The authors analyzed retinas of the cystathionine β-synthase mouse model of endogenous hyperhomocysteinemia and report increased mitochondrial fission as a novel mechanism of homocysteine-induced toxicity to retinal ganglion cells.
Abstract
Purpose.
To evaluate the effect of excess homocysteine on the regulation of retinal ganglion cell mitochondrial dynamics.
Methods.
Mice deficient in cystathionine-β-synthase (cbs) were used as a model of hyperhomocysteinemia. Gene and protein expression analyses of Opa1 and Fis1 were performed on cbs+/−neural retinas. Mitochondria within retinal ganglion cell axons underwent systematic ultrastructural analysis to measure area, length, width, and the distance between the mitochondria and the axon wall. Primary mouse ganglion cells were cultured, treated with homocysteine, and assessed for levels of Opa1 and Fis1 protein, the number of mitochondria per length of neurite, and levels of cleaved caspase-3.
Results.
Opa1 and Fis1 protein levels in cbs+/− neural retinas were elevated to 191.00% ± 26.40% and 226.20% ± 4.57%, respectively, compared with wild-type. Mitochondria of cbs+/− retinas were smaller in all parameters studied, including area (0.32 ± 0.01μm2 vs. 0.42 ± 0.02 μm2), compared with wild-type. Primary ganglion cells treated with homocysteine had elevations in Opa1 and Fis1 proteins, a significantly higher number of mitochondria per length of neurite (0.1781 ± 0.017 vs. 0.1156 ± 0.012), and significantly higher levels of cleaved caspase-3 compared with control.
Conclusions.
This study provides the first evidence that homocysteine-induced ganglion cell loss involves the dysregulation of mitochondrial dynamics, both in vivo and in vitro. The present data suggest increased mitochondrial fission as a novel mechanism of homocysteine toxicity to neurons. Of particular relevance are glaucoma and Alzheimer's disease, neurodegenerative diseases that are associated with hyperhomocysteinemia and, more recently, have implicated increased mitochondrial fission in their pathogeneses.
Homocysteine is a non-proteinogenic amino acid that is an intermediate in methionine and cysteine metabolism. Severe elevations in plasma homocysteine (hyperhomocysteinemia) are caused by homozygous mutations in the regulatory enzymes involved in homocysteine metabolism; these enzymes include methionine synthase, methylene tetrahydrofolate reductase, and cystathionine β-synthase.1 Moderate hyperhomocysteinemia occurs more commonly and is caused by heterozygous mutations in these regulatory enzymes or by nutritional deficiencies in the vitamins folate, B12, and B6. Recently, several clinical studies reported a correlation between elevated plasma homocysteine and retinal degenerative disorders, including open-angle glaucoma, maculopathy, and diabetic retinopathy.2–9
Our laboratory has investigated extensively the potential of homocysteine to induce toxicity to the retina using both in vitro and in vivo models.10–13 Our earliest in vivo study documented that intravitreal injection of a high level of homocysteine (200 μM) led to abundant cell death in the ganglion cell layer.13 To specifically analyze the effect of endogenous elevation of homocysteine on the retina, our laboratory recently used a mutant mouse model of hyperhomocysteinemia developed in the laboratory of Nobuyo Maeda.14 The mouse harbors a deletion of the gene encoding cystathionine β-synthase (cbs), an enzyme required for the conversion of homocysteine to cysteine. Using the heterozygous mutant mouse (cbs+/−) as a model of moderate hyperhomocysteinemia (∼4- to 7-fold elevation in plasma homocysteine), we found that ganglion cell viability was decreased by approximately 20%; even greater neuronal death was observed in mice with higher levels of this amino acid.10 These studies described for the first time that endogenous elevation in plasma homocysteine induces ganglion cell loss. To investigate potential mechanisms of homocysteine-induced ganglion cell toxicity, we performed microarray analysis on neural retinas of cbs+/− mice. The expression of numerous genes was altered in these mice; two genes of particular interest whose expression levels were changed in the neural retina of the cbs+/− mouse were opa1 and fis1.10
Opa1 and Fis1 proteins are involved in mitochondrial fission and fusion; Opa1 is a modulator of mitochondrial fusion, and Fis1 is the rate-limiting protein in mitochondrial fission.15 Mitochondria are the primary energy-producing organelles and are dynamic in that they require a balance of fission and fusion processes to function properly.15 Excessive fission results in mitochondrial fragmentation and eventual cellular apoptosis, whereas excessive fusion results in elongated mitochondria and inhibition of adequate energy production. Given that retinal ganglion cells are neurons that are highly active, as evidenced by a high metabolic rate, they are especially sensitive to alterations in mitochondrial dynamics.16,17 Deficiencies in mitochondrial fission and fusion processes have been implicated in the pathogenesis of several neurodegenerative disorders, including glaucoma, a disease characterized specifically by retinal ganglion cell loss.16,18–20 In the present study, we test the hypothesis that elevation in homocysteine impairs the balance of mitochondrial fission and fusion in retinal ganglion cells, resulting in excessive mitochondrial fission and cellular apoptosis.
Methods
Animals
The development of cbs+/− mice has been described.10 For these studies, heterozygous mice were given tap water containing methionine (final concentration, 0.5%) starting from the weaning period to elevate plasma homocysteine to approximately sevenfold that of wild-type mice.21 Maintenance and treatment of animals adhered to the institutional guidelines for the humane treatment of animals and to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Quantitative Reverse-Transcriptase Polymerase Chain Reaction
Neural retinas were isolated from wild-type and cbs+/− mice; after homogenization, RNA was extracted using reagent (TRIzol; Invitrogen, Carlsbad, CA). Total RNA was converted to cDNA using reverse transcriptase (SuperScript II; Invitrogen, Carlsbad, CA) and oligo(dT)12–18 primer (Invitrogen). Real-time PCR was performed using a SYBR Green fluorescein mix from ABgene (Absolute QPCR; Thermo Scientific, Surrey, UK) and a real-time PCR detection system (I-Cycler; Bio-Rad, Hercules, CA). The primers used to detect isoforms of opa1 and transcript variants of fis1 are listed in Table 1. PCR was performed for 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds; melt curve analysis confirmed the purity of the end products. Resultant CT values were normalized to 18s and analyzed using the comparative CT method to obtain fold-changes in gene expression.22 PCR analysis was performed twice using the pooled wild-type and cbs+/− samples, and the Student's t-test was used to compare the two groups.
Table 1.
Primers Used for Quantitative Real-time PCR Analysis
| Primers Used to Detect Isoforms of opa1 | |
| OPAexon4–5_F: | 5′-TGCAGGTTCACCTGGAGAAAC-3′ |
| OPAexon4b_F: | 5′-CCCAAATTGGTTAGTGAAGTCC-3′ |
| OPAexon5–6_R: | 5′-TGTCTGACACCTTCCTGTAATGCTTGT-3′ |
| OPAexon5b_R: | 5′-GCTTCTGTTGGGCATAGCTC-3′ |
| Isoform 1: primer pair OPAexon4–5_F & OPAexon5–6_R | |
| Isoform 5: primer pair OPAexon4b_F & OPAexon5–6_R | |
| Isoform 7: primer pair OPAexon4–5_F & OPAexon5b_R | |
| Isoform 8: primer pair OPAexon4b_F & OPAexon5b_R | |
| Primers Used to Detect Transcript Variants of fis1 | |
| Transcript variant 1 | Fwd: 5′-AGGCCGTGCTGAACGAGCTG-3′ |
| Rev: 5′-GGTAGTTGCCCACGGCCAGG-3′ | |
| Transcript variant 2 | Fwd: 5′-CCTCGGGTGCAGGGGTCCAT-3′ |
| Rev: 5′-GCAACAGCTCCTCCAGCAGCAC-3′ | |
Immunohistochemical Studies
Immunohistochemical analysis was performed on frozen sections of intact retina, as described previously.23 Sections were incubated overnight in a humidified chamber at 4°C with a mouse monoclonal antibody against Opa1 (1:100) (BD Biosciences, San Jose, CA) or a rabbit polyclonal antibody against Fis1 (1:1000) (Abcam, Cambridge, MA). For the detection of immunopositive signals, retinal sections were incubated with Oregon Green–conjugated goat anti–mouse IgG (1:1000) or Alexa Fluor 555–conjugated donkey anti–rabbit IgG (1:1000; Invitrogen) secondary antibody. Slides were washed in PBS, incubated with a 1:24,000 dilution of Hoechst stain, coverslipped, and viewed with an epifluorescence microscope (AxioPlan-2; Zeiss, Oberkochen, Germany) equipped with a high-resolution monochromatic (AxioCam HR; Zeiss) camera and operated using the corresponding software program (AxioVision, version 4.6.3.0; Zeiss).
Isolation and Treatment of Primary Ganglion Cells
Primary ganglion cells were isolated from the retinas of 2- to 4-day-old C57BL/6J mice that were the offspring of breeding pairs purchased from Harlan Sprague-Dawley Inc. (Indianapolis, IN). Immunopanning procedures and verification of purity of the cells have been described in detail.12 Cells were treated with 50 μM homocysteine (Sigma-Aldrich Chemical, St. Louis, MO) for 0, 3, 6, 9, 12, and 15 hours; protein was isolated and examined for varying levels of Opa1 and Fis1.
Immunoblotting of Opa1, Fis1, and Cleaved Caspase-3
Protein extraction from neural retinas isolated from wild-type mice and cbs+/− mice or from control and homocysteine-treated primary ganglion cells were subjected to Western blot analysis to detect Opa1 and Fis1 levels following our published method.23 Briefly, protein samples were subjected to SDS-PAGE and transferred to nitrocellulose membranes, which were then incubated with a monoclonal mouse anti–Opa1 antibody (1:1000; BD Biosciences), a polyclonal rabbit anti–Fis1 antibody (1:500; Abcam) (for neural retinas), a polyclonal rabbit anti–Fis1 antibody (1:250) (BioVision, Mountain View, CA) (for primary ganglion cells), or a polyclonal rabbit anti–cleaved caspase-3 antibody (1:500) at 4°C overnight, followed by an HRP-conjugated goat anti–mouse IgG (1:5000) or goat anti–rabbit IgG antibody (1:3000). Proteins were visualized with the ECL Western blot detection system. Membranes were reprobed with mouse monoclonal anti–β-actin antibody (1:5000) as a loading control. Immunoblotting was repeated at least three times; one-way ANOVA and the Student's t-test were used to compare protein band densities as statistical analyses.
Electron Microscopy
Mice, which had been maintained for 5 weeks on a high methionine diet, were perfused with a 2% paraformaldehyde/2% glutaraldehyde in 0.1 M sodium cacodylate buffer solution. Eyes were enucleated and fixed for 1 hour at room temperature in 2% paraformaldehyde/2% glutaraldehyde in 0.1 M sodium cacodylate buffer in sucrose (pH 7.2) and postfixed for 1 hour with osmium tetroxide. Eyes were punctured at the limbus, fixed overnight, and washed in sodium cacodylate buffer. Then they were dehydrated in serial ethanol (70%–100%), infiltrated with propylene oxide, and infiltrated with Epon and propylene oxide (1:1) at room temperature for 1 hour Subsequently, fresh Epon was added, and polymerization was carried out overnight. Tissue was embedded in an embedding resin mixture (EM-bed 812/Araldite-502; Electron Microscopy Sciences, Hatfield, PA). Thin sections (90 nm) were cut with a diamond knife on an ultramicrotome (EM UC6; Leica Microsystems, Inc., Bannockburn, IL) and placed on copper grids. Sections were stained with uranyl acetate and lead citrate and viewed using a transmission electron microscope (JEM 1230; JEOL USA, Inc., Peabody, MA) at 110 kV and imaged with a CCD camera (UltraScan4000/First Light Digital Camera Controller; Gatan Inc., Pleasanton, CA).
To examine retinal ganglion cell mitochondria, we analyzed the nerve fiber layer (composed of retinal ganglion cell axons); 10 images of the nerve fiber layer were taken at random for each eye at a magnification of 2500×, and mitochondria were examined. An individual mitochondrion within the ganglion cell axon was identified by the presence of a double membrane and inner membrane folds (mitochondrial cristae). Mitochondria were systematically evaluated using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) according to four measurement parameters: total area of mitochondrial cross-section, length, width, and distance to nearest axon wall; length and width were defined as the longest and shortest distance, respectively, that passed through the center of the mitochondrion. The first 100 mitochondria (progressing from left to right in each image) were analyzed for each eye to avoid selection bias; the Student's t-test was used to test for significance in differences in measurements obtained for wild-type and cbs+/− mice.
Dye Analysis
Primary ganglion cells were cultured and treated for 18 hours with control media or media containing 50 μM homocysteine. During the last 30 minutes of treatment, cells were incubated with media containing 200 nM dye (MitoTracker Green FM; Invitrogen). After treatment, cells were fixed with 4% paraformaldehyde, washed with PBS, and fixed for visualization using an epifluorescence microscope (AxioPlan-2; Zeiss) equipped with a high-resolution monochromatic (AxioCam HR; Zeiss) camera and operated using the corresponding software program (AxioVision, version 4.6.3.0; Zeiss). The number of total mitochondria per neurite length was quantified using this program. The Student's t-test was used to test for significance in difference between control and homocysteine-treated groups; P < 0.05 was considered significant.
Results
Evaluation of Opa1 and Fis1 Expression in Neural Retina of cbs+/− Mice
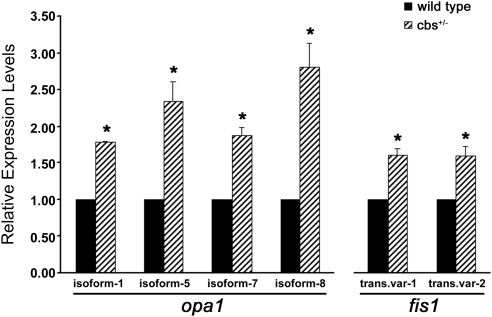
Microarray analysis of cbs+/− mouse neural retina had suggested an alteration in the expression of two genes associated with mitochondrial dynamics, opa1 and fis1, compared with wild-type.10 To confirm and evaluate directly these changes in gene expression, quantitative real-time PCR analysis for isoforms and transcript variants of opa1 and fis1 was performed using neural retina isolated from 8-week-old wild-type and cbs+/− mice (Fig. 1). Expression levels of all four isoforms of opa1 were elevated in cbs+/− retinas compared with wild-type retinas. Levels of isoforms-1 and -7 were increased approximately 1.80-fold and ∼1.90 -fold of wild-type, respectively (P < 0.002 and P < 0.015), whereas isoforms-5 and -8 were increased more robustly (2.35 ± 0.27-fold and 2.81 ± 0.32-fold of wild-type expression; P < 0.037 and P < 0.030). Both transcript variants of fis1 modulate mitochondrial fission, and the expression of these was increased in cbs+/− retinas in comparison with wild-type retinas (transcript variant 1: 1.61 ± 0.09-fold of wild-type expression, P < 0.020; transcript variant 2: 1.60 ± 0.14-fold of wild-type expression, P < 0.049).
Figure 1.
Expression of Opa1 and Fis1 is increased in neural retinas of cbs+/− mice. Fold change in expression of Opa1 isoforms and Fis1 transcript variants as determined by quantitative real-time polymerase chain reaction analysis. Expression levels in retinas of cbs+/− mice were as follows: Opa1-isoform 1, 1.79 ± 0.01 of wild-type, P < 0.0002; Opa1-isoform 5, 2.35 ± 0.27 of wild-type, P < 0.037; Opa1-isoform 7, 1.87 ± 0.11 of wild-type, P < 0.016; Opa1-isoform 9, 2.81 ± 0.32 of wild-type, P < 0.03; Fis1-transcript variant 1, 1.61 ± 0.09 of wild-type, P < 0.02; Fis1-transcript variant 2, 1.60 ± 0.14 of wild-type, P < 0.05. *Significantly different from wild-type.
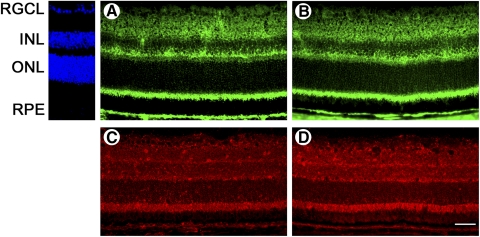
Retinal cryosections of 8-week-old wild-type and cbs+/− mice were subjected to immunohistochemical analysis (Fig. 2). Evaluation of Opa1 in the wild-type retina revealed that the protein was present ubiquitously; protein expression was detected in the retinal ganglion cell layer, mitochondria-rich inner segments of photoreceptor cells, RPE cell layer, and inner and outer plexiform layers (Fig. 2A). Localization of Opa1 in cbs+/− retinas was similar to that in wild-type retinas (Fig. 2B). Immunohistochemistry of wild-type retinas using an antibody against Fis1 detected Fis1 throughout the retina, including the ganglion cell layer, inner plexiform and nuclear layers, outer plexiform layer, inner segments of photoreceptor cells, and RPE cell layer (Fig. 2C). The expression pattern of Fis1 in cbs+/− mice remained comparable to that in wild-type mice (Fig. 2D), and levels of the protein appeared elevated compared with wild-type.
Figure 2.
Opa1 and Fis1 protein localization in retinas of cbs+/− mice remains similar to wild-type. Retinal cryosections from wild-type mice (A, C) and cbs+/− mice (B, D) at 8 weeks of age. Opa1 protein (green fluorescence) localization is similar in the retinas of wild-type mice (A) and of cbs+/− mice (B). Localization of Fis1 protein (red fluorescence) remains comparable in retinas of wild-type mice (C) and cbs+/− mice (D). Levels of Fis1 are elevated in retinas of cbs+/− mice compared with wild-type. Scale bar, 25 μm. RGCL = retinal ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer.
To measure Opa1 and Fis1 protein levels, we performed immunoblotting analysis on protein isolated from neural retinas of 8-week-old wild-type and cbs+/− mice (Figs. 3A, 3B). Semiquantitative evaluation of Opa1 protein levels in wild-type and cbs+/− retinas showed that retinal expression of Opa1 protein increased slightly, but significantly, in mice with hyperhomocysteinemia. Densitometry analysis revealed that Opa1 band density in cbs+/− retinas was 191.00% ± 26.40% that of wild-type (P < 0.02). Protein levels of Fis1 were also elevated in retinas of cbs+/− mice compared with wild-type (226.20% ± 4.57% of wild-type; P < 0.001); this finding corroborated the data obtained from immunohistochemical analysis. Several protein bands (particularly at 64 kDa) were visualized when immunoblotting against Fis1; Fis1 must form oligomers to mediate mitochondrial fission,24 and the numerous protein bands are evidence of this polymerization in vivo. To comprehensively investigate the effect of hyperhomocysteinemia on mitochondrial dynamics, the expression of other proteins known to regulate mitochondrial fission and fusion was determined (Fig. 3C). Expression levels of other fusion proteins (MFN1, MFN2) as well as fission protein (DRP1) remained unaltered in the neural retinas of cbs+/− mice.
Figure 3.
Opa1 and Fis1 protein levels are increased in neural retinas of cbs+/− mice. Representative Western blots of Opa1 (Mr ∼80–100 kDa), Fis1 (Mr ∼17 kDa), MFN1 (Mr ∼84 kDa), MFN2 (Mr ∼86 kDa), and DRP1 (Mr ∼82 kDa) showing increased levels of Opa1 (1.91 ± 0.26 of wild-type, P < 0.019) and Fis1 (2.26 ± 0.05 of wild-type, P < 0.0001) in retinas of cbs+/− mice. Levels of Mfn1, Mfn2, and Drp1 remained unchanged compared with wild-type.
Ultrastructural Analysis of Mitochondrial Morphology in Retinal Ganglion Cells of cbs+/− Mice
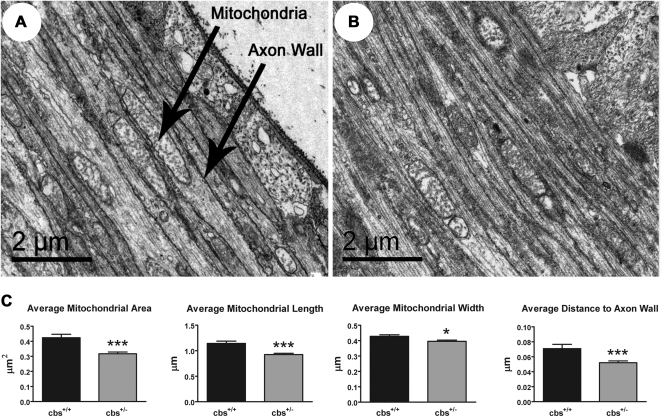
After confirmation that mRNA and protein expression of opa1 and fis1 was increased in the retinas of cbs+/− mice, we asked whether changes in expression of these proteins would alter the shape and distribution of mitochondria within the retina. We were particularly interested in examining mitochondria within retinal ganglion cells. Data collected from this comprehensive analysis of retinal ganglion cell mitochondria in wild-type and cbs+/− mice are shown in Figure 4. Mitochondria of wild-type and cbs+/− retinal ganglion cells ranged in shape from elongated and oval to circular (Figs. 4A, 4B). Variation in mitochondrial shape was expected because we were examining a 3D structure using 2D imaging methods. Morphometric examination revealed that mitochondria in the nerve fiber layer of cbs+/− mice were significantly smaller and were in closer proximity to the axon wall than their wild-type counterparts (Fig. 4C). The average mitochondrial area of retinal ganglion cell mitochondria within cbs+/− mice was 0.32 ± 0.01 μm2 compared with an average of 0.42 ± 0.02 μm2 in wild-type mice (P < 0.0001). Mitochondrial length and width were significantly reduced in cbs+/− mice compared with wild-type mice (length, 0.93 ± 0.03 μm vs. 1.14 ± 0.04 μm [P < 0.0001]; width, 0.40 ± 0.01 μm vs. 0.43 ± 0.01 μm [P < 0.01] in cbs+/− and wild-type mice, respectively). Although mitochondria were smaller in cbs+/− mice, they were positioned closer to the ganglion cell axon wall than mitochondria of wild-type mice; the distance to the closest axon wall was 0.05 ± 0.00 μm in cbs+/− mice compared with 0.07 ± 0.01 μm in wild-type mice (P < 0.0005). Collectively, these data suggest that there is a structural change in the morphology of axonal mitochondria of ganglion cells in the cbs+/− mouse.
Figure 4.
Mitochondria within nerve fiber layer of cbs+/− mice are smaller than in wild-type mice. Electron microscopic images of mitochondria within nerve fiber layer of wild-type mice (A) and cbs+/− mice (B). Evaluation of several parameters revealed that mitochondria of cbs+/− mice are smaller than mitochondria of wild-type mice (C). Average mitochondrial area: wild-type (0.424 ± 0.022 μm2) versus cbs+/− (0.317 ± 0.012 μm2), P < 0.0001; average mitochondrial length: wild-type (1.144 ± 0.044 μm) versus cbs+/− (0.925 ± 0.025 μm), P < 0.0001; average mitochondrial width: wild-type (0.428 ± 0.010 μm) versus cbs+/− (0.396 ± 0.008 μm), P < 0.0123; average distance from axon wall: wild-type (0.071 ± 0.006 μm) versus cbs+/− (0.052 ± 0.002), P < 0.0005.
Immunoblotting Analysis of Opa1 and Fis1 in Homocysteine-Treated Retinal Ganglion Cells
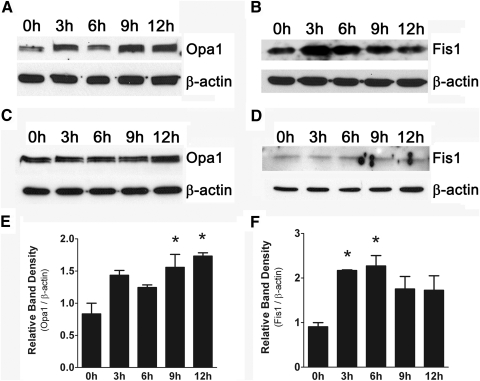
We then asked whether levels of Opa1 and Fis1 in retinal ganglion cells could be altered by direct exposure to excess homocysteine. We treated primary ganglion cells freshly isolated from neonatal mouse retinas with 50 μM homocysteine for 0, 3, 6, 9, and 12 hours. Protein was isolated from these cells, and immunoblotting was performed using antibodies against Opa1 and Fis1 (Figs. 5A, 5B). Levels of Opa1 were increased, and expression peaked at 9 to 12 hours after homocysteine exposure. Levels of Fis1 were also increased in response to homocysteine exposure; expression peaked earlier (3–6 hours) during the course of treatment. Cells treated with media containing no homocysteine for the same time points served as control; protein was isolated, and immunoblotting was performed against Opa1 and Fis1 (Figs. 5C, 5D), confirming that there was no change in the levels of either protein in the absence of homocysteine exposure. Densitometric analysis of Opa1 and Fis1 levels for cells treated with homocysteine (Figs. 5E, 5F) revealed that levels of Opa1 were significantly elevated at 9 to 12 hours after homocysteine exposure (levels at 9 and 12 hours were 1.56 ± 0.20-fold and 1.73 ± 0.05-fold, respectively, higher than levels at time 0 hour; P < 0.019) and that levels of Fis1 were significantly elevated at 3 to 6 hours after homocysteine exposure (levels at 3 and 6 hours were 2.17 ± 0.10-fold and 2.27 ± 0.01-fold, respectively, higher than levels at time 0 hour; P < 0.025). Taken together, these data suggest that alterations in Opa1 and Fis1 in retinal ganglion cells are directly modulated by exposure to excess homocysteine.
Figure 5.
Opa1 and Fis1 protein levels are increased in primary ganglion cells after exposure to 50 μM homocysteine. Representative Western blot analysis depicting increased Opa1 protein at 9 to 12 hours (A) and increased Fis1 protein at 3 to 6 hours (B). Cells exposed to control media show no change in Opa1 or Fis1 protein levels (C, D). Densitometric analysis (E, F) of Opa1 and Fis1 protein bands for cells treated with homocysteine: Opa1 (9 hours: 1.56 ± 0.20-fold higher than levels at time 0 hour, 12 hours: 1.73 ± 0.05-fold higher than levels at time 0 hour; P < 0.019) and Fis1 (3 hours: 2.17 ± 0.10-fold higher than levels at time 0 hour, 6 hours: 2.27 ± 0.01-fold higher than levels at time 0 hour; P < 0.025). *Significantly different from baseline (time 0 hour).
Analysis of Alterations in Mitochondrial Dynamics and Cell Viability in Homocysteine-Treated Retinal Ganglion Cells
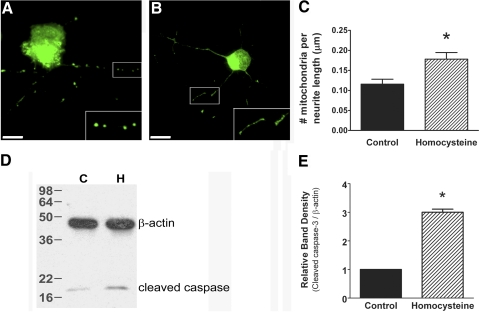
To determine whether the observed increases in Opa1 and Fis1 protein after exposure to elevated homocysteine would alter mitochondrial dynamics, primary ganglion cells were treated with homocysteine for 18 hours and coincubated with dye (MitoTracker Green FM; Invitrogen) for direct visualization of mitochondria. Representative images of control and homocysteine-treated cells are shown in Figure 6. Primary ganglion cells treated with homocysteine (Fig. 6B) contain mitochondria that appear smaller and more numerous than control cells (Fig. 6A). Quantification of the number of mitochondria per length of neurite (Fig. 6C) revealed a higher density of mitochondria in ganglion cells treated with homocysteine than in control cells (0.1781 ± 0.017 vs. 0.1156 ± 0.012, respectively; P < 0.016), suggesting an increase in mitochondrial fission processes.
Figure 6.
Exposure of primary ganglion cells to 50 μM homocysteine induces mitochondria that are smaller and more numerous and increases levels of cleaved caspase-3. Representative images of primary ganglion cells loaded with dye; no treatment (A) versus 50 μM homocysteine (B). Scale bar, 10 μM. (inset) Higher magnification of boxed area. Quantification of the number of mitochondria per length of neurite (C) shows that mitochondrial number is higher in primary ganglion cell neurites after treatment with 50 μM homocysteine (no treatment, 0.1156 ± 0.012, 50 μM; homocysteine, 0.1781 ± 0.017; P < 0.016). Immunoblot analysis performed on protein isolated from primary ganglion cells after exposure to 50 μM homocysteine revealed higher levels of cleaved caspase-3 than in control primary ganglion cells (C, control; H, homocysteine-treated) (D). Densitometric analysis of cleaved caspase-3. (E) Control, 1.00 ± 0.00; homocysteine-treated, 3.00 ± 0.11; P < 0.003.
We then asked whether the increase in mitochondrial fission would coincide with an elevation in markers of apoptosis, such as cleaved caspase-3. Primary ganglion cells were cultured and treated with homocysteine for 18 hours and protein isolated. Immunoblot analysis showed that levels of cleaved caspase-3 were significantly elevated in homocysteine-treated cells compared with control; densitometric analysis confirmed these findings (3.00 ± 0.11 vs. 1.00 ± 0.00, respectively; P < 0.003) (Figs. 6D, 6E). These data strongly suggest a link between homocysteine-induced acceleration of mitochondrial fission and subsequent ganglion cell apoptosis.
Discussion
Mitochondria are the primary energy-producing organelles within neurons and are required for oxidative phosphorylation, intracellular calcium homeostasis, and regulation of cellular pH.25 Dysfunction of these organelles leads to cellular apoptosis through mechanisms such as intracellular reactive oxygen species generation and induction of the intrinsic apoptotic pathway.26 Mitochondrial dysfunction is implicated in the pathogenesis of many neurodegenerative disorders, including glaucoma, Alzheimer's disease, and Parkinson's disease.19,27 Interestingly, these diseases have been associated with elevated levels of plasma homocysteine.28–31 Our laboratory has investigated mechanisms of cell death induced by exposure to homocysteine in neurons, particularly retinal ganglion cells. Recently, we characterized the retinal phenotype of the cbs mouse, a mouse model of hyperhomocysteinemia, and found that retinal ganglion cells were particularly susceptible in this model. Our earlier microarray analysis identified genes involved in mitochondrial dynamics that were altered in retinas of these mice; these studies were the springboard for the current analysis of the mitochondrial genes opa1 and fis1.10 We asked whether the balance of mitochondrial dynamics was altered in the cbs mouse model of hyperhomocysteinemia. Three notable findings emerged from this work. First, we found that Opa1 and Fis1 expression was increased in the neural retinas of cbs+/− mice. Second, we demonstrated that the elevations in expression coincided with increased mitochondrial fission within axons of retinal ganglion cells of cbs+/− mice. Third, we found that exposure to homocysteine (at 50-μM concentrations) was sufficient to generate an increase in mitochondrial fission in primary retinal ganglion cells and a concomitant increase in apoptotic processes.
The finding that opa1 isoforms-7 and -8 were elevated in the cbs+/− retina in addition to opa1 isoforms-1 and -5 suggested that Opa1 may be functioning as a regulator of apoptosis and a modulator of mitochondrial fusion. Opa1 undergoes proteolytic cleavage to generate both membrane-bound and soluble forms; the membrane-bound form is attached to the inner mitochondrial membrane and modulates mitochondrial fusion, whereas the soluble form is located in the inner mitochondrial space and maintains the morphology of mitochondrial cristae.15 One study demonstrated that overexpression of exon 5b (present in isoforms-7 and -8) resulted in altered mitochondrial cristae morphology, release of cytochrome c, and increased cellular apoptosis.32 It is established that mutations in opa1 are associated with autosomal dominant optic atrophy (DOA), a progressive disease characterized by preferential loss of ganglion cells, degeneration of the optic nerve, and nerve fiber layer thinning.33–35 Indeed, autosomal DOA is the most common hereditary optic neuropathy in the general population and a major cause of inherited blindness, reinforcing further the central role played by mitochondrial dynamics in regulating retinal ganglion cell survival for both inherited and sporadic optic neuropathies.
Fis1 expression was also upregulated in the present study. Both transcript variants of fis1 contribute to its function as a regulator of mitochondrial fission; studies have shown that overexpression of this protein leads to excessive mitochondrial fragmentation and cellular apoptosis.15 Thus, the data indicated that dysfunction of cbs increases the expression of opa1 and fis1 in the retina and may potentiate cellular apoptosis. These alterations in gene expression were borne out by increased levels of the proteins they encode, as demonstrated by immunoblot analysis with antibodies against Opa1 and Fis1. Our immunohistochemical studies of Opa1 and Fis1 showed comparable patterns of localization in retinas of wild-type and cbs+/− mice. Fis1 immunohistochemical data mirrored the protein elevation detected by immunoblotting, although this was not observed with Opa1. Indeed, our rationale for performing the immunoblotting for these two proteins was prompted by the variable sensitivity of immunohistochemistry that has been described by others.36,37 Other proteins involved in regulating mitochondrial dynamics (MFN1, MFN2, and DRP1) were not altered by immunoblot analysis. Given that the function of Opa1 is not solely to regulate mitochondrial fusion and that Fis1 is the rate-limiting protein in mitochondrial fission (though DRP1 is found ubiquitously throughout the cell),15 it is understandable that the expression of MFN1, MFN2, and DRP1 does not appear to depend on Opa1 and Fis1 levels. We also examined alterations in expression of other regulators of mitochondrial fission and fusion proteins, but they were not found to be significantly altered by microarray analysis of the cbs+/− mouse retina. Of the proteins known to process and regulate mitochondrial fusion proteins (PHB1, PHB2, YME1L, paraplegin, MIB, Oma1, and PARL)38,39 and the proteins known to regulate mitochondrial fission proteins (endophilinB1, PKCδ, Mff, and Sumo1),38,40,41 only the expressions of Oma1 and Sumo1 were found to be significantly altered by a factor of −2.33 and +2.36, respectively.10 Oma1 is thought to regulate the cleavage of the long form of Opa1 to the short form of Opa138 and Sumo1 is known to stabilize DRP1.38 The lack of significant alteration in other regulators of mitochondrial dynamics further highlights Opa1 and Fis1 as key players in the modulation of mitochondrial health in this model system.
To determine whether the alteration in levels of Opa1 and Fis1 would induce a change in mitochondrial morphology, we systematically examined the shape and distribution of mitochondria. Given that morphometric analysis of cbs+/− retinas had revealed an approximately 20% decrease in the number of cells in the retinal ganglion cell layer,10 we focused our study on mitochondria within retinal ganglion cell axons. Measurements performed at the ultrastructural level revealed that retinal ganglion cell mitochondria of cbs+/− mice were smaller and were located closer to the axon wall than their wild-type counterparts. The presence of smaller mitochondria within axons of retinal ganglion cells strongly supports an increase in mitochondrial fission processes as a potential mechanism of retinal ganglion cell damage in the cbs mutant mouse.
The cbs mutant mouse is a useful model of elevated homocysteine, the excess of which is due to decreased activity of the cystathionine β-synthase enzyme. We predicted that the phenotypic changes observed in cbs+/− and cbs−/− retinas,10 including alterations of mitochondrial proteins observed in the present study, are due to elevated homocysteine levels (rather than secondarily to the decrease in enzyme activity). To investigate directly the effects of excess homocysteine on mitochondrial dynamics, we cultured retinal ganglion cells from wild-type neonatal mice, exposed them to 50 μM homocysteine for various lengths of time, and analyzed levels of Opa1 and Fis1 protein. Immunoblotting demonstrated increases in Opa1 and Fis1 protein in retinal ganglion cells exposed to homocysteine. This is the first report of alterations of these proteins caused by hyperhomocysteinemia in a mammalian system and the first report of the potential of hyperhomocysteinemia to alter directly mitochondrial dynamics. To determine whether the homocysteine-mediated increase in Opa1 and Fis1 proteins would affect mitochondrial dynamics in vitro, we directly visualized mitochondria within ganglion cell neurites with dye (MitoTracker Green FM; Invitrogen). The finding that mitochondria in homocysteine-treated cells were more numerous than in control cells further supported the hypothesis that homocysteine mediates retinal ganglion cell toxicity by altering the balance of mitochondrial fusion and fission. Furthermore, we showed that homocysteine-induced ganglion cell death occurs in an apoptotic fashion. Taken together, these findings strongly suggest that retinal ganglion cell damage by homocysteine involves accelerated mitochondrial fission and an increased propensity for apoptotic cell death. It is noteworthy that mice deficient in Opa1 (Opa1±), which have been used as a model of DOA, demonstrate increased mitochondrial fission in retinal ganglion cells.42 In the cbs+/− mice, there was an increase in mitochondrial fission, yet the levels of Opa1 were increased. An increase in Opa1 would typically be expected to result in mitochondrial fusion (not fission). This enigmatic finding may be explained by the concomitant elevation of Fis1, the key rate-limiting protein responsible for regulating mitochondrial fission. Alternatively, the fact that there was a twofold increase in expression of Opa1 could also have contributed to the observed increase in mitochondrial network fragmentation. Although Opa1 is a pro-fusion mediator, the balance is delicate, and there is evidence to suggest that suprathreshold levels can have a detrimental effect on mitochondrial network morphology.32 The data underscore the complexity involved in balancing mitochondrial fusion and fission.
The significance of this work stems from the observation that homocysteine perturbs the balance of mitochondrial fusion and fission in vivo. This study provides the first evidence of a link between hyperhomocysteinemia and neuronal death caused by mitochondrial dysfunction. Of particular relevance are age-associated neuronal disorders such as glaucoma and Alzheimer's disease, which have implicated hyperhomocysteinemia as a contributing/compounding factor to neuronal toxicity.28–31 In summary, our earlier analysis of the retinal transcriptome of the cbs+/− mouse disclosed altered expression of genes encoding two mitochondrial proteins, Opa1 and Fis1. The present study explored that observation comprehensively and has provided the first ultrastructural evidence of altered mitochondria in the presence of excess homocysteine. Our work furthers the understanding of the damaging potential of hyperhomocysteinemia to neurons (especially ganglion cells) and sheds light on novel mechanisms of homocysteine toxicity to neurons.
Footnotes
Supported by National Institutes of Health Grant R01 EY 012830.
Disclosure: P.S. Ganapathy, None; R.L. Perry, None; A. Tawfik, None; R.M. Smith, None; E. Perry, None; P. Roon, None; B.R. Bozard, None; Y. Ha, None; S.B. Smith, None
References
- 1. Mudd SH, Skovby F, Levy HL, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37:1–31 [PMC free article] [PubMed] [Google Scholar]
- 2. Heuberger RA, Fisher AI, Jacques PF, et al. Relation of blood homocysteine and its nutritional determinants to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2002;76:897–902 [DOI] [PubMed] [Google Scholar]
- 3. Axer-Siegel R, Bourla D, Ehrlich R, et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol. 2004;137:84–89 [DOI] [PubMed] [Google Scholar]
- 4. Tsina EK, Marsden DL, Hansen RM, Fulton AB. Maculopathy and retinal degeneration in cobalamin C methylmalonic aciduria and homocystinuria. Arch Ophthalmol. 2005;123:1143–1146 [DOI] [PubMed] [Google Scholar]
- 5. Seddon JM, Gensler G, Klein ML, Milton RC. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am J Ophthalmol. 2006;141:201–203 [DOI] [PubMed] [Google Scholar]
- 6. Bleich S, Jünemann A, von Ahsen N, et al. Homocysteine and risk of open-angle glaucoma. J Neural Transm. 2002;109:1499–1504 [DOI] [PubMed] [Google Scholar]
- 7. Yang G, Lu J, Pan C. The impact of plasma homocysteine level on development of retinopathy in type 2 diabetes mellitus. Zhonghua Nei Ke Za Zhi. 2002;41:34–38 [PubMed] [Google Scholar]
- 8. Ates O, Azizi S, Alp HH, et al. Decreased serum paraoxonase 1 activity and increased serum homocysteine and malondialdehyde levels in age-related macular degeneration. Tohuku J Esp Med. 2009;217:17–22 [DOI] [PubMed] [Google Scholar]
- 9. Rochtchina E, Wang JJ, Flood VM, Mitchell P. Elevated serum homocysteine, low serum vitamin B12, folate, and age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:344–346 [DOI] [PubMed] [Google Scholar]
- 10. Ganapathy PS, Moister B, Roon P, et al. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest Ophthalmol Vis Sci. 2009;50:4460–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin PM, Ola MS, Agarwal N, Ganapathy V, Smith SB. The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res Mol Brain Res. 2004;123:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007;48:4785–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore P, El-sherbeny A, Roon P, Schoenlein PV, Ganapathy V, Smith SB. Apoptotic cell death in the mouse retinal ganglion cell layer is induced in vivo by the excitatory amino acid homocysteine. Exp Eye Res. 2001;73:45–57 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe M, Osada J, Aratani Y, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845 [DOI] [PubMed] [Google Scholar]
- 16. Delettre C, Lenaers G, Griffoin JM, et al. Nuclear gene-OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210 [DOI] [PubMed] [Google Scholar]
- 17. Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Chan DC. Critical dependence of neurons on mitochondrial dynamics. Curr Opin Cell Biol. 2006;18:453–459 [DOI] [PubMed] [Google Scholar]
- 19. Tezel G; Fourth ARVO/Pfizer Ophthalmics Research Institute Conference Working Group The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1001–1012 [DOI] [PubMed] [Google Scholar]
- 20. Rosenberg EA, Sperazza LC. The visually impaired patient. Am Fam Physician. 2008;77:1431–1436 [PubMed] [Google Scholar]
- 21. Dayal S, Bottiglieri T, Arning E, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–1209 [DOI] [PubMed] [Google Scholar]
- 22. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 23. Dun Y, Duplantier J, Roon P, Martin PM, Ganapathy V, Smith SB. Serine racemase expression and D-serine content are developmentally regulated in neuronal ganglion cells of the retina. J Neurochem. 2008;104:970–978 [DOI] [PubMed] [Google Scholar]
- 24. Serasinghe MN, Yoon Y. The mitochondrial outer membrane protein hFis1 regulates mitochondrial morphology and fission through self-interaction. Exp Cell Res. 2008;314:3494–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osborne NN. Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757 [DOI] [PubMed] [Google Scholar]
- 26. Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Aging Dev. 2010;13:536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci. 2010;67:3435–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turgut B, Kaya M, Arslan S, Demir T, Güler M, Kaya MK. Levels of circulating homocysteine, vitamin B6, vitamin B12, and folate in different types of open-angle glaucoma. Clin Interv Aging. 2010;5:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clement CI, Goldberg I, Healey PR, Graham SL. Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma. 2009;18:73–78 [DOI] [PubMed] [Google Scholar]
- 30. Zhuo JM, Praticò D. Normalization of hyperhomocysteinemia improves cognitive deficits and ameliorates brain amyloidosis of a transgenic mouse model of Alzheimer's disease. FASEB J. 2010;24:3895–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tu MC, Huang CW, Chen NC, et al. Hyperhomocysteinemia in Alzheimer dementia patients and cognitive decline after 6 months follow-up period. Acta Neurol Taiwan. 2010;19:164–173 [PubMed] [Google Scholar]
- 32. Olichon A, Bachouri G, Baricault L, Delettre C, Belenguer P, Lenaers G. Opa1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restriction function in apoptosis. Cell Death Differ. 2007;14:682–692 [DOI] [PubMed] [Google Scholar]
- 33. Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies: disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lenaers G, Reynier P, ElAchouri G, et al. OPA1 functions in mitochondria and dysfunctions in optic nerve. Int J Biochem Cell Biol. 2009;41:1866–1874 [DOI] [PubMed] [Google Scholar]
- 35. Yu-Wai-Man P, Bailie M, Atawan A, Chinnery PF, Griffiths PG. Pattern of retinal ganglion cell loss in dominant optic atrophy due to OPA1 mutations. Eye (Lond). 2011;25:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leong AS, Leong TY. Standardization in immunohistology. Methods Mol Biol. 2011;724:37–68 [DOI] [PubMed] [Google Scholar]
- 37. Mahler M, Ngo JT, Schulte-Pelkum J, Luettich T, Fritzler MJ. Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Res Ther. 2008;10:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011;149:241–251 [DOI] [PubMed] [Google Scholar]
- 39. Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563–569 [DOI] [PubMed] [Google Scholar]
- 40. Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase Cδ under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22:256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takahashi Y, Karbowski M, Yamaguchi H, et al. Loss of Bif-2 suppresses Bax/Bak conformations change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams PA, Morgan JE, Votruba M. Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal ganglion cell dendropathy. Brain. 2010;133:2942–2951 [DOI] [PubMed] [Google Scholar]