Compared with cytokeratin (CK)19, CK7 is the more reliable marker for distinguishing between the corneal and conjunctival epithelium, particularly in patients with limbal stem cell deficiency.
Abstract
Purpose.
To present cytokeratin (CK)7 (OV-TL 12/30 clone) as a newly identified, reliable marker for distinguishing between the conjunctival and corneal surface epithelia, which will contribute to the precise diagnosis of limbal stem cell deficiency (LSCD).
Methods.
Corneal and conjunctival epithelial imprints from 12 cadaveric bulbi and from 9 patients with clinically diagnosed LSCD were used for CK7 and CK19 immunocytochemistry. Specimens on nitroacetate cellulose filter papers obtained from the patients were stained with a combination of periodic acid-Schiff (PAS) and Gill's modified Papanicolaou stains, to assess the presence of goblet cells (GCs).
Results.
CK7 was present in almost all superficial conjunctival epithelial cells from the cadaveric specimens. No immunostaining was observed on the corneal surface. A prominent sharp border of stain was found between the positive conjunctiva and the completely negative epithelium of the central cornea. A more gradual centrifugal decrease in the number of positive cells between the conjunctiva and cornea was observed for CK19. Several CK19-positive cells were detected in the central corneal epithelium. All corneal specimens from affected eyes (unilateral as well as bilateral LSCD patients) revealed strong positivity for CK7, and GCs were present in only 78% of patients.
Conclusions.
In cases in which GCs are severely decreased or are absent from the conjunctival surface, the detection of CK7 (OV-TL 12/30 clone) clearly confirms the overgrowth of the conjunctival epithelium over the cornea. Moreover, CK7 is a more reliable marker for distinguishing between the corneal and conjunctival epithelia compared with CK19.
The corneal and conjunctival epithelia cooperate to provide a biodefense system for the anterior surface of the eye and, together with the tear film, contribute to the maintenance of the optically smooth ocular surface.1,2 Physiologic corneal epithelial homeostasis is maintained mostly by the proliferation and migration of limbal epithelial stem cells, although, in their absence, the corneal epithelium can be renovated by the basal cells of the central epithelium as well.3–5
In cases in which the corneolimbal cells are not able to maintain the replacement and regeneration of the corneal epithelium, limbal stem cell deficiency (LSCD) arises. The most common causes of LSCD are related to external factors that destroy limbal epithelial stem cells, such as chemical or thermal injury and ultraviolet or ionizing radiation. Moreover, LSCD occurs as a consequence of aniridia, Stevens-Johnson syndrome, cicatrization of the ocular surface, ocular mucous membrane pemphigoid, neurotrophic keratopathy, or peripheral inflammatory diseases. In addition, multiple surgical procedures including cataract, pterygium surgery, keratoplasty, and cryotherapies applied to the limbal region and also contact lens wear can lead to primary destruction and hypofunction and consequently to the gradual or total loss of limbal epithelial stem cells (LESCs).6–9
The main characteristics of LSCD are conjunctival epithelial ingrowth over the corneal surface (conjunctivalization), vascularization, chronic inflammation, recurrent or persistent epithelial defects, and corneal opacities.7 Limbal tissue grafting from an undamaged paired eye in the case of unilateral LSCD (autotransplantation) or ex vivo cultured limbal epithelial cell transplantation in the case of bilateral LSCD (allotransplantation) have become commonly used surgical techniques for corneal surface reconstruction,10 because vascularization and inflammation increase the risk of allograft rejection after penetrating keratoplasty.11
The detection of goblet cells (GCs) on corneal imprints using conventional cytological staining (hematoxylin-eosin, PAS, Papanicolaou staining) has been the only useful laboratory criterion for the diagnosis of LSCD for a long time.7,9,12,13 Impression cytology of the ocular surface is a simple, fast and, for the patient, relatively noninvasive method of obtaining a sufficient number of cells for laboratory confirmation of LSCD.14 Difficulties with the diagnosis occur when the conjunctival surface is so damaged that the GCs are absent or very rare in this area and consequently are undetectable on the corneal surface. In such cases, the diagnosis has to be made on the basis of differences between the phenotypes of the corneal and conjunctival epithelia.15,16
