flip and facilitates formation of the death-inducing signaling complex.27,28 Caspase-8 can specifically target and cleave the X-linked inhibitor of apoptosis (XIAP), leading to XIAP inactivation.29 Activated caspase-8 cleaves additional downstream caspases, including caspase-3.28 Caspases ultimately elicit the morphologic hallmarks of apoptosis, including cell rounding, cytoskeletal collapse, cytoplasmic condensation, DNA fragmentation, and formation of apoptotic bodies that are rapidly phagocytosed and digested by macrophages or neighboring cells.11 In this study, we found that DS significantly increased caspase-8 expression and activity in B6 mice, whereas it has no such effect in B6γKO mice. Exogenous administration of IFN-γ increased caspase-8 expression and activity in both strains under dry eye. This indicates that caspase-8 may play a key role in dry eye–induced apoptosis, which can be further exacerbated by IFN-γ. Caspase-8 may provide a new target for the treatment of dry eye–induced epithelial disease.
The second predominant apoptotic pathway is the intrinsic or “mitochondrial” pathway.11 Most inducers of the mitochondrial apoptotic pathway inhibit prosurvival proteins such as Bcl2 and Bcl-xL and trigger the depolarization of the mitochondrial membrane potential.29,30 In some cell types, caspase-8 can also induce the cleavage of the proapoptotic protein Bid.31 The truncated form of Bid (t-Bid) has the ability to interact with the mitochondria and induce apoptosis.31 Activation of the mitochondrial apoptotic pathway leads to the release of the mitochondrial protein cytochrome c and the recruitment of caspase-9 to form the apoptosome complex.32 Cleavage and activation of caspase-9 also results in the subsequent activation of effector caspases-3, -6, and -7.31,32 Although DS promoted the migration of IFN-γ+ cells into goblet cell zones of the conjunctiva and increased the concentration of IFN-γ in tears,17 we found that it had no effect on AC-caspase-9 expression and activity in both strains of mice. However, the exogenous administration of IFN-γ increased caspase-9 expression and activity in both strains. These results suggest that IFN-γ–induced apoptosis in conjunctival epithelia through the caspase-9–mediated intrinsic apoptotic pathway may be highly concentration dependent. In addition, whether IFN-γ–induced activation of the intrinsic apoptotic pathway is triggered by Bcl2 and Bcl-xL inhibition or caspase-8 activation requires further investigation.
Although apoptosis is a part of the normal process of epithelial cell renewal, in excess it is pathologic. This study shows that dry eye induces conjunctival apoptosis through the caspase-8–mediated extrinsic pathway and that the Th-1 cytokine IFN-γ can exacerbate conjunctival apoptosis through dual apoptotic pathways in dry eye. The relationship between ocular surface inflammation and apoptosis in dry eye has been the subject of much investigation. Expression of proapoptotic markers (Fas, Fas ligand, APO2.7, CD40, and CD40 ligand) by the conjunctival epithelium in dry eye has been found to positively correlate with the expression of HLA-DR class II antigen, an immune activation marker in dry eye patients.33 After 6 months of therapy with cyclosporine A, the levels of cell membrane markers for apoptosis (i.e., Fas) and inflammation, such as HLA-DR, were significantly reduced.34 Although it is still to be determined whether apoptosis is a result or a cause of ocular inflammation in dry eye disease, this study provides evidence that the Th-1 cytokine IFN-γ plays a significant role in the pathologic apoptosis in the conjunctiva that develops in dry eye, characterized by increased Th-1 and Th-17 CD4+ T cell infiltration on the ocular surface. Our study provides new insight that immune-based ocular surface inflammation in dry eye promotes apoptosis of the tear-secreting ocular surface epithelia.
Footnotes
Supported by National Institutes of Health Grant EY11915 (SCP), Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
Disclosure: X. Zhang, None; W. Chen, None; C.S. De Paiva, None; R.M. Corrales, None; E.A. Volpe, None; A.J. McClellan, None; W.J. Farley, None; D.-Q. Li, None; S.C. Pflugfelder, None
References
- 1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326 [DOI] [PubMed] [Google Scholar]
- 2. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren's syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957 [DOI] [PubMed] [Google Scholar]
- 3. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589 [DOI] [PubMed] [Google Scholar]
- 4. de Paiva CS, Hwang CS, Pitcher JD, III, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2010;49:246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoon KC, de Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569 [DOI] [PubMed] [Google Scholar]
- 6. de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen W, Zhang X, Zhang J, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci. 2008;49:1386–1391 [DOI] [PubMed] [Google Scholar]
- 8. Chen W, Zhang X, Liu M, et al. Trehalose protects against ocular surface disorders in experimental murine dry eye through suppression of apoptosis. Exp Eye Res. 2009;89:311–318 [DOI] [PubMed] [Google Scholar]
- 9. Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–129 [DOI] [PubMed] [Google Scholar]
- 10. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005;24:80–85 [DOI] [PubMed] [Google Scholar]
- 11. Chawla-Sarkar M, Lindner DJ, Liu YF, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249 [DOI] [PubMed] [Google Scholar]
- 12. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614 [PubMed] [Google Scholar]
- 13. Rice BA, Foster CS. Immunopathology of cicatricial pemphigoid affecting the conjunctiva. Ophthalmology. 1990;97:1476–1483 [DOI] [PubMed] [Google Scholar]
- 14. Caproni M, Torchia D, Schincaglia E, et al. The CD40/CD40 ligand system is expressed in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis spectrum. Br J Dermatol. 2006;154:319–324 [DOI] [PubMed] [Google Scholar]
- 15. Rojas B, Cuhna R, Zafirakis P, et al. Cell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81:313–325 [DOI] [PubMed] [Google Scholar]
- 16. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795 [DOI] [PubMed] [Google Scholar]
- 17. de Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007;48:2553–2560 [DOI] [PubMed] [Google Scholar]
- 18. Abu-Helu RF, Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. Induction of salivary gland epithelial cell injury in Sjogren's syndrome: in vitro assessment of T cell-derived cytokines and Fas protein expression. J Autoimmun. 2001;17:141–153 [DOI] [PubMed] [Google Scholar]
- 19. Wen LP, Madani K, Fahrni JA, Duncan SR, Rosen GD. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol. 1997;273:L921–L929 [DOI] [PubMed] [Google Scholar]
- 20. Trautmann A, Kruger K, Akdis M, et al. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol. 2005;138:142–150 [DOI] [PubMed] [Google Scholar]
- 21. Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301 [DOI] [PubMed] [Google Scholar]
- 22. Lowe B, Avila HA, Bloom FR, Gleeson M, Kusser W. Quantitation of gene expression in neural precursors by reverse-transcription polymerase chain reaction using self-quenched, fluorogenic primers. Anal Biochem. 2003;315:95–105 [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease: a better indicator than tear mucin. Arch Ophthalmol. 1983;101:1284–1287 [DOI] [PubMed] [Google Scholar]
- 24. Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14:299–302 [PubMed] [Google Scholar]
- 25. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–337 [DOI] [PubMed] [Google Scholar]
- 26. Ahn EY, Pan G, Vickers SM, McDonald JM. IFN-gamma upregulates apoptosis-related molecules and enhances Fas-mediated apoptosis in human cholangiocarcinoma. Int J Cancer. 2002;100:445–451 [DOI] [PubMed] [Google Scholar]
- 27. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304 [DOI] [PubMed] [Google Scholar]
- 28. Ping L, Ogawa N, Sugai S. Novel role of CD40 in Fas-dependent apoptosis of cultured salivary epithelial cells from patients with Sjogren's syndrome. Arthritis Rheum. 2005;52:573–581 [DOI] [PubMed] [Google Scholar]
- 29. Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060 [DOI] [PubMed] [Google Scholar]
- 30. Hawkins CJ, Vaux DL. Analysis of the role of bcl-2 in apoptosis. Immunol Rev. 1994;142:127–139 [DOI] [PubMed] [Google Scholar]
- 31. Ridley SH, Sarsfield SJ, Lee JC, et al. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165–3173 [PubMed] [Google Scholar]
- 32. Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132 [DOI] [PubMed] [Google Scholar]
- 33. Brignole F, Pisella PJ, Goldschild M, De Saint JM, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41:1356–1363 [PubMed] [Google Scholar]
- 34. Brignole F, Pisella PJ, De Saint JM, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42:90–95 [PubMed] [Google Scholar]