Our studies indicate that ethyl pyruvate prevents lipopolysaccharide-induced nuclear factor kappa B–dependent ocular inflammatory signals in cultured cells and in rat eyes. Therefore, supplementation of ethyl pyruvate could be a novel approach to control ocular inflammation, especially uveitis.
Abstract
Purpose.
Recent studies indicate that ethyl pyruvate (EP) exerts anti-inflammatory properties; however, the effect of EP on ocular inflammation is not known. The efficacy of EP in endotoxin-induced uveitis (EIU) in rats was investigated.
Methods.
EIU in Lewis rats was developed by the subcutaneous injection of lipopolysaccharide (LPS; 150 μg). EP (30 mg/kg body weight) or its carrier was injected intraperitoneally 1 hour before or 2 hours after lipopolysaccharide injection. Animals were killed after 3 and 24 hours followed by enucleation of eyes and collection of the aqueous humor (AqH). The number of infiltrating cells and levels of proteins in the AqH were determined. The rat cytokine/chemokine multiplex method was used to determine level of cytokines and chemokines in the AqH. TNF-α and phospho-nuclear factor kappa B (NF-κB) expression in ocular tissues were determined immunohistochemically. Human primary nonpigmented ciliary epithelial cells (HNPECs) were used to determine the in vitro efficacy of EP on lipopolysaccharide-induced inflammatory response.
Results.
Compared to controls, AqH from the EIU rat eyes had a significantly higher number of infiltrating cells, total protein, and inflammatory cytokines/chemokines, and the treatment of EP prevented EIU-induced increases. In addition, EP also prevented the expression of TNF-α and activation of NF-κB in the ciliary bodies and retina of the eye. Moreover, in HNPECs, EP inhibited lipopolysaccharide-induced activation of NF-κB and expression of Cox-2, inducible nitric oxide synthase, and TNF-α.
Conclusions.
Our results indicate that EP prevents ocular inflammation in EIU, suggesting that the supplementation of EP could be a novel approach for the treatment of ocular inflammation, specifically uveitis.
Ethyl pyruvate (EP) is a membrane-permeant aliphatic ester derived from the endogenous metabolite pyruvic acid (Fig. 1). Exogenous EP has the potential to augment intracellular pyruvate levels, which enable the cells to protect themselves from reactive oxygen species (ROS)-mediated damage.1–3 However, several studies have shown that EP as an intact ester also has direct pharmacologic effects, which have been recently reviewed by Kao and Fink.4 The pharmacologic effects of EP are quite diverse, and include downregulation of the secretion of proinflammatory cytokines, enhanced antitumor immunity, amelioration of redox-mediated damage to cells and tissues, inhibition or promotion of apoptosis based on the circumstances, and support of cellular adenosine triphosphate synthesis. Recently, a number of studies have shown that EP is a robust and potent anti-inflammatory agent in pathologic conditions, such as hemorrhagic shock, sepsis, systemic inflammation, colitis, pancreatitis, and pulmonary fibrosis.5–13 These reports indicate that the anti-inflammatory effects of EP are caused by downregulation of the proinflammatory transcription factor nuclear factor kappa B (NF-κB) and the expression of NF-κB–dependent proinflammatory marker proteins, such as TNF-α, IL-6, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS).5,6 In addition, a few studies indicate its potential in preventing sugar and oxidative stress–induced cataract formation and toxicity to some of the ocular tissues.14–16 Although the role of EP in various inflammatory conditions has been examined, its potential anti-inflammatory role in the prevention of ocular inflammation has never been explored.
Figure 1.
Structure of ethyl pyruvate.
Inflammation has been implicated in pathogenic mechanisms in various vision-threatening ocular diseases, such as uveitis,17 diabetic retinopathy,18 and AMD.19 Among all ocular inflammatory diseases, uveitis is considered as a potent, blindness causing intraocular condition.20,21 So far, the etiology of the uveitis is yet to be understood. Nevertheless, autoimmune disorders, infections, exposure to toxins, and many other unknown factors are believed to cause uveitis.17 A potential common pharmacologic strategy against uveitis is likely to contain suppression and regulation of intraocular inflammation. Various chemical mediators play key roles in ocular inflammation, particularly uveitis. The key inflammatory molecules include cytokines IL-622 and TNF-α,23 the arachidonic acid cascade enzyme COX-224 and its major metabolite prostaglandin E2,25 monocyte chemotactic protein (MCP)-1,26 angiotensin II type 1 receptor,27,28 intracellular signaling pathways Janus kinase and signal transducer and activator of transcription (JAK/STAT)22,27 and IκB/NF-κB,29 and intercellular adhesion molecule (ICAM)-1.30 A number of antioxidants that can prevent the NF-κB–dependent expression of inflammatory cytokines have been shown to have potent antiocular inflammatory actions.31 Antioxidants like benfotiamine, guggulsterone, curcumin, lutein, and reservatrol have been shown to prevent endotoxin-induced uveitis (EIU) in experimental animals.31 EP has now well established as an antioxidant with potent anti-inflammatory agent in various nonocular conditions. However, the efficacy of EP in ocular inflammatory pathologic conditions such as uveitis is not known. We investigated the efficacy of EP in preventing ocular inflammation in rats. Our results indicate that supplementation of EP prevents lipopolysaccharide (LPS)-induced ocular inflammation leading to uveitis in rats.
Materials and Methods
Materials
EP and LPS (Escherichia coli 0111:B4 strain) were purchased from Sigma-Aldrich (Saint Louis, MO). Human primary nonpigmented ciliary epithelial cells (HNPECs) and culture media were obtained from ScienCell Research Laboratories (Carlsbad, CA). TNF-α ELISA kit was purchased from BD Biosciences (San Diego, CA). The MILLIPLEX MAG rat cytokine/chemokine magnetic bead panel along with Luminex xMAP detection method was purchased from Millipore Corporation (Billerica, MA). Rabbit polyclonal iNOS, goat polyclonal Cox-2, rabbit polyclonal phospho-p65 (Ser 536), mouse monoclonal p65 (F-6), goat polyclonal TNF-α, and mouse monoclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH; A-3) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). All other reagents obtained from Sigma-Aldrich were of highest purity grade.
Animals
The handling, treatment, and procedures on animals were carried out according to the ARVO Statement for the use of Animals in Ophthalmic and Vision Research. Adult male Lewis rats (8–10 weeks old, 150 to 200 g) were purchased and kept in 12-hour light/12-hour dark cycles for 3 days to acclimatize in the animal house at the University of Texas Medical Branch, Galveston. Rats were randomly divided into five groups (n = 6). LPS was injected subcutaneously (150 μg/kg body weight) to induce uveitis as described previously.32 The intraperitoneal injection of EP (30 mg/kg body weight) was given 1 hour before and 2 hours after LPS injection. For the dose-dependent study, three different doses of EP (15, 30, and 60 mg/kg body weight) were administered 1 hour before LPS induction. Rats in the control group were injected with the vehicle (Ringer's lactate solution) only. The animals were killed at 3 and 24 hours after LPS injection. The aqueous humor (AqH) was collected from some of the eyes immediately by an anterior chamber puncture with a 30-gauge needle under a surgical microscope. After determination of the number of infiltrating cells and protein concentration in AqH, the samples were kept at −80°C until further use. The rest of the eyes were transferred immediately into 4% paraformaldehyde for immunohistochemical analyses.
Determination of Infiltrating Cells and Total Proteins in AqH
The AqHs were diluted in an equal amount of Trypan blue solution followed by infiltrating cell counting under the light microscope using hemocytometer. The total protein concentration in the AqH samples was measured with a colorimetric protein assay kit (Bio-Rad, Hercules, CA).
Determination of Cytokines/Chemokines in AqH
The levels of cytokines/chemokines in the AqH were determined by the MILLIPLEX MAG rat cytokine/chemokine magnetic bead panel along with Luminex xMAP detection method as per manufacturer's protocol. The results were expressed in picograms per milliliter.
Paraffin Embedding of Eyes
The enucleated eyes from the rats were fixed in 4% paraformaldehyde for 24 hours. After fixing, the eyes were washed in ice cold PBS (3×) and immediately transferred in 70%, 90%, and 100% reagent histologic grade alcohol for 24 hours each, followed by embedding in paraffin. Sagittal sections of 5 μm were cut and used for histologic analysis.
Immunofluorescence Studies
The eye sections were warmed at 60°C for 1 hour in the oven, deparaffinized in xylene, rehydrated by passing through 100%, 95%, 80%, and 70% reagent alcohol, and washed with deionized water. Heat-induced epitope recovery was used as sections were submerged in 250 mL of 1× Antigen Retrieval Citrate Buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0) and steam-heated in a standard steamer (food model, Black & Decker) for 15 minutes After the antigen retrieval, the sections were rinsed in PBS twice and incubated with blocking buffer (2% BSA, 0.1% Triton X-100, 2% normal rabbit immunoglobulin G or 2% normal goat serum) overnight at 4°C. The sections were incubated with primary antibodies against TNF-α or phospho-p65 antibodies (dilution 1:200) for overnight and washed with PBS (3 × 5 minutes each). The sections were incubated in respective Alexa Fluor-488 or Alexa Fluor-594 secondary antibodies (dilution 1:200) for 1 hour at room temperature followed by washing with PBS (3 × 5 minutes each). The sections were mounted with Vectashield mounting media containing 4′,6-diamidino-2-phenylindole and covered with a coverslip. The sections were examined by fluorescent microscopy (EPI-800 microscope; Nikon, Tokyo, Japan) and photographed with a digital camera (Olympus) fitted to the microscope.
In Vitro Cell Culture Study
Primary HNPECs were cultured per the supplier's protocol using the epithelial cell media containing basal medium, antibiotics, epithelial cell growth supplement, and fetal bovine serum. The cells were grown in a humidified incubator at 37°C and 5% CO2. All incubations were performed in the serum-free media. The cells were pretreated with 40 μM of EP for 30 minutes and subsequently stimulated with 1 μg/mL LPS for various time intervals as stated in the figure legends.
Western Blot Analysis
After the treatment, HNPECs were washed twice with ice cold PBS and lysed in radioimmunoprecipitation assay buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1:100 dilution of protease inhibitor cocktail (Sigma-Aldrich). The protein levels were measured from the supernatant and aliquots were diluted with 2× SDS sample buffer and boiled for 5 minutes The lysates were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). The membranes were then incubated in blocking solution containing 5% weight per volume dried fat-free milk and 0.1% vol/vol Tween-20 in Tris-buffered saline. Subsequently, the membranes were incubated with anti-iNOS, -Cox-2, -phospho-p65, -p65 and -GAPDH antibodies. The membranes were then probed with horseradish peroxidase–conjugated secondary antibody (GE Health Care, Piscataway, NJ) and visualized by chemiluminescence (Pierce Biotechnology, Rockford, IL). To determine translocation of NF-κB from the cytoplasm to the nucleus, HNPECs were treated for different time intervals followed by extraction of cytoplasmic and nuclear proteins per the manufacturer's protocol using a nuclear extraction kit (Cayman Chemicals, Ann Arbor, MI).
Reactive Oxygen Species Measurement
The HNPECs were plated (50,000 cells per well) in a 24-well plate. Serum starved HNPECs were incubated with various concentrations (10, 20, and 40 μM) of EP for 1 hour 5 μg per milliliter followed by stimulation with LPS for an additional hour. Cells incubated without LPS and EP served as control. After incubation, cells were washed with PBS three times and 10 μM H2DCFDA dye was added and incubated for 30 minutes at 37°C. Fluorescence was measured at 485 nm excitation and 535 nm emission using a microplate reader (BioTek, Winooski, VT).
Measurement of TNF-α
The level of TNF-α in the culture media (stored at −80°C) was assessed with a commercially available ELISA kit according to the manufacturer's instructions.
Cell Viability Assay
HNPECs were plated (10,000 cells/well) in a 96-well plate. After 24 hours, cells were serum starved in 0.1% FBS medium and 5 μg/mL LPS was added to the media for 24 hours after pretreating the cells with 40 μM EP for 1 hour. Cells incubated without LPS and EP served as control. Cell viability was determined by CellTiter 96 AQueous one solution cell proliferation assay kit. CellTiter reagent was added to the culture well, incubated for 3 hours, and absorbance was recorded at 490 nm using a 96-well micro plate reader (BioTek).
Statistical Analysis
Data are expressed as the mean ± SD. One-way ANOVA was used to compare inflammatory markers. P < 0.05 was considered statistically significant.
Results
EP Suppresses LPS-Induced Inflammatory Cell Infiltration and Protein Concentration in Rat AqH
Because increased infiltration of inflammatory cells in AqH is hallmark of uveitis, at first we have examined the efficacy of EP, injected before LPS induction, in preventing the infiltration of cells in AqH. At 24 hours after LPS injection, the number of infiltrating cells in AqH was quantified by manually counting the cells with a hemocytometer under a stereoscope microscope. The manual cell counting revealed a significant (∼160-fold) LPS-induced increase in the AqH infiltration of the inflammatory cells, which were significantly (>70%) reduced by EP (Fig. 2B). There were no significant infiltrated cells in the control or EP-treated groups (Fig. 2B). Moreover, the total protein concentration in the AqH of LPS group rat eyes was also increased significantly (∼25-fold) compared to control group. However, EP significantly suppressed (∼60%) elevation in protein concentration in the AqH (Fig. 2C). Similarly, a significant reduction of infiltrating cells (>60%) and protein levels (∼45%) were also observed in the AqH of EIU plus EP postinjection (2 hours after LPS) rat eyes (Figs. 2D, 2E). In addition, EP-rendered protection was also observed in a dose-dependent manner (Figs. 2F, 2G). The lowest dose of EP (15 mg/kg body weight) partially suppressed the infiltrating cells and protein levels in the AqH and was not statistically significant. However, higher EP dosages (30 and 60 mg/kg body weight) significantly (P < 0.001) suppressed infiltrating cells and protein levels in EIU rat eye AqH (Figs. 2F, 2G). Our studies also indicate that 30 mg per kg body weight of EP caused significant suppression of EIU, which is almost similar to 60 mg per kg body weight, indicating that in rats, 30 mg per kg body weight of EP is the optimal dose required to significantly prevent LPS-induced ocular inflammation. These results indicate a preventive role of EP in LPS-induced inflammatory cell infiltration in the aqueous chamber and an elevation of inflammatory proteins in the AqH of rat eyes.
Figure 2.
EP prevents LPS-induced inflammatory cell infiltration and increase in protein concentration in rat AqH. (A–C) LPS was injected in rats pretreated (1 hour before LPS injection) without or with EP (30 mg/kg body weight) to induce uveitis. (A) Serial sections of paraformaldehyde-fixed rat eyes enucleated 24 hours after EIU-induction were stained with hematoxylin–eosin and were observed under a light microscope to examine the infiltration of inflammatory cells in the anterior chamber. (B) The infiltrated inflammatory cells and (C) total protein concentration in the rat AqH at 24 hours. (D and E) Uveitis was induced in rats by injecting with LPS followed by treatment (2 hours after LPS injection) without or with EP (30 mg/kg body weight). (D) The infiltrated inflammatory cells and (E) total protein concentration in the rat AqH at 24 hours. (F and G) LPS was injected in rats pretreated (1 hour before LPS) without or with EP (0, 15, 30, and 60 mg/kg body weight) to examine the dose-dependent effects of EP on EIU. (F) The infiltrated inflammatory cells and (G) total protein concentration in the rat AqH at 24 hours. Trypan blue exclusion cell counting method was used to determine number of infiltrating cells, and the Bradford method was used to measure total protein concentration. Results are expressed as the mean ± SD (n = 6). *P < 0.001 vs. the control group; **P < 0.01 vs. the EIU group. Magnification: (A), ×200.
Effect of EP on LPS-Induced Inflammatory Cytokines and Chemokines in Rat AqH
Because increased protein levels in the AqH are related to the increased breach of inflammatory cytokines and chemokines, we next determined the levels of various inflammatory cytokines and chemokines in rat AqH. A magnetic bead array–based rat specific inflammation kit was used to measure 23 inflammatory marker proteins from a single sample. The results obtained were analyzed by 3.1 xPONENT software and expressed in picograms per milliliter. As shown in Figure 3, in EIU rat AqH a significant (P < 0.001 or P < 0.01) increase in the levels of inflammatory proteins such as growth-related oncogene/KC, IL-1β, IL-6, IL-18, leptin, MCP-1, MIP-1α, RANTES, and TNF-α were observed. However, pretreatment with EP suppressed the LPS-induced secretion of cytokines and chemokines significantly (P < 0.001 or P < 0.01) in the AqH of rat eyes (Fig. 3). In addition, no significant changes in the levels of granulocyte colony-stimulating factor, granulocyte-macrophage colony stimulating factor, IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IL-17, interferon gamma-induced protein 10 kDa, and VEGF were observed in the LPS and EP-treated rat eye AqH (data not shown).
Figure 3.
EP Inhibits LPS-induced increase in cytokines and chemokines in EIU eye AqH. Inflammatory cytokine and chemokine levels in the AqH collected 24 hours after LPS injection were measured by using the MILLIPLEX MAG rat cytokine/chemokine magnetic bead panel as described in the methods. Results are expressed as the mean ± SE (n = 3; AqH was pooled from 2 rats for each data point); *P < 0.001 or **P < 0.01 vs. the control group; *P < 0.001 or **P < 0.01 vs. EIU group. The results were analyzed and expressed in picograms per milliliter.
Effect of EP on LPS-Induced Expression of TNF-α in the Rat Ocular Tissues
Because EP significantly suppressed the elevation in the levels of various inflammatory markers, we next determined the effect of EP on the expression of major proinflammatory cytokine TNF-α in the ocular tissues of EIU rat eyes. As shown in Figure 4, EIU caused an increased expression of TNF-α in the ciliary body and the retina which was significantly suppressed by EP.
Figure 4.
EP suppresses the expression of TNF-α in ocular tissues of EIU rats. Serial sections of paraformaldehyde-fixed rat eyes enucleated 24 hours after EIU were immunostained with DAPI and antibodies against TNF-α. The DAPI and antibody staining intensity was observed by fluorescent microscope. Representative images are shown (n = 4). C, Control; EP, ethyl pyruvate; EIU, endotoxin-induced uveitis; EIU + EP, endotoxin-induced uveitis + ethyl pyruvate; IMNC, isotype-matched negative control. Magnification: ×400.
Effect of EP on Endotoxin-Induced NF-κB Activation in the EIU Rat Ocular Tissues
The transcription factor NF-κB is well known to transcribe the genes responsible for various inflammatory markers. Therefore, we next investigated the effect of EP on endotoxin-induced activation of NF-κB in the rat eye tissues. The paraffin sections were probed with antibodies against phospho-p65 (the active subunit of NF-κB). After 3 hours of LPS-induction, phospho-NF-κB–positive cells were observed in the iris–ciliary body complex in the anterior region and the retina in the posterior region of the eye (Fig. 5). However, pretreatment of EP significantly suppressed the LPS-induced increase in the number of phospho-NF-κB–positive cells in the anterior and posterior regions of EIU rat eyes (Fig. 5).
Figure 5.
EP suppresses the activation of NF-κB in ocular tissues of LPS-induced EIU. Serial sections of paraformaldehyde-fixed rat eyes enucleated 3 hours after EIU induction were immunostained with DAPI and antibodies against phospho-p65. The DAPI and antibody staining intensity was observed by fluorescent microscope. Representative images are shown (n = 4). C, Control; EP, ethyl pyruvate; EIU, endotoxin-induced uveitis; EIU + EP, endotoxin-induced uveitis + ethyl pyruvate; IMNC, isotype-matched negative control. Magnification: ×400.
Effect of EP on LPS-Induced Inflammatory Response in HNPECs
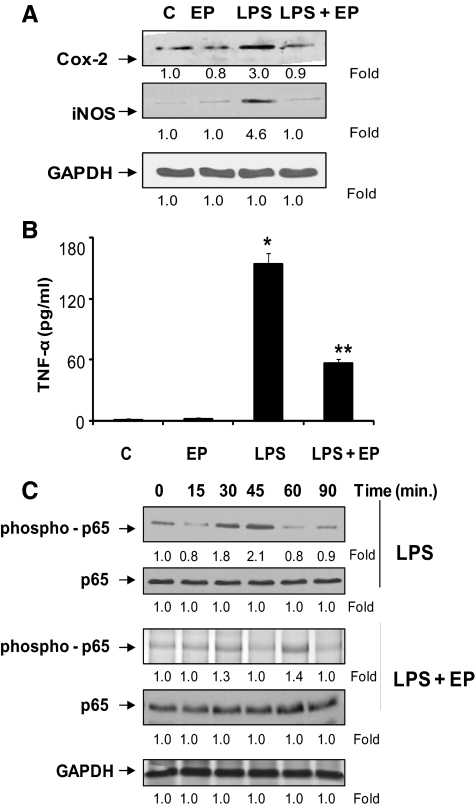
Ocular ciliary epithelial cells are a major source of inflammatory markers secreted into the AqH during uveitis. We used HNPECs as an in vitro model to study the efficacy of EP as an anti-inflammatory agent. When HNPECs were stimulated with LPS, there was ∼3.0- and ∼4.6-fold increase in the expression of inflammatory Cox-2 and iNOS proteins, respectively (Fig. 6A). The increased inflammatory response of HNPECs was also confirmed by determining the levels of TNF-α in the culture media. There was a significant (P < 0.01) increase in the level of TNF-α in culture media on LPS treatment compared to the control group (Fig. 6B). However, EP significantly suppressed the expression of Cox-2 and iNOS in HNPECs and the secretion of TNF-α level in culture media (Figs. 6A, 6B). Because the transcription factor NF-κB is known to transcribe inflammatory markers such as TNF-α, Cox-2, and iNOS, we next examined the effect of EP on LPS-induced NF-κB activation in HNPECs. Results shown in Figure 6C indicate that LPS caused a time-dependent activation of NF-κB and preincubation of EP prevented the LPS-induced increase in the NF-κB activation. These results suggest that EP prevents the expression of inflammatory markers by suppressing the activation of NF-κB in HNPECs. We next examined the effect of EP on LPS-induced translocation of NF-κB from the cytoplasm to the nucleus (Fig. 7A). Our results shown in Figure 7A indicate that EP significantly prevented LPS-induced nuclear translocation of NF-κB. Because NF-κB has been shown to be a redox sensitive transcription factor, we next examined the efficacy of EP in the prevention of LPS-induced ROS formation in HNPECs. Our results shown in Figure 7B indicate that EP prevents LPS-induced ROS levels. In addition, we have also examined the efficacy of EP in preventing LPS-induced HNPEC cell death. The results shown in Figure 7C show that EP significantly prevented LPS-induced cell death in HNPECs.
Figure 6.
EP prevents inflammatory response in HNPECs stimulated with LPS. (A) Growth-arrested HNPECs pretreated without or with EP (40 μM) were incubated with 1 μg/mL of LPS for 24 hours. The expression of iNOS and Cox-2 from cell lysates was determined by Western blot analysis using specific antibodies. (B) The levels of TNF-α in the culture media was determined with ELISA kit. Data are expressed as the mean ± SD (n = 6). *P < 0.001 vs. the control group; **P < 0.01 vs. the LPS group. (C) Growth-arrested HNPECs pretreated with or without EP (40 μM) were incubated with 1 μg/mL of LPS for 0 to 90 minutes. The expression levels of phospho- and total-p65 were determined by Western blot analysis using specific antibodies.
Figure 7.
EP prevents LPS-induced nuclear translocation of p65, ROS generation and HNPECs death. (A) Growth-arrested HNPECs pretreated with or without EP (40 μM) were incubated with 1 μg/mL of LPS for 0 to 60 minutes The translocation of p65 from cytoplasm to nucleus was determined by Western blot analysis using specific antibodies. (B) Growth-arrested HNPECs pretreated with or without EP (10, 20, and 40 μM) were incubated with 5 μg/mL of LPS for 1 hour. The ROS measurement was performed using H2DCFDA dye. (C) Growth-arrested HNPECs pretreated with or without EP (40 μM) were incubated with 5 μg/mL of LPS for 24 hours. The cell viability was determined by MTT assay. Data are expressed as the mean ± SD (n = 4). *P < 0.001 vs. the control group; **P < 0.01 vs. the LPS group. EP, Ethyl pyruvate.
Discussion
As a product of glycolysis and the starting substrate of the Kreb's cycle, pyruvate plays a key role in intermediary metabolism. It is also an important endogenous scavenger of ROS such as H2O2, OH., and peroxynitrite. Therefore, pyruvate has been studied extensively as a protective agent for cellular systems exposed to oxidative stress conditions in a variety of in vitro and in vivo models of diverse pathologic conditions.4 However, the use of pyruvate as a potential therapeutic agent is limited because of its poor stability in aqueous solutions. Therefore, a closely related and more aqueous stable form of pyruvate is warranted. Recently, an ester form of pyruvate (EP) believed to be more stable than an anionic form has been developed. Several studies revealed that EP is more effective in ameliorating oxidative stress-induced injuries or tissue alterations than pyruvate itself.14,15,33 Moreover, four crucial findings by Ulloa et al.7 have established EP as an excellent anti-inflammatory agent. These are: (1) EP inhibits the LPS-induced secretion of the proinflammatory TNF-α in a concentration-dependent fashion. (2) Treatment with EP improves survival in mice challenged with a lethal dose of LPS or rendered septic by cecal ligation and perforation (CLP). Importantly, the therapeutic benefit of EP is evident in the CLP model of polymicrobial sepsis even though it injected 24 hours after the onset of infection. (3) EP inhibits secretion of the proinflammatory DNA-binding protein, high mobility group box 1 (HMGB1) in vitro and in vivo conditions. (4) EP attenuates the activation of two key proinflammatory signaling pathways, NF-κB and p38 mitogen-activated protein kinase (MAPK). These earlier findings by Ulloa et al.7 have been confirmed and extended by numerous follow-up studies.8–13,34 However, the therapeutic efficacy of EP in ocular diseases has not been explored extensively. Recently, few studies reported that EP could prevent corneal wound healing,35 oxidative damage to lens and sugar and galactose cataracts in rodents.14–16,36 Moreover, in rats, the instillation of 5% EP (50 g/L or 430 mM) is well tolerated over a 40-day experimental period, and penetration of EP through the cornea is rapid, with pyruvate concentration in the AqH reaching 7 mM after 15 minutes.16 Similarly, topical administration of EP in mouse eyes caused an increase in the levels of pyruvate in the AqH and in the lens, the peak concentrations being 4.7 and 3.6 mM, respectively.37 These studies suggest that EP could also be an efficient therapeutic agent for the intervention of other inflammatory ocular conditions.
The present study using an EIU model revealed that endotoxin-induced infiltration of the inflammatory cells, an increase in the protein concentration, and the levels of various inflammatory cytokines/chemokines in the rat eye AqH were significantly inhibited by EP. The underlying mechanism for an anti-inflammatory role of EP has been shown to be primarily through the inhibition of redox sensitive transcription factor NF-κB. In an in vitro model using RAW 264.7 cells, Han et al.38 showed that EP could inhibit the NF-κB pathway through direct modification of p65. Moreover, their results suggest that EP inhibits DNA binding by covalently modifying p65 at Cys.38 The modification of the sulfhydryl-containing compound by EP has been shown by others as well.39 Similarly, in an in vivo murine model of hemorrhagic shock, Yang et al.5 showed that resuscitation with a solution containing EP downregulated activation of the proinflammatory transcription factor NF-κB and the expression of several proinflammatory proteins, such as TNF-α, IL-6, Cox-2, and iNOS in the liver and intestinal mucosa. Consistent with these previous in vitro and in vivo studies, our study also shows that EP inhibits the activation of NF-κB in vivo in the EIU model and in vitro cell culture model.
Moreover, EP was also observed to inhibit expression of iNOS and Cox-2 in HNPECs. These results suggest that EP prevents the NF-κB–dependent expression of iNOS and Cox-2 and therefore production of nitric oxide (NO) and prostaglandin (PGE2). Our results are in agreement with previous studies, which showed the suppression of iNOS and Cox-2 expression and subsequent NO and PGE2 production by EP in vitro and in vivo.38,40 Elevated levels of NO have been detected in the AqH in uveitis patients with Behçet's disease.41 Moreover, the iNOS inhibitor NG-nitro-L-arginine (L-NAME) prevents the development of uveitis by inhibiting iNOS activity.42 Further, NO could activate Cox enzymes followed by a marked increase in the PGE2 production.43 Because ciliary epithelial cells are known to maintain AqH homeostasis and are considered a major source of inflammatory marker release in AqH,44 we have also investigated the mechanism of EP in LPS-induced inflammatory response in HNPECs in vitro. Our results suggest that EP significantly suppressed the NF-κB dependent expression of iNOS and Cox-2 in HNPECs. EP also suppressed the LPS-induced elevated levels of TNF-α in the culture media.45 Our previous studies and other investigators have shown increased levels of TNF-α in the AqH and an elevated expression in ocular tissues in EIU model45–48 and in eyes of patients with Behçet's disease.49 The crucial role of TNF-α in the ocular pathologies has been further substantiated by diminished inflammation in TNF receptor–deficient mice in immune complex–induced uveitis.48 In the present study, EP decreased TNF-α levels induced by LPS in vitro and in vivo.
The expression of inflammatory markers, such as iNOS, Cox-2, and TNF-α, warrants activation of NF-κB50 which have been demonstrated in various ocular tissues of uveitis models.46,47 LPS and TNF-α have been shown to activate NF-κB in mouse lens and human lens epithelial cells and HNPECs, respectively.51,52 Since we have observed that EP suppressed iNOS, Cox-2, and TNF-α in this study, we have investigated upstream signaling events in EIU rats, such as phosphorylation of NF-κB. Our results show that EP suppresses NF-κB phosphorylation and thereby activation in HNPECs on LPS-stimulation in a ROS-dependent manner. Our results are in agreement with others who have shown that the treatment with EP prevents activation of NF-κB.5,38 In summary, our results indicate that EP supplementation prevents the ocular inflammatory response evoked by LPS in cultured cells and in the rat model of EIU.
Footnotes
Supported in part by National Institutes of Health Grants EY018591 and GM71036 (KVR) and Wilkin AMD Fund and Research to Prevent Blindness (FJGMV).
Disclosure: N.M. Kalariya, None; A.B.M. Reddy, None; N.H. Ansari, None; F.J.G.M. VanKuijk, None; K.V. Ramana, None.
References
- 1. Holleman MAF. Notice sur l'action de l'eau oxygénée sur les acétoniques et sur le dicétones. Recl Trav Chim Pays-bas Belg. 1904;23:169–171 [Google Scholar]
- 2. Ervens B, Gligorovski S, Herrmann H. Temperature dependent rate constants for hydroxyl radical reactions with organic compounds in aqueous solutions. Phys Chem Chem Phys. 2003;5:1811–1824 [Google Scholar]
- 3. Varma SD, Hegde KR. Lens thiol depletion by peroxynitrite. Protective effect of pyruvate. Mol Cell Biochem. 2007;298:199–204 [DOI] [PubMed] [Google Scholar]
- 4. Kao KK, Fink MP. The biochemical basis for the anti-inflammatory and cytoprotective actions of ethyl pyruvate and related compounds. Biochem Pharmacol. 2010;80:151–159 [DOI] [PubMed] [Google Scholar]
- 5. Yang R, Gallo DJ, Baust JJ, et al. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2002;283:G212–G222 [DOI] [PubMed] [Google Scholar]
- 6. Venkataraman R, Kellum JA, Song M, Fink MP. Resuscitation with Ringer's ethyl pyruvate solution prolongs survival and modulates plasma cytokine and nitrite/nitrate concentrations in a rat model of lipopolysaccharide-induced shock. Shock. 2002;18:507–512 [DOI] [PubMed] [Google Scholar]
- 7. Ulloa L, Ochani M, Yang H, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansson AS, Johansson-Haque K, Okret S, Palmblad J. Ethyl pyruvate modulates acute inflammatory reactions in human endothelial cells in relation to the NF-kappaB pathway. Br J Pharmacol. 2008;154:1318–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong W, Cai B, Pena G, et al. Ethyl pyruvate prevents inflammatory responses and organ damage during porcine hemorrhage. Shock. 2010;34:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai B, Chen F, Lin X, et al. Anti-inflammatory adjuvant in resuscitation fluids improves survival in hemorrhage. Crit Care Med. 2009;37:860–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davé SH, Tilstra JS, Matsuoka K, et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang YZ, Ling Y, Yin T, et al. Delayed ethyl pyruvate therapy attenuates experimental acute pancreatitis via reduced serum high mobility group box 1 levels in rats. World J Gastroenterol. 2008;14:4546–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamada N, Maeyama T, Kawaguchi T, et al. The role of high mobility group box 1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:440–447 [DOI] [PubMed] [Google Scholar]
- 14. Varma SD, Devamanoharan PS, Ali AH. Prevention of intracellular oxidative stress to lens by pyruvate and its ester. Free Radic Res. 1998;28:131–135 [DOI] [PubMed] [Google Scholar]
- 15. Varma SD, Devamanoharan PS, Rutzen AR, Ali AH, Henein M. Attenuation of galactose-induced cataract by pyruvate. Free Radic Res. 1999;30:253–263 [DOI] [PubMed] [Google Scholar]
- 16. Devamanoharan PS, Henein M, Ali AH, Varma SD. Attenuation of sugar cataract by ethyl pyruvate. Mol Cell Biochem. 1999;200:103–109 [DOI] [PubMed] [Google Scholar]
- 17. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308 [DOI] [PubMed] [Google Scholar]
- 18. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 19. Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397 [DOI] [PubMed] [Google Scholar]
- 20. Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Read RW. Uveitis: advances in understanding of pathogenesis. Curr Rheumatol Rep. 2006;8:260–266 [DOI] [PubMed] [Google Scholar]
- 22. Izumi-Nagai K, Nagai N, Ozawa Y, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170:2149–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koizumi K, Poulaki V, Doehmen S, et al. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191 [DOI] [PubMed] [Google Scholar]
- 24. Shiratori K, Ohgami K, Ilieva IB, Koyama Y, Yoshida K, Ohno S. Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by alpha-melanocyte-stimulating hormone. Invest Ophthalmol Vis Sci. 2004;45:159–164 [DOI] [PubMed] [Google Scholar]
- 25. Bellot JL, Palmero M, Garcia-Cabanes C, Espi R, Hariton C, Orts A. Additive effect of nitric oxide and prostaglandin-E2 synthesis inhibitors in endotoxin-induced uveitis in the rabbit. Inflamm Res. 1996;45:203–208 [DOI] [PubMed] [Google Scholar]
- 26. Mo JS, Matsukawa A, Ohkawara S, Yoshinaga M. Role and regulation of IL-8 and MCP-1 in LPS-induced uveitis in rabbits. Exp Eye Res. 1999;68:333–340 [DOI] [PubMed] [Google Scholar]
- 27. Kurihara T, Ozawa Y, Shinoda K, et al. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest Ophthalmol Vis Sci. 2006;47:5545–5552 [DOI] [PubMed] [Google Scholar]
- 28. Nagai N, Oike Y, Noda K, et al. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:2925–2931 [DOI] [PubMed] [Google Scholar]
- 29. Nagai N, Izumi-Nagai K, Oike Y, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350 [DOI] [PubMed] [Google Scholar]
- 30. Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566 [PubMed] [Google Scholar]
- 31. Yadav UCS, Kalariya NM, Ramana KV. Emerging role of antioxidants in the protection of uveitis complications. Curr Med Chem. 2011;18:931–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalariya NM, Shoeb M, Reddy AB, Zhang M, van Kuijk FJ, Ramana KV. Prevention of endotoxin-induced uveitis in rats by plant sterol guggulsterone. Invest Ophthalmol Vis Sci. 2010;51:5105–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513–1518 [DOI] [PubMed] [Google Scholar]
- 34. Huang LF, Yao YM, Zhang LT, Dong N, Yu Y, Sheng ZY. The effect of high mobility group box 1 protein on activity of regulatory T cells after thermal injury in rats. Shock. 2009;31:322–329 [DOI] [PubMed] [Google Scholar]
- 35. Harvey SA, Guerriero E, Charukamnoetkanok N, Piluek J, Schuman JS, Sundarraj N. Responses of cultured human keratocytes and myofibroblasts to ethyl pyruvate: a microarray analysis of gene expression. Invest Ophthalmol Vis Sci. 2010;51:2917–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varma SD, Hegde KR, Kovtun S. Oxidative damage to lens in culture: reversibility by pyruvate and ethyl pyruvate. Ophthalmologica. 2006;220:52–57 [DOI] [PubMed] [Google Scholar]
- 37. Hegde KR, Kovtun S, Varma SD. Intraocular penetration of pyruvate following its topical administration in mice. Mol Cell Biochem. 2010;338:87–90 [DOI] [PubMed] [Google Scholar]
- 38. Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105 [DOI] [PubMed] [Google Scholar]
- 39. Song M, Kellum JA, Kaldas H, Fink MP. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J Pharmacol Exp Ther. 2004;308:307–316 [DOI] [PubMed] [Google Scholar]
- 40. Genovese T, Esposito E, Mazzon E, et al. Beneficial effects of ethyl pyruvate in a mouse model of spinal cord injury. Shock. 2009;32:217–227 [DOI] [PubMed] [Google Scholar]
- 41. Duygulu F, Evereklioglu C, Calis M, Borlu M, Cekmen M, Ascioglu O. Synovial nitric oxide concentrations are increased and correlated with serum levels in patients with active Behçet's disease: a pilot study. Clin Rheumatol. 2005;24:324–330 [DOI] [PubMed] [Google Scholar]
- 42. Mandai M, Yoshimura N, Yoshida M, Iwaki M, Honda Y. The role of nitric oxide synthase in endotoxin-induced uveitis: effects of NG-nitro L-arginine. Invest Ophthalmol Vis Sci. 1994;35:3673–3680 [PubMed] [Google Scholar]
- 43. Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coca-Prados M, Escribano J, Ortego J. Differential gene expression in the human ciliary epithelium. Prog Retin Eye Res. 1999;18:403–429 [DOI] [PubMed] [Google Scholar]
- 45. Santos Lacomba M, Marcos Martin C, Gallardo Galera JM, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–255 [DOI] [PubMed] [Google Scholar]
- 46. Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Franks WA, Limb GA, Stanford MR, et al. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11:187–191 [DOI] [PubMed] [Google Scholar]
- 49. Tugal-Tutkun I, Mudun A, Urgancioglu M, et al. Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behçet's disease: an open-label trial. Arthritis Rheum. 2005;52:2478–2484 [DOI] [PubMed] [Google Scholar]
- 50. Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268 [DOI] [PubMed] [Google Scholar]
- 51. Alexander G, Carlsen H, Blomhoff R. Strong in vivo activation of NF-kappaB in mouse lenses by classic stressors. Invest Ophthalmol Vis Sci. 2003;44:2683–2688 [DOI] [PubMed] [Google Scholar]
- 52. Dudek EJ, Shang F, Taylor A. H2O2-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med. 2001;31:651–658 [DOI] [PubMed] [Google Scholar]