The proteins that allow such a distinction to be made belong to the family of intermediate filaments: cytokeratins (CKs).16 CK3 and CK19 are considered to be especially suitable markers for discriminating between the corneal and conjunctival epithelia. CK3 and its pair-mate CK12 are corneal epithelium-specific proteins and are found in all layers of the normal human corneal epithelium, particularly in the suprabasal and superficial layers. The expression of CK3 decreases toward the limbal surface and conjunctiva, where it is absent or present in only a few cells.17,18 Conversely, CK19 is considered a major component of the conjunctival epithelium.18–20 It is abundantly expressed throughout all conjunctival layers,15,16,21,22 but its presence decreases centripetally toward the limbal epithelium and the peripheral cornea and finally, according to most authors, disappears in the central corneal epithelium.18,19,23 On the other hand, some studies have described CK19-positive cells in the central cornea as well.23–25 Because of the opposing directions of the labeling gradients for CK3 and CK19, these CKs are most often used for distinguishing between corneal and conjunctival epithelium and finally for the diagnosis of LSCD.15,16
CK7, similar to CK8, -18, -17, and -19, is a typical simple epithelial CK.26,27 Moreover, CK7 and -19 are characteristic of the glandular epithelium of the lung, breast, and cervix among other tissues.28 The expression of CK7 in the conjunctiva has been described by Krenzer and Freddo.29 Elder et al.16 found CK7 in the basal and suprabasal epithelial cells of the central cornea, whereas, in contrast, it was not detected in any layer of the central corneal epithelium by Moroi et al.30
The purpose of this study was to detect CK7 on the ocular surface and to investigate whether this CK may be used as a more reliable marker for distinguishing between the corneal and conjunctival epithelia, particularly in patients with LSCD.
Materials and Methods
The study followed the standards of the Ethics Committee of the General Teaching Hospital and Charles University, Prague, and adhered to the tenets set out in the Declaration of Helsinki. All cadaveric bulbi, with no known eye disease, were obtained from the Ocular Tissue Bank Prague.
Control Samples
Imprints of the central corneal epithelium, peripheral corneal epithelium, and upper bulbar conjunctiva from six cadaveric bulbi (age, 39–61 years; mean age, 53.8 ± 8.4) were prepared and consequently used for indirect fluorescent immunocytochemistry to detect CK7 and CK19.
Epithelial imprints of the central cornea and conjunctiva from another six cadaveric bulbi (age, 26–80 years; mean age, 48.5 ± 17.1) were used for mRNA isolation and subsequently for the evaluation of CK7 expression by semiquantitative reverse transcription–polymerase chain reaction (RT-PCR).
In addition, epithelial imprints of the central cornea and conjunctiva from three different cadaveric bulbi (age, 46–79 years; mean age, 66.0 ± 17.6) were used for Western blot analysis. The time between death, impression cytology, and storage did not exceed 24 hours.
Finally, two cadaveric corneoscleral buttons (age, 27 and 61 years) were used for the detection of CK7 on cryosections by indirect fluorescence immunohistochemistry to visualize CK7 throughout the whole corneoconjunctival epithelial layer. Immediately after surgery, the buttons were dissected into four parts, snap frozen in liquid nitrogen, embedded in optimal cutting temperature compound, and stored at −70°C. The tissue was cryosectioned radially at a thickness of 7 μm, to evaluate all corneal, limbal, and perilimbal conjunctival layers; three sections were mounted per slide (Superfrost Plus; Fischer Scientific, Pittsburgh, PA). The immunohistochemistry procedure was performed as described previously.31 A cadaveric sample of human breast tissue, which was used as a positive control, was received from the Institute of Forensic Medicine and Toxicology of the First Faculty of Medicine and General University Hospital, Prague.
Patient Samples
Nine patients from the Department of Ophthalmology, General Teaching Hospital, and First Faculty of Medicine, Charles University, Prague (age, 1–62 years; mean age, 43 ± 20.4) with bilateral or unilateral diagnosed LSCD, were sent to the Laboratory of the Biology and Pathology of the Eye and examined by impression cytology to confirm the LSCD diagnosis. At first the LSCD diagnosis was based on clinical examination (biomicroscopy), where five patients presented with unilateral damage (three with chemical burns, one with a thermal burn, and one with injury of unknown etiology) and four patients with bilateral damage (all from chemical burns).
Impression Cytology
To prevent the contamination of the cornea by epithelial cells released from the ocular surface, cadaveric bulbi were carefully rinsed with PBS before imprinting and processing. Membranes used for the peripheral cornea imprints were first marked with two holes, which were imprinted on the limbal area and then used as markers for distinguishing between the corneal and conjunctival side.
Imprints were obtained using a sterile, single-packed membrane (10 mm diameter; Millicell CM, PICM 01250; Millipore, Bedford, MA), which was pressed on the ocular surface for 5 seconds. The membranes were then stored in their original packaging at −80°C until processing.
Impression cytology of the patients was performed bilaterally from the cornea and upper bulbar conjunctiva after the application of 0.4% oxybuprocaine hydrochloride eye drops as topical anesthesia. For GC evaluation in the patients, impression cytology was performed bilaterally on the cornea and upper bulbar conjunctiva, using nitroacetate cellulose filter papers (GSWP 0.4700, pore size 0.22 μm). The specimens were stained with a combination of periodic acid-Schiff (PAS) and Gill's modified Papanicolaou stains.32–34
Indirect Fluorescence Immunocytochemistry
The membranes (Millicell; Millipore) with the corneoconjunctival epithelium of six cadaveric bulbi and all the patients were released from their plastic holders (by treatment with acetone for 1 minute) and placed cell side up on round 12-mm coverslips. Then, the cells were rinsed in phosphate-buffered saline (PBS) and permeabilized in 0.2% Triton X-100. After they were washed, the membranes were exposed to a blocking solution (2.5% bovine serum albumin in PBS) for 20 minutes and then incubated with the primary antibodies diluted in PBS containing 0.1% bovine serum albumin for 1 hour at room temperature. The mouse monoclonal antibodies anti-CK7 (1:50, clone OV-TL12/30) and anti-CK 19 (1:50, clone RCK 108; DakoCytomation, Glostrup, Denmark) were used (for the patient samples only the CK7 antibody was used). The membranes were washed in PBS and incubated with fluorescein isothiocyanate–conjugated anti-mouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. After they were rinsed in PBS, the membranes were mounted with propidium iodide (Vectashield; Vector Laboratories, Inc. Burlingame, CA) to counterstain the DNA within the nuclei.
Specimen Assessment
All specimens were examined by light and fluorescence microscopy (model BX51; Olympus Co., Tokyo, Japan) at a magnification of 100× to 1000×. Images were taken with one of two cameras (CCD-1300; VDS Vosskühler GmbH, Germany, and ProgRes C12plus, Jenoptik, Laser.Optik.Systeme GmbH, Jena, Germany). An image-analysis system (NIS Elements; Laboratory Imaging, Za Drahou, Czech Republic) was used for cell analysis. The central and peripheral corneal and conjunctival epithelia were evaluated separately. At least 200 epithelial cells per assessed area were examined (in two cases with an insufficient number of cells per imprint, only 100 cells were examined). The number of GC and the percentage of CK7- and CK19-positive cells were calculated.
Semiquantitative Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted from imprints of the central corneal epithelium and upper bulbar conjunctival epithelium of six cadaveric bulbi (RNeasy Plus Micro Kit; Qiagen, Hilden, Germany). Six microliters of total RNA were reverse transcribed into cDNA in a 20-μL reaction mixture (SuperScript III/RNase OUT Enzyme Mix; Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Subsequently, individual samples were amplified with the following specific oligonucleotides for CK7 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH; synthesized by Generi Biotech, Hradec Králové, Czech Republic): human CK7, sense primer 5′-cag gac cct caa tga gacg-3′, antisense primer 5′-cca ggg agc gac tgt tgt-3′; and human GAPDH, sense primer 5′-AGC CAC ATC GCT CAG ACAC-3′, antisense primer 5′-GCC CAA TAC GAC CAA ATCC-3′. Reverse transcription reactions (65°C for 5 minutes, 50°C for 50 minutes, and 85°C for 5 minutes), with oligo(dT)20 were followed by 30 PCR cycles (initial denaturation 94°C for 3 minutes, 94°C for 45 seconds, 63°C for 30 seconds, and 72°C for 30 seconds) and a final extension step (10 minutes at 70°C). PCR products were analyzed by ethidium bromide–stained 2% agarose gel electrophoresis.
Western Blot Analysis
To prepare whole-cell extracts, we treated corneal and conjunctival epithelial cells on membranes (Millicell; Millipore) in lysis buffer containing 0.2% Triton X-100, 10% glycerol, 0.5 mM EDTA, 1 mM dithiothreitol, and protease inhibitors in PBS, followed by centrifugation for 15 minutes at 14,000g. The protein concentration was determined with a commercial kit (BCA Protein Assay Kit; Pierce, Rockford, IL). Equal volumes of protein extract and sample buffer were mixed, reduced by 5% β-mercaptoethanol, and fractionated on 5% to 10% SDS polyacrylamide gels.35 After electrophoresis was complete, the proteins were transferred to nitrocellulose membranes (Serva Electroforesis GmbH, Heidelberg, Germany) and blocked with 5% nonfat dry milk in PBS containing 0.05% Tween-20 (PBS-T) at 4°C overnight. After the membranes were washed in PBS-T, they were probed with mouse antibodies against CK7 (1:1000, clone OV-TL12/30; DakoCytomation) and β-actin (1:2000; Abcam, Cambridge, UK) for 2 hours at room temperature. After another wash in PBS-T, the membranes were incubated with peroxidase conjugated goat anti-mouse antibody (1:12,000, ImmunoPure; Pierce Biotechnology) with 1% BSA for 45 minutes at room temperature and washed with PBS-T. Positive reactions were visualized with an enhanced chemiluminescence technique (SuperSignalWest Femto Maximum Sensitivity Substrate kit; Pierce Biotechnology, Rockford, IL) for 5 minutes and examined with a membrane documentation system (Syngene Chemigenius-Q and the GeneSnap program; Synoptics Ltd., Cambridge, UK).
Results
Controls
The results of the immunocytochemical analysis of the corneal and conjunctival epithelia of controls stained with antibodies to CK7 (OV-TL 12/30) and CK19 are shown in Table 1.
Table 1.
Presence of CK-7 and -19 in Different Areas of the Corneal and Conjunctival Control Samples
| Samples | Central Cornea | Peripheral Cornea/Limbal Area |
Conjunctiva | |
|---|---|---|---|---|
| Corneal Side | Limbal Side | |||
| CK7 | ||||
| Co1 | N | N | 100 | 95 |
| Co2 | 1 | N | 100 | 95 |
| Co3 | 1 | 2 | 100 | 100 |
| Co4 | N | 5 | 95 | 80 |
| Co5 | N | N | 95 | 95 |
| Co6 | N | N | 95 | 100 |
| CK19 | ||||
| Co1 | 45 | 50 | 90 | 95 |
| Co2 | 60 | 35 | 95 | 100 |
| Co3 | 40 | 40 | 100 | 100 |
| Co4 | 30 | 50 | 95 | 90 |
| Co5 | 30 | 45 | 80 | 90 |
| Co6 | 30 | 55 | 65 | 65 |
Data are the percentage of positive cells. N, negative.
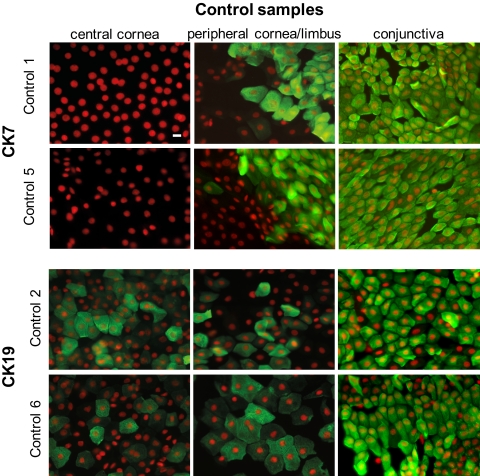
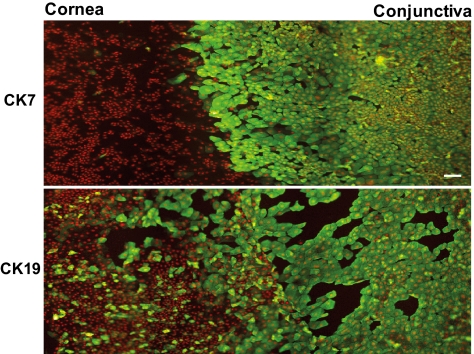
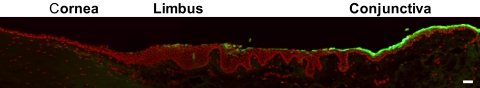
No CK7 signal was detected in the central and pericentral corneal epithelium of cadaveric samples (Fig. 1), except in two (control [Co]2, Co3) where less than 1% of the cells revealed positivity. These cells formed clusters above the completely negative corneal epithelial monolayer. A similar situation was observed in the peripheral cornea. The limbal side and the conjunctival epithelium revealed CK7 staining in almost 100% of the cells (Fig. 1). A prominent sharp border of the CK7 staining was noted in most of the control specimens between the negative peripheral corneal epithelium and the strongly positive conjunctival epithelium (Fig. 2).
Figure 1.
Indirect fluorescence immunocytochemistry on membranes. Expression of CK7 and -19 (FITC, green) in the control samples. The nuclei were counterstained with propidium iodide (red). Scale bar, 10 μm.
Figure 2.
Differences between the localization of CK7 and -19 (FITC, green) in the superficial epithelium of a control central cornea and conjunctiva. A prominent, sharp border between the absence and presence of the CK7 signal was noted, compared with the gradual decrease in CK19 staining. The nuclei were counterstained with propidium iodide (red). Scale bar, 50 μm.
CK19 staining was most prominent in the conjunctiva (90% of the cells), then gradually decreased in the peripheral and central cornea. The positivity for CK19 declined without any sharp border, and some CK19-positive cells were found in the central corneal epithelium as well (Figs. 1, 2).
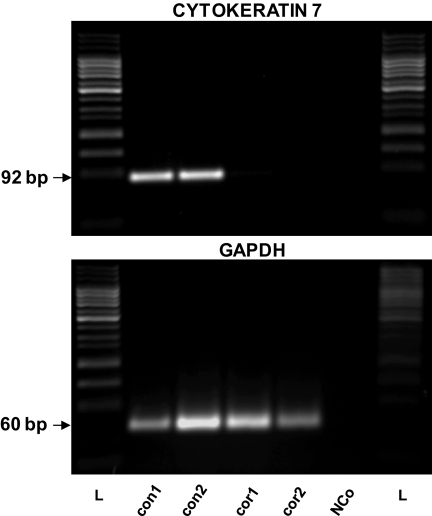
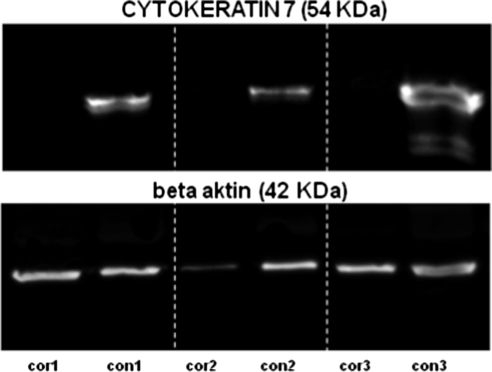
CK7 mRNA was found in each of the six samples of conjunctival epithelium using semiquantitative RT-PCR as was GAPDH mRNA, which served as an internal control. No CK7 expression was found in five samples of the central corneal epithelium, whereas the sixth sample of the central cornea exhibited the weak expression of CK7. Representative results are shown in Figure 3. Using Western blot analysis, CK7 (54 kDa) was detected in the surface conjunctival cells only, whereas the corneal epithelium was completely negative (Fig. 4).
Figure 3.
Expression of CK7 in two representative samples of surface conjunctival epithelium (con) and in two samples of corneal surface epithelium (cor) determined by RT-PCR. GAPDH was used as an internal control. NCo, negative control (reaction without sample cDNA), a marker for internal contamination; L, 50-bp DNA ladder (25–1000 bp).
Figure 4.
Expression of CK7 protein (54 kDa) in three different samples of corneal (cor) and conjunctival surface epithelium (con) as determined by Western blot analysis. β-Actin (42 kDa) was used as an internal control.
On cryosections, the central corneal epithelium was completely negative, and positivity for CK7 first appeared in the superficial epithelial layer of the limbal area, then strongly increased in the upper layer of the conjunctival epithelium (Fig. 5).
Figure 5.
Detection of CK7 (FITC, green) on a radial cryosection of a corneoscleral button. Nuclei were counterstained with propidium iodide (red). Scale bar, 50 μm.
Patients
The percentage of CK7-positive cells and the presence of GCs on the ocular surface of patients are shown in Table 2.
Table 2.
The Presence of CK7 and GCs in Patients with Unilateral and Bilateral LSCD
| Unaffected Cornea |
LSCD Cornea |
LSCD Conjunctiva |
||||
|---|---|---|---|---|---|---|
| CK7 | GC | CK7 | GC | CK7 | GC | |
| Unilateral damage | ||||||
| P1 | – | NS | 50 | − | 100 | − |
| P2 | – | − | 80 | + | 95 | + |
| P3 | – | − | 100 | + | 100 | + |
| P4 | – | NS | 75 | − | 70 | + |
| P5 | – | − | 50 | − | 70 | − |
| LSCD Cornea (RE) |
LSCD Cornea (LE) |
LSCD Conjunctiva |
||||
|---|---|---|---|---|---|---|
| CK7 | GC | CK7 | GC | CK7 | GC | |
| Bilateral damage | ||||||
| P6 | 95 | + | 100 | − | 100 LE | + |
| P7 | 90 | + | 100 | − | 95 LE | + |
| P8 | 95 | + | 100 | − | 100 RE | + |
| P9 | 80 | + | 90 | + | 80 RE | + |
+, presence of cells; −, absence of cells; RE, right eye; LE, left eye; NS, no specimen available.
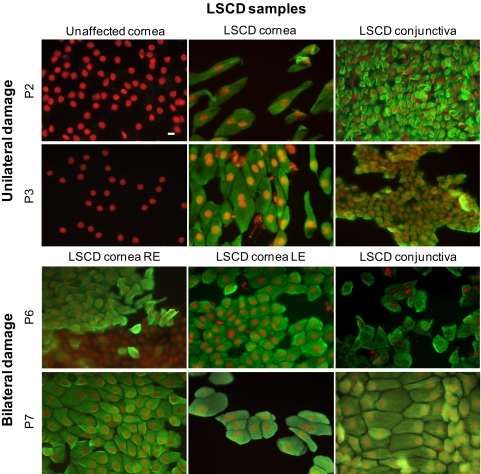
No CK7 or GCs were detected in the unaffected eyes of patients with unilateral damage. All affected corneas of patients with unilateral or bilateral LSCD revealed strong positivity for CK7 in 50% to 100% of the surface epithelial cells, whereas GCs were found only in 7 (54%) of 13 affected corneas.
CK7 was present in each of the nine tested conjunctivas from LSCD eyes, and GCs were present in only seven (78%; Table 2, Fig. 6).
Figure 6.
Ocular surface of patients (P) with LSCD. No CK7 (FITC, green) was present in the unaffected corneas. CK7 was expressed by normal conjunctiva as well as conjunctival cells that overgrew the corneas of the LSCD eyes. Indirect fluorescence immunocytochemistry was performed on the membranes, and nuclei were counterstained with propidium iodide (red). RE, right eye; LE, left eye. Scale bar, 10 μm.
Discussion
In this study, we present evidence that an anti-CK7 antibody (OV-TL12/30 clone) reacts almost exclusively with cells of the human conjunctival surface epithelium, but not with corneal epithelial cells. Because of this reactivity, the detection of CK7 becomes a new and reliable approach for the detection of the overgrowth of conjunctival cells over the corneal epithelium during LSCD.
The results reported up to now concerning the presence of CK7 in the anterior part of the eye are controversial. Some authors found CK7 only in GCs,29 some found it in the conjunctiva as well as the central and pericentral cornea,16 and some found no CK7 signal in the central part of the cornea.30 These discrepancies may be explained by the existence of conformation-dependent epitopes or the existence of isoforms for individual CK polypeptides.36
In the experiments presented herein, CK7 unambiguously and intensely stained the surface epithelial layer of the conjunctiva but not the superficial corneal epithelium. We used cryosections to determine the precise location of the border between areas with positive and negative CK7 staining. Finally, we did not detect a CK7 signal in any layer of the corneal epithelium, but such a signal was found in the superficial layers of the conjunctival epithelium with a sharp border in the limbal area. The same results (CK7 expression in the conjunctiva but not in the cornea) were obtained using RT-PCR and Western blot analysis. With RT-PCR, only one corneal sample revealed weak positivity for CK7. This could be caused by the contamination of the corneal sample with conjunctival cells during impression cytology. Western blot analysis showed one positive band in the distinct and specific area of 54 kDa in the surface conjunctival epithelium of all three samples, compared with the completely negative corneal epithelium.
Commercially available anti-keratin antiserums can exhibit great variability with respect to reactivity, quality, and methodological approach, together with the condition of the tissue used.31,37,38 The OV-TL 12/30 clone of CK7 antibody was described as a chain-specific monoclonal antibody that stains intermediate filament structures.39 Moreover, OV-TL 12/30 antibody was found to specifically react with CK7 on electrophoretically separated cytoskeletal preparations (one- and two-dimensional immunoblots) of human cell lines.40 Differences in the reactivity patterns of individual CK7 antibodies were explained as the likely result of epitope masking.40 This fact led us to use other available clones of anti-CK7 antibodies (clone C-68; Exbio, Prague, Czech Republic, and clone LP5K, Chemicon International Inc., Temecula, CA). None of the other tested antibodies showed such pronounced positivity present throughout the whole conjunctival surface as did the OV-TL12/30 clone. Both of these antibodies detected a much lower number of positive epithelial cells on the conjunctival surface (∼25% of the superficial conjunctival cells, data not shown). Based on these findings, we consider the OV-TL12/30 clone of the CK7 antibody to be a new and reliable marker for distinguishing between conjunctival and corneal surface epithelia in the diagnosis of LSCD.
In our findings, the OV-TL12/30 anti-CK7 antibody, used as a diagnostic marker for detecting the overgrowth of conjunctival cells over the cornea, was more effective than the antibodies commonly used to detect CK19, the expression of which decreased centripetally from the conjunctiva toward the peripheral, pericentral, and central corneal epithelium. In addition, the border between the cornea and conjunctiva was more clearly delineated by CK7 than by CK19. In addition, we unambiguously showed that the detection of CK7-positive conjunctival epithelium on the corneal surface was more specific than the detection of GCs, which were not present either on the conjunctival or the corneal surface, particularly in the severe stages of ocular damage. We believe that our technique could be easily and widely used by other laboratories.
Finally, if impression cytology is considered as a diagnostic tool for the confirmation of LSCD, we recommend performing a morphologic assessment (including cell morphology and GC evaluation) on nitroacetate cellulose filters, together with immunofluorescent examination of CK7 on the corneal surface, especially using the OV-TL 12/30 clone of the anti-CK7 antibody.
Acknowledgments
The authors thank the Department of Ophthalmology, 1st Medical Faculty of Charles University and General Teaching Hospital in Prague for cooperation with this study and for the clinical assessment of LSCD.
Footnotes
Supported by Research Project MSM0021620806 of the Ministry of Education, Youth and Sports of the Czech Republic and by Project 260501 from Charles University in Prague.
Disclosure: K. Jirsova, None; L. Dudakova, None; S. Kalasova, None; V. Vesela, None; S. Merjava, None
References
- 1. Sack RA, Nunes I, Beaton A, Morris C. Host-defense mechanism of the ocular surfaces. Biosci Rep. 2001;21:463–480 [DOI] [PubMed] [Google Scholar]
- 2. Thoft RA, Friend J, Kenyon KR. Ocular surface response to trauma. Int Ophthalmol Clin. 1979;19:111–131 [PubMed] [Google Scholar]
- 3. Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49:5279–5286 [DOI] [PubMed] [Google Scholar]
- 4. Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–863 [DOI] [PubMed] [Google Scholar]
- 5. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443 [PubMed] [Google Scholar]
- 6. Foster CS, Sainz De La Maza M. Ocular cicatricial pemphigoid review. Curr Opin Allergy Clin Immunol. 2004;4:435–439 [DOI] [PubMed] [Google Scholar]
- 7. Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485 [DOI] [PubMed] [Google Scholar]
- 8. Solomon A, Espana EM, Tseng SC. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology. 2003;110:93–100 [DOI] [PubMed] [Google Scholar]
- 9. Sridhar MS, Vemuganti GK, Bansal AK, Rao GN. Impression cytology-proven corneal stem cell deficiency in patients after surgeries involving the limbus. Cornea. 2001;20:145–148 [DOI] [PubMed] [Google Scholar]
- 10. Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52:483–502 [DOI] [PubMed] [Google Scholar]
- 11. Tseng SH, Yen JS, Chien HL. Lens epithelium in senile cataract. J Formos Med Assoc. 1994;93:93–98 [PubMed] [Google Scholar]
- 12. Maskin SL, Heitman KF, Lawton AW, Yee RW. Diagnostic impression cytology for external eye disease. Cornea. 1989;8:270–273 [PubMed] [Google Scholar]
- 13. Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981;21:135–142 [PubMed] [Google Scholar]
- 14. Egbert PR, Lauber S, Maurice DM. A simple conjunctival biopsy. Am J Ophthalmol. 1977;84:798–801 [DOI] [PubMed] [Google Scholar]
- 15. Donisi PM, Rama P, Fasolo A, Ponzin D. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22:533–538 [DOI] [PubMed] [Google Scholar]
- 16. Elder MJ, Hiscott P, Dart JK. Intermediate filament expression by normal and diseased human corneal epithelium. Hum Pathol. 1997;28:1348–1354 [DOI] [PubMed] [Google Scholar]
- 17. Jirsova K, Neuwirth A, Kalasova S, Vesela V, Merjava S. Mesothelial proteins are expressed in the human cornea. Exp Eye Res. [DOI] [PubMed] [Google Scholar]
- 18. Pitz S, Moll R. Intermediate-filament expression in ocular tissue. Prog Retin Eye Res. 2002;21:241–262 [DOI] [PubMed] [Google Scholar]
- 19. Kasper M, Moll R, Stosiek P, Karsten U. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry. 1988;89:369–377 [DOI] [PubMed] [Google Scholar]
- 20. Kivela T, Uusitalo M. Structure, development and function of cytoskeletal elements in non-neuronal cells of the human eye. Prog Retin Eye Res. 1998;17:385–428 [DOI] [PubMed] [Google Scholar]
- 21. Chen WY, Mui MM, Kao WW, Liu CY, Tseng SC. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Curr Eye Res. 1994;13:765–778 [DOI] [PubMed] [Google Scholar]
- 22. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24 [DOI] [PubMed] [Google Scholar]
- 23. Lauweryns B, van den Oord JJ, De Vos R, Missotten L. A new epithelial cell type in the human cornea. Invest Ophthalmol Vis Sci. 1993;34:1983–1990 [PubMed] [Google Scholar]
- 24. Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47:4780–4786 [DOI] [PubMed] [Google Scholar]
- 26. Leube RE, Bosch FX, Romano V, Zimbelmann R, Hofler H, Franke WW. Cytokeratin expression in simple epithelia. III. Detection of mRNAs encoding human cytokeratins nos. 8 and 18 in normal and tumor cells by hybridization with cDNA sequences in vitro and in situ. Differentiation. 1986;33:69–85 [DOI] [PubMed] [Google Scholar]
- 27. Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramaekers F, Huysmans A, Schaart G, Moesker O, Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987;170:235–249 [DOI] [PubMed] [Google Scholar]
- 29. Krenzer KL, Freddo TF. Cytokeratin expression in normal human bulbar conjunctiva obtained by impression cytology. Invest Ophthalmol Vis Sci. 1997;38:142–152 [PubMed] [Google Scholar]
- 30. Moroi SE, Gokhale PA, Schteingart MT, et al. Clinicopathologic correlation and genetic analysis in a case of posterior polymorphous corneal dystrophy. Am J Ophthalmol. 2003;135:461–470 [DOI] [PubMed] [Google Scholar]
- 31. Merjava S, Neuwirth A, Mandys V, Jirsova K. Cytokeratins 8 and 18 in adult human corneal endothelium. Exp Eye Res. 2009;89:426–431 [DOI] [PubMed] [Google Scholar]
- 32. Gill GW, Frost JK, Miller KA. A new formula for a half-oxidized hematoxylin solution that neither overstains nor requires differentiation. Acta Cytol. 1974;18:300–311 [PubMed] [Google Scholar]
- 33. Martinez AJ, Mills MB, Jaceldo KB, et al. Standardization of conjunctival impression cytology. Cornea. 1995;14:515–522 [PubMed] [Google Scholar]
- 34. Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–733 [DOI] [PubMed] [Google Scholar]
- 35. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685 [DOI] [PubMed] [Google Scholar]
- 36. Kasper M, Stosiek P, Lane B. Cytokeratin and vimentin heterogeneity in human cornea. Acta Histochem. 1992;93:371–381 [DOI] [PubMed] [Google Scholar]
- 37. Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A. 2005;102:9523–9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mygind H, Nielsen B, Moe D, Clausen H, Dabelsteen E, Clausen PP. Antikeratin antibodies in routine diagnostic pathology: a comparison of 10 different commercial antikeratins. Apmis. 1988;96:1009–1022 [PubMed] [Google Scholar]
- 39. van Niekerk CC, Jap PHK, Thomas CMG, et al. Marker profile of mesothelial cells versus ovarian carcinoma cells. Int J Cancer. 1989;43:1065–1071 [DOI] [PubMed] [Google Scholar]
- 40. Ramaekers F, van Niekerk C, Poels L, et al. Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol. 1990;136:641–655 [PMC free article] [PubMed] [Google Scholar